Abstract
Is it important to adopt the perspective of the model when learning a new skill? Is the “mirror system” equally involved when the teacher is facing or side-by-side with students? In this functional MRI study, we measured the cerebral hemodynamic changes in participants who watched video-clips depicting simple hand or foot actions. The participants either watched passively or imitated these actions. Half the video-clips depicted actions filmed from the perspective of the participant (1st-person perspective) and half from a frontal view as if watching someone else (3rd-person perspective). Behavioral results showed that latency to imitate was significantly shorter for the 1st-person perspective than the 3rd-person perspective. Functional imaging results demonstrate that the observation of intransitive actions engaged primary visual and extrastriate visual areas, but not the premotor cortex. Imitation vs. observation of actions yielded enhanced signal in the contralateral somatosensory and motor cortices, cerebellum, left inferior parietal lobule and superior parietal cortex, and left ventral premotor cortex. Activity in the lateral occipital cortex around the extrastriate body area was significantly enhanced during imitation, as compared to observation of actions confirming that this region involvement reaches beyond the perception of body parts. Moreover, comparisons of the two visual perspectives showed more activity in the left sensory-motor cortex for 1st-person, even during observation alone, and in the lingual gyrus for 3rd-person perspective. These findings suggest that the 1st-person perspective is more tightly coupled to the sensory-motor system than the 3rd-person perspective, which requires additional visuospatial transformation. Published by Elsevier Inc.
Keywords: Imitation, Perspective-taking, 1st-person vs. 3rd-person visual perspective, Extrastriate body area, Functional MRI
Introduction
There has been an upsurge of interest for the neurophysiological investigation of imitation, which derives from at least three sources (see Brass and Heyes, 2005; Meltzoff and Decety, 2003; Meltzoff and Prinz, 2002). First, human newborns imitate, suggesting a common representation mediating the observation and execution of human action (Meltzoff, 2005; Meltzoff and Moore, 1997). Second, the discovery of mirror neurons in the monkey has provided a physiological model for the basic mechanism of this perception-action coupling, which is involved in imitation and action understanding (Rizzolatti and Craighero, 2004; Rizzolatti et al., 2001; Rumiati and Bekkering, 2003; Williams et al., 2001). These mirror neurons, located in the ventral premotor cortex (area F5), as well as in parietal area PF, fire both when the monkey carries out a goal-directed action and when it observes the same action performed by another individual. Subsequent observations (Umilta et al., 2001; Kohler et al., 2002) strongly suggest that mirror neurons represent general aspects of actions such as the goal or its consequences rather than some other more elementary property of the movements (e.g., specific motor commands, muscle activity, movement direction). Third, new neuroimaging methods have become available to examine the anatomical areas involved in perception-action coupling in humans under diverse sophisticated paradigms (Decety, 2006; Decety and Chaminade, 2005; Iacoboni, 2005; Jackson and Decety, 2004).
In humans, a large number of neurophysiological studies demonstrated that a motor resonance mechanism in the premotor and the posterior parietal cortices exists when subjects observe goal-directed actions being executed by other people (e.g., Baldissera et al., 2001; Buccino et al., 2001; Fadiga et al., 1995; Grafton et al., 1996; Hamzei et al., 2003; Hari et al., 1998) or even when only the goal of these action is visible (Chao and Martin, 2000; Grèzes and Decety, 2001a; Grèzes et al., 2003a,b). This motor resonance mechanism is also involved in action imitation as reported by various functional imaging studies (e.g., Buccino et al., 2004; Chaminade et al., 2002; Decety et al., 1997, 2002; Iacoboni et al., 1999; Nishitani and Hari, 2000; Tanaka et al., 2001; Tanaka and Inui, 2002; Williams et al., 2005). Furthermore, interference between action observation and action execution has been demonstrated in a number of behavioral studies (Brass et al., 2001; Castiello, 2003; Kilner et al., 2003; Prinz, 2005), which also suggests a direct link between perception and action.
Together, monkey and humans data have often been interpreted in favor of the direct-matching hypothesis, which states that we understand actions by mapping the visual representation of the observed action onto our motor representation of the same action (Rizzolatti et al., 2001). Nevertheless, there remain unresolved issues not accounted for by the current mirror neuron system interpretation such as, for instance, whether an action needs to be directed towards a goal object for this system to be involved (i.e., ‘transitive’ action). Moreover, human brain imaging studies have not been as clear cut as the monkey literature showing that the ventral premotor cortex is involved whether the movements were goal-directed (e.g., Grèzes et al., 2003a,b) or not (e.g., Iacoboni et al., 1999, Koski et al., 2003; Tanaka and Inui, 2002). While it appears clear the action mirror system is involved when a goal is present, it remains uncertain whether it still is when the action to be imitated is ‘intransitive’. Intransitive actions are particularly interesting because they are simple, easy to execute, and constitute the first actions that newborns are able to imitate (Meltzoff and Moore, 1977, 1997).
Another aspect of imitation that has been less investigated is the effect of the visual perspective of the model on the neural basis for this type of behavior. Despite various definitions of imitation, most scholars agree that this capacity requires that one maps one’s own behavior onto the behavior observed in another individual. Therefore, visual perspective transformation is a crucial aspect for matching one’s own body to that of another person. It remains uncertain whether mirror neurons are differentially involved by varying visual perspectives. The goal of the act is invariant regardless of which perspective it is viewed from. Assuming that mirror neurons code the goal of an action and not the movements to achieve it, which is the traditional assumption in the literature, they would not be sensitive to the visual perspective of the model. Most functional neuroimaging investigations have presented movements or actions via static images or video-clips shown from a 3rd-person visual perspective only, i.e., the model facing the imitator.
In this experiment, we presented participants with a series of simple hand and foot intransitive actions (such as rotations, lateral of vertical movements) from two different visual perspectives and which they had to imitate or observe. One perspective (1st person) did not require spatial transformation because the model was presented as if the camera was in the viewer’s eyes. In the other perspective (3rd person), the model was presented from the front, as if looking in a mirror or seeing someone else’s limbs (Fig. 1). We predicted that both task and perspective differences will lead to motor-areas-related changes. At the behavioral level, we predicted that, when the model’s perspective matched that of the participants (1st-person perspective), the time taken to initiate the same movement should be shorter than when the model perspective is seen from the front (3rd-person perspective), because the motor representation of the action should be more readily available and thus lead to a more robust pattern of activation within motor-related structures. At the hemodynamic level, it was therefore expected that imitation and observation (which also evoke motor representations) in the 1st-person perspective yield stronger activation in the sensory-motor regions including primary motor cortex, supplementary motor area and cerebellum. This pattern was also predicted when comparing imitation to observation of action.
Fig. 1.
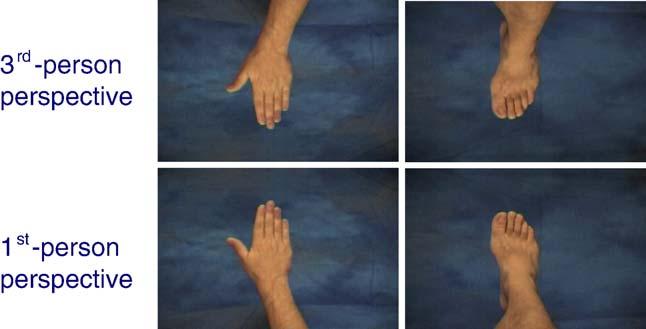
Static illustration of the video-clips used in the experiment, as they were shown to the participants. Top row: 3rd-person visual perspective; bottom row: 1st-person visual perspective.
In visual areas, greater hemodynamic response was predicted especially in areas of the lateral occipito-temporal region specialized for processing body parts (Chan et al., 2004), when spatial transformations are needed in imitation (3rd-person perspective). Moreover, specific involvement of the right temporo-parietal junction was expected during 3rd-person perspective based on previous work on perspective-taking (e.g., Ruby and Decety, 2001). It is known that the posterior parietal cortex plays a fundamental role in motor representation (Halsband et al., 2001; Rushworth et al., 2003; Sirigu et al., 1996) and bodily awareness (Berlucchi and Aglioti, 1997). Although there is no dispute that this region has an important role in action understanding and imitation (e.g., Buxbaum et al., 2005; Meltzoff and Decety, 2003; Chaminade et al., 2005), its contribution to different visual perspectives remains to be clarified. An area in the posterior parietal cortex at the temporo-parietal junction seems to mediate a specialized neural mechanism for egocentric perspective transformations (Jeannerod, 2003). We thus expected to detect signal changes related to the different visual perspectives in this region. Finally, the fact that the actions used in the study are “intransitive”, that is, they do not involve any objects, reduces the potential participation of the inferior frontal gyrus (the human homologue region of monkey area F5) during conditions of visual observation of the model.
Material and methods
Subjects
Sixteen healthy individuals aged 29 ± 6.5 years were recruited (8 females, 8 males). Participants gave informed consent according to the declaration of Helsinki. They were paid for their participation. The study was approved by the local ethics committee.
Stimuli and task
A series of 5-s video-clips showing different hand and foot actions performed by a human model were used. Examples of the videos are shown in Fig. 1. Half of the clips were from the 1st-person visual perspective and half from the 3rd-person perspective (see Fig. 1), and, within each perspective, half the clips were of hands and half of feet movements. Three different types of movements were shown, each executed in two directions for a total of 6 different movements: rotations (clockwise and anti-clockwise), horizontal movement (initiated on the left or the right), and vertical movements (initiated towards the top or the bottom). The video-clips were edited to show only the limbs of the model centered against a blue background. Each clip was comprised of two repetitions of the same action.
In the scanner, the participants were shown the video-clips and instructed to either watch the actions (Observation) or to imitate on-line the actions (Imitation) by starting as quickly as possible. Note that imitation of the 1st-person perspective model meant that the subject was watching and doing the actions from the same perspective they saw on the video-clip, but with their own body; whereas imitation of the 3rd-person perspective involved reproducing an action while observing another person do it, as if you are facing that person. Finally, a baseline condition consisted on watching a static cross on a blue background.
Functional MRI procedure
Participants took part in five EPI runs following a 2 × 2 factorial design (Perspective = 1st-person or 3rd person × Task = Imitation or Observation). Each run comprised 18 blocks of three trials. Each block consisted of 3 trials of the same perspective and the same task but with one of each different type of movements (rotation, horizontal, vertical). A trial consisted of 2 repetitions of the exact same movement for a total duration of 5 s. Each of the 4 following conditions was repeated 4 times in a run: (1) observe 1st-person, (2) observe 3rd-person, (3) imitate 1st-person, (4) imitate 3rd-person, and a fifth condition, consisting of a visual baseline (static cross) was repeated twice in each run. Half the blocks presented hand stimuli, half feet stimuli, randomized across sessions. The participants were instructed via a 1-s screen at the beginning of each block to either “watch” the actions (or the cross) shown without moving their limbs (observation 1st-person, observation 3rd-person) or “imitate” these actions in synchrony with the model (imitation 1st-person, imitation 3rd-person). Participants were provided with several training trials prior to the scanning sessions in order to learn to comply with the task and perform the actions as instructed.
Participants’ behavior was videotaped with a digital camera during scanning. These recordings were later coded for errors (i.e., whether the action imitated was the correct one and whether movement occurred during observation trials). Response latencies were also scored; frame-by-frame analysis of the videotaped records was conducted (one frame = 1/30th s) to evaluate the delay between the beginning of the model’s action and that of the participant. Response latencies were submitted to a 2 (Perspective) × 2 (Limb) ANOVA.
Functional MRI data acquisition and analysis
MRI data were acquired on a 3-T head-only Siemens Magnetom Allegra System equipped with a standard quadrature head coil. Changes in blood-oxygenation-level-dependent (BOLD) T2*-weighted MR signal were measured using a gradient echo-planar imaging (EPI) sequence (repetition time TR = 2000 ms, echo time TE = 30 ms, FoV = 192 mm, flip angle 80°, 64 × 64 matrix, 34 slices/slab, slice thickness 4.5 mm, no gap, voxel size = 3.0 × 3.0 × 4.5 mm). For each scan, a total of 166 EPI volume images were acquired along the AC-PC plane. Structural MR images were acquired with an MPRAGE sequence (TR = 2500, TE = 4.38, FoV = 256 mm, flip angle = 8°, 256 × 256 matrix, 160 slices/slab, slice thickness = 1 mm, no gap).
Image processing was carried out using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK), implemented in MATLAB 6.5 (Mathworks Inc. Sherborn, MA). Images were realigned and normalized using standard SPM procedures. The normalized images of 2 × 2 × 2 mm were smoothed by a FWHM 8 × 8 × 8 Gaussian kernel. A first fixed effect level of analysis was computed subject-wise using the general linear model with hemodynamic response function modeled as a boxcar function whose length covered the three successive trials of the same condition. The five conditions described previously were included in the model. Note that the decision not to include Limb as a separate factor stems from the facts that changes in perspective and task were the main questions of interest, and the results of preliminary analyses revealed potential power issues. First-level contrasts were introduced in second-level random-effect analysis to allow for population inferences. Main effects were computed using one-sample t test, including all subjects for each of the contrasts of interest, which yielded a statistical parametric map of the t statistic (SPM t). A voxel-level threshold of P < 0.001 uncorrected for multiple comparisons (t = 3.73) and extend threshold of k = 10 were used to identify regions where significant hemodynamic changes were observed. Unless otherwise specified, all spatial localizations were made using MNI coordinates.
Results
Behavioral results
The video analysis of participants’ behavior shows that the overall error rate for the imitation trials was very low, 3.9%. No errors were made on the limb (hand of foot) or the type of movement (rotation, vertical, horizontal); the only errors were in the direction of the movement (e.g., rotation clockwise as opposed to counterclockwise). This demonstrates that participants understood the directions and performed the task efficiently. Interestingly, however, 90% of those errors were made during the 3rd-person condition.
As expected, the analysis of the response latencies showed a significant main effect for visual perspective. Participants were significantly faster in imitating a model seen from the 1st-person versus 3rd-person perspective [F(1,15) = 31.62, P < 0.001] (Fig. 2). In fact, every subject showed this effect. This fits our hypothesis that the more visuospatial similarity between the model and the observer, the easier the movement is initiated. Other studies have previously shown that compatibility between the movement to be executed and the model is a factor that significantly influences motor performance (e.g., Brass et al., 2001; Craighero et al., 2002; Heyes et al., 2005; Vogeley et al., 2004; Vogt, 2003). Participants were also faster at imitating with the hand versus the foot [F(1, 15) = 8.39, P < 0.05] which makes sense given the differences in motor control. There was no significant Visual Perspective × Limb interaction.
Fig. 2.
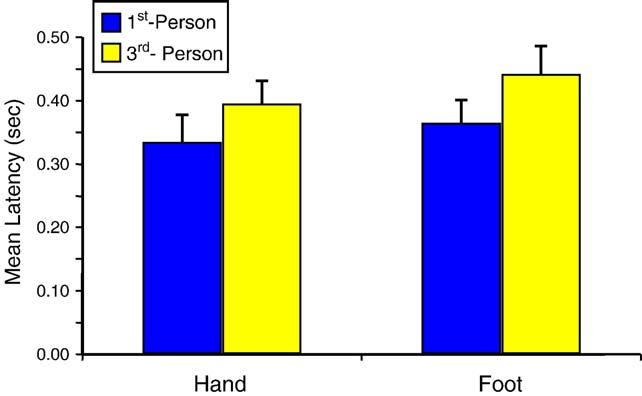
Latency to imitate the actions (+SE) as a function of perspective from which the model was seen (1st vs. 3rd person) and limb used.
Functional imaging results
The functional imaging data are presented in the following order: (1) main/simple effects related to the task, comparing Observation and Imitation and comparing both to the Baseline condition, (2) simple/main effects related to the visual perspective, comparing 1st-person and 3rd-person perspectives and comparing both to the Baseline condition, and (3) interactions between Task and Perspective factors.
Effects of task
The imitation conditions versus the visual baseline (static cross) showed the extensive neural network involved in action production including the left motor and somatosensory cortices, bilateral inferior parietal lobule, superior parietal cortex, and cerebellum (Fig. 3). Distinct activation sites were detected in the foot (x = -6, y = -32, z = 78) and hand (x = -32, y = -22, z = 60) areas of the somatosensory motor cortex. Moreover, significant bilateral activation was found at the lateral occipital cortex near the temporal junction, including MT and the extrastriate body area (EBA; e.g., Downing et al., 2001).
Fig. 3.
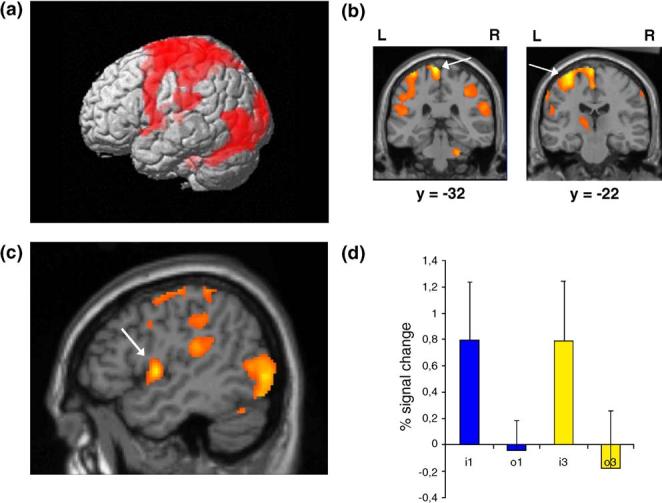
(a) Results from the Imitation vs. Baseline contrast showing the network involved in action production, including the left inferior parietal lobule and superior parietal cortex, cerebellum, and (b) motor and somatosensory cortices [foot x = -6, y = -32, z = 78 (b), and hand area x = 32 y = 22, z = 60]. (c) Peak of activity in the ventral premotor cortex (x = -48, y =0, z = 2) and (d) percent signal change (+SD) in this area for the different conditions (i1 = imitate 1st-person; o1 = observe 1st-person; i3 = imitate 3rd-person; o3 = observe 3rd-person). Results are superimposed on the MNI MRI template.
The cerebral activity during Observation irrespective of the visual perspective of the model contrasted with the visual baseline resulted in different clusters of activation in the cuneus (BA 18) and extensive bilateral activation at the occipito-temporal junction. The latter clusters were centered around the EBA in both hemispheres [(x = -50, y = -74, z = 2) and (x = 48, y = -72, z = 2)]. No signal change was found in the ventral premotor cortex, even at a very liberal threshold (P < 0.1 uncorrected).
Signal change in EBA was greater in the Imitation than in the Observation conditions in both hemispheres (Fig. 4). Percentage signal changes were extracted for each session separately, averaged for each subject, and analyzed separately for each hemisphere. Values in the right EBA for each Task (Imitation, Observation) and each Perspective (1st-person, 3rd-person) were submitted to a 2 × 2 repeated measures ANOVA. The results showed a significant effect of Task [F(1,15) = 7.17, P < 0.05], but not of Perspective [F(1,15) < 1, ns] and no significant interaction between these variables [F(1,15) < 1, ns]. The same pattern of results was found for the left EBA (not shown).
Fig. 4.
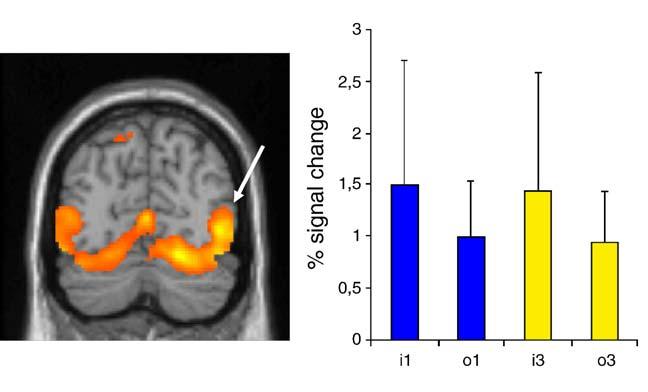
Peak of activation in the right extrastriate body area (EBA) in the Imitation vs. Baseline contrast and percent signal change (+SD) in the same region (x = 48, y = -72, z = 2) for the different conditions (i1 = imitate 1st-person; o1 = observe 1st-person; i3 = imitate 3rd-person; o3 = observe 3rd-person). Results are superimposed on the MNI MRI template. Note that the same pattern of result was found for the left EBA.
The main effect of Task (Imitation vs. Observation) collapsed over the different perspectives showed a number of motor-related areas including primary motor cortex, supplementary motor area (SMA), dorsal and ventral premotor cortex (precentral operculum), thalamus, and cerebellum (Table 1). Additional peaks were found in the parietal cortex, notably in the right inferior and superior parietal lobules and the precuneus. The reverse contrast (Observation vs. Imitation) did not yield any significant peaks.
Table 1.
Main effect of Task: brain regions that showed significantly more activation during the Imitation than during the Observation condition for both visual perspectives collapsed
| Region | L/R | x | y | z | t value |
|---|---|---|---|---|---|
| Precentral gyrus — front operc — PMv | R | 58 | 16 | -6 | 5.41 |
| Precentral gyrus — PMv | L | -56 | 12 | -8 | 5.55* |
| Inferior frontal gyrus | L | -60 | 10 | 30 | 4.13 |
| Middle frontal gyrus — PMd | L | -56 | 4 | 42 | 4.64 |
| Middle frontal gyrus — PMd | R | 46 | 2 | 60 | 4.16 |
| Precentral gyrus —PMv | L | -48 | 0 | 2 | 4.99 |
| Thalamus | L | -12 | -6 | 16 | 4.26 |
| Middle frontal gyrus — PMd | L | -24 | -8 | 74 | 9.00* |
| Medial frontal gyrus — SMA | L | -4 | -14 | 78 | 9.90* |
| Cerebellum — lobule III | R | 22 | -30 | -34 | 4.70 |
| Primary motor cortex | L | -4 | -32 | 78 | 8.95* |
| Inferior parietal lobule | R | 52 | -38 | 60 | 4.35 |
| Inferior parietal lobule | R | 44 | -38 | 46 | 4.21 |
| Cerebellum — lobule IV | R | 4 | -50 | -4 | 4.78 |
| Cerebellum — lobule VI | L | -38 | -56 | -30 | 3.95 |
| Superior parietal lobule | R | 8 | -58 | 70 | 4.35 |
| Cerebellum — lobule VI | R | 34 | -60 | -26 | 4.53 |
| Precuneus | R | 10 | -70 | 62 | 4.47 |
The peaks are ordered from the most rostral to the most caudal. Localization of the cerebellum peaks was done based on Schmahmann et al. (2000).
P <0.05 corrected.
When each condition of imitation was contrasted with its corresponding observation condition (i.e., imitation 1st-person vs. observation 1st-person perspective, and imitation 3rd-person vs. observation 3rd-person perspective), thus removing the visual input from the model, significant activation was detected in the left ventral premotor cortex, the SMA proper, the cerebellum (mostly in the ipsilateral hemisphere), as well as the primary somatosensory and motor cortices (in the hand and foot areas). Although this network was similar for the contrast in both perspective conditions, one major difference was that activation was more bilateral and extended to the left secondary somatosensory cortex (SII) in the 1st-person perspective task contrast (Fig. 5).
Fig. 5.
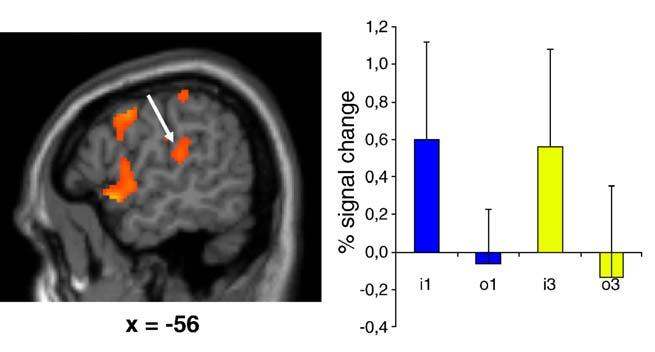
Peak of activation in the left SII in the contrast Imitation 1st-person vs. Observation 1st-person perspective and percent signal change (+SD) in the same region (x = -56, y = -26, z = 18; t = 4.40) for the different conditions (i1 = imitate 1st-person; o1 = observe 1st-person; i3 = imitate 3rd-person; o3 = observe 3rd-person). Results are superimposed on the MNI MRI template.
Effects of perspective
The contrasts of 1st-person perspective vs. Baseline and 3rd-person perspective vs. Baseline yielded very similar results and very extensive bilateral activation of the occipital regions and occipito-parietal junction, as well as motor-related areas, mainly in the left hemisphere. Comparison of the two visual perspectives, collapsed over the different tasks, was then directly performed (1st-person vs. 3rd-person perspective) and showed peaks of activation in the cuneus bilaterally, right orbitofrontal cortex, right middle temporal gyrus, left inferior frontal gyrus, and most interestingly in the left post- and precentral gyri (Fig. 6 and Table 2). The reverse contrast (3rd-person vs. 1st-person perspective) yielded a single cluster in the lingual gyrus (x = 4, y = below -78, z = -2 )which fell our conservative extend threshold (Table 2).
Fig. 6.
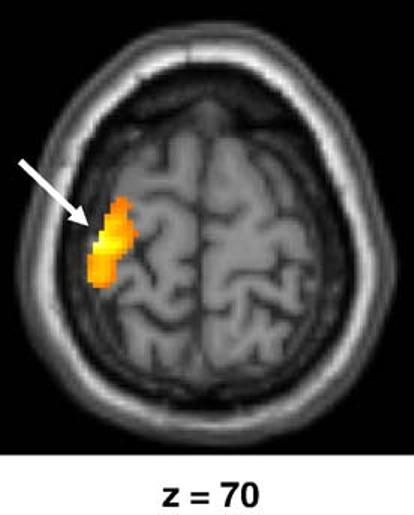
Left sensory-motor cortex activation (x = -36, y = -22, z = 70; t = 7.59) in the contrast 1st-person perspective vs. 3rd-person perspective, irrespective of the task. Results are superimposed on the MNI MRI template.
Table 2.
Effects of perspective
| Region | L/R | x | y | z | t value |
|---|---|---|---|---|---|
| (a) Main effect of Perspective: brain areas that showed significantly more activation during 1st person than 3rd-person perspective for both tasks combined | |||||
| Medial frontal gyrus (BA11) | R | 4 | 56 | -16 | 5.04 |
| Inferior frontal gyrus | L | -26 | 30 | -22 | 5.87 |
| Cingulate gyrus — OFC (BA 25) | R | 4 | 8 | -10 | 3.97a |
| Precentral gyrus — M1 | L | -28 | -12 | 72 | 5.57 |
| Precentral gyrus — M1 | L | -36 | -22 | 70 | 7.59* |
| Postcentral gyrus | L | -34 | -32 | 72 | 5.96 |
| Middle temporal gyrus (BA 21) | R | 62 | -50 | -2 | 4.36 |
| Cuneus (BA 18) | R | 6 | -92 | 20 | 9.16* |
| Cuneus (BA 18) | L | -4 | -100 | 6 | 5.34 |
| (b) 3rd-person vs. 1st-person perspective | |||||
| Lingual gyrus (BA18) | R | 4 | -78 | -2 | 4.02a |
| (c) Imitate 1st-person vs. Imitate 3rd-person perspective | |||||
| Frontal pole (BA 10-11) | L-R | 0 | 60 | -8 | 5.49 |
| Orbitofrontal (BA 25) | L | -4 | 14 | -8 | 4.37 |
| Postcentral gyrus | L | -46 | -18 | 62 | 7.83 |
| Precentral gyrus — M1 | L | -32 | -20 | 72 | 7.36 |
| Cuneus (BA 17-18) | R | 2 | -96 | -16 | 9.54* |
| Cuneus (BA 17-18) | L | -10 | -100 | -8 | 5.15 |
| Cuneus (BA 17-18) | R | 12 | -100 | -2 | 5.20 |
| (d) Observe 1st-person vs. Observe 3rd-person perspective | |||||
| Precentral gyrus | L | -36 | -22 | 70 | 4.73 |
| Cuneus (BA 18) | R | 4 | -90 | 18 | 6.53 |
| Cuneus (BA 18) | L | -6 | -100 | 6 | 4.74 |
| (e) Imitate 3rd-person vs. Imitate 1st-person perspective | |||||
| Inferior frontal gyrus (BA 47) | R | 34 | 20 | -16 | 4.93 |
| Cerebellum — lobule III | R | 8 | -44 | -26 | 4.98 |
| Lingual gyrus | L | -22 | -62 | -10 | 4.32 |
| Lingual gyrus | R | 4 | -76 | 0 | 6.27 |
| Lingual gyrus | R | 20 | -76 | -6 | 5.87 |
| Lingual gyrus | L | -14 | -82 | -10 | 6.64 |
| Superior occipital gyrus | R | 32 | -82 | 22 | 5.05 |
| Superior occipital gyrus | L | -42 | -88 | 16 | 3.96a |
The peaks are ordered from the most rostral to the most caudal peak.
The extent of this peak did not meet our criteria of k > 10.
P < 0.05.
Separate contrasts between perspectives, for each task, yielded peaks in the frontal pole, left orbitofrontal cortex, cuneus bilaterally, and left pre- and postcentral gyrus for Imitate 1st-person vs. Imitate 3rd-person perspective (Table 2), while the Observe 1st-person vs. Observe 3rd-person perspective contrast resulted in significant activity in the cuneus bilaterally and left precentral gyrus (Table 2).
The reverse contrasts comparing imitation following a model seen from the 3rd-person and seen from a 1st-person perspective led to an increase in the right inferior frontal gyrus, the cerebellar vermis, the right superior occipital gyrus (temporo-occipital junction), and in a number of peaks in the lingual gyrus (Table 2). Comparison between observation of the actions seen from the 3rd-person perspective and seen from the 1st-person perspective did not yield any significant peak of hemodynamic change.
Interaction effects
The different interactions between Task and Perspective were investigated using the contrast [(1st-person Imitate-1st-person Observe)-(3rd-person Imitate-3rd-person Observe)], which did not yield any significant results, and the contrast [(3rd-person Imitate -3rd-person Observe) -(1st-person Imitate-1st-person Observe)], which yielded a few small clusters in the temporal cortex in both hemispheres, only one of which in the left temporal pole reached the cluster significance threshold (x = -44, y = 16, z = -34).
Discussion
The behavioral data provided initial evidence that the imitation of models seen from a 1st-person and 3rd-person perspective involves slightly different neural processes. Imitation based on a 1st-person view of the model was initiated faster than a 3rd-person view of the model and also seemed to generate fewer errors. This result fits our hypothesis that the more visuospatial similarity between the imitator and the model to imitate, the easier the task, in line with the “Like-Me” mechanism of imitation (Meltzoff, 2005; Meltzoff and Decety, 2003). Our current findings fit well with other studies that have also shown that compatibility between the movement to be executed and the model is a factor that significantly influences motor performance (e.g., Brass et al., 2001; Craighero et al., 2002; Heyes et al., 2005; Vogeley et al., 2004; Vogt, 2003).
The functional imaging data support the notion that the two different tasks (Imitation vs. Observation) and the two visual perspectives (1st-person vs. 3rd-person) each rely on partly distinct neural mechanisms.
Imitation vs. Observation
Observation of intransitive actions
The observation of hand and foot movements involved the extrastriate body area, which is consistent with a growing body of literature attributing a selective role in the perception of human body parts to this region (e.g., Downing et al., 2001). However, the functional validity of the activation reported in this region cannot be fully assessed since the coordinates were not predetermined through a localizer session, which would for instance involve complex visual stimuli other than body parts for each participant. Even though exact localization is not possible, it is likely that the activation reported in this study lies within EBA, based on the knowledge provided by a series of studies examining this region (Downing et al., 2001; Astafiev et al., 2004).
Interestingly, no signal change was observed in either the ventral premotor cortex or in the inferior frontal gyrus during observation of actions. This suggests that the simple intransitive actions used in this study do not tap into the mirror neuron system in the premotor cortex. Such a result is compatible with the finding that cells in the monkey’s ventral premotor cortex fire when the observed action is directed to an object but does not respond to the sight of the hand or the object alone. The mirror neurons in monkey premotor cortex respond only to transitive actions (e.g., a hand grasping a peanut) and not to intransitive ones (the hand moving in the absence of an object). It may be puzzling that our results are compatible with monkey data but are not fully in line with some of the previous human findings. It has been reported in humans that the premotor mirror system is activated when participants watch pantomime (the goal is not shown, only imagined, or evoked, see Decety et al., 1997; Grèzes et al., 1999) and even when they watch meaningless body movements (Fadiga et al., 1995).
However, compatible with our current findings, it was recently proposed that Broca’s area participates not so much in the observation of simple intransitive actions but in movement preparation, such as is involved in delayed execution (Makuuchi, 2005). Moreover, it has been suggested that the left inferior frontal gyrus comes into play in tasks that involve selection (even if not involving semantic retrieval) (Zhang et al., 2004). These two aspects are not present in the observation alone task, but they are in the imitation task, and, as such, these recent proposals are in line with what we have observed (see also Brass and Heyes, 2005).
Imitation of intransitive actions
When each condition of imitation was contrasted with its corresponding observation condition (e.g., imitation from a 1st-person visual perspective versus observation from a 1st-person visual perspective), thus controlling for neural responses to the visual input from the model, significant activation was detected in the left ventral premotor cortex, the SMA proper, the cerebellum (mostly in the ipsilateral hemisphere), as well as the contralateral primary somatosensory and motor cortices (in the hand and foot areas). This network was similar for imitation in both perspective conditions. One difference between these conditions was that the secondary somatosensory cortex (SII) was significantly activated only in the 1st-person perspective contrast. Even though this finding was obtained through a simple effect in the absence of a significant interaction, it is in line with the hypothesis that 1st-person perspective facilitates the integration of kinesthetic information, which is both sensory and motor in nature. This latter interpretation is further supported in the direct comparison between the perspective conditions (see next section).
Part of the left ventral premotor cortex (inferior precentral gyrus) was also activated during imitation. It has been argued that both action recognition and language production share a common system underpinned by the inferior frontal gyrus (Rizzolatti and Arbib, 1998). But, in the context of our study, it is unlikely that activation of this region can be interpreted in relation to its proposed role in speech, and no signal change was detected in this region or the more anterior and lateral region corresponding to Brodmann’s area 44 during the action recognition conditions alone (the observation conditions). Indeed, the coordinates of this region correspond to the inferior and medial part of the precentral sulcus (precentral operculum), a region that responds strongly during action execution of both hand and foot movements (Rijntjes et al., 1999; Kollias et al., 2001) and less during observation (Grèzes et al., 2003a,b; Grèzes and Decety, 2001b).
Our results demonstrate that the EBA responds more to the imitation of intransitive movements than to passive observation of the same movements. This finding supports a recent fMRI study demonstrating that EBA responds not only during the observation of other people’s body parts, but also during goal-directed movements of the observer, even in the absence of visual feedback from her/his movements (Astafiev et al., 2004). These authors consequently proposed an extended role for the EBA, involving this region not only in the visual perception of body parts but also in the planning, execution, and imagination of actions with different limbs. The magnitude of the percent signal change in the observation and imitation conditions in the present study also supports the view of Astafiev and colleagues who proposed an incremental modulation of the activity in EBA according to the condition involving body parts: the weakest response is expected during visual attention, while the strongest response would stem from performing actions while visual feedback of the movement is available. It is thus likely that the EBA is important not only for the visual processing of body parts, but also for making an ‘interpersonal registration,’ i.e., automatically mapping the visual representation of another’s body to one’s own body, which can then be used for one’s own action plans (see Thomas et al., in press). In this sense, EBA could provide initial input for a larger mirror system involved in interpersonal somatotopic map used in both perception and action.
1st-person vs. 3rd-person perspectives
1st-person perspective, the self
Consistent with the main effect of Perspective, direct comparison between observation of the actions seen from the 1st-person perspective and observation of the actions seen from the 3rd-person perspective resulted in the activation in the visual cortex bilaterally, as well as in the contralateral sensory -motor cortex. Finding a difference in visual areas between conditions that involve distinct visual stimuli is not surprising. However, the significant activation of the precentral gyrus for the 1st-person perspective, even in the absence of producing movement, is an interesting finding and deserves further consideration. Moreover, this activity was restricted to the left hemisphere, contralateral to the movements, as would be expected during the execution of movements.
A number of TMS studies have documented that observing and imagining actions can be sufficient to induce changes in the sensory-motor cortex (e.g., Clark et al., 2003), which is compatible with our current results as well as those of Maeda et al. (2001) who used TMS to assess whether action observation enhances cortico-spinal excitability. These authors tested the specificity of this effect and the role played by the orientation of the observer. They found that observation of a movement enhances motor output to the muscles involved in the movement and facilitates the observed action. Furthermore, they showed evidence for the high degree of specificity of this observation-induced motor cortical modulation. The degree of modulation was dependent on hand orientation, and thus modulation was maximal when the observed action corresponded to the orientation of the observer.
One may argue that activity in the left sensory -motor cortex is related to small movements that may have been produced by the subjects. However, performance of the subject was monitored and filmed during scanning, and no noticeable movements of the target limbs were produced by subjects during the observation conditions. It remains plausible that some subtle muscle contractions occurred during the observation of the 1st-person perspective actions. However, such a finding would prove interesting in itself because it would suggest that the 1st-person perspective and not the 3rd-person perspective tends to induce nonvoluntary muscle contractions in a condition where subjects were instructed not to move and no visible movement was produced.
The fact that greater activation of the sensory -motor cortex during 1st-person perspective was not restricted to the observation conditions but also observed during imitation also supports the interpretation that the motor representation is differentially modulated by variation of the visual perspective. This interpretation also suggests that the motor representation based on 1st-person perspective action may involve more kinesthetic components than that based on 3rd-person perspective, similarly to what is proposed for visual and kinesthetic motor imagery by Solodkin et al. (2004). Finally, activity of the somatosensory cortex is often reported in studies of perspective-taking when the perspective of the self is taken as opposed to the perspective of someone else (Ruby and Decety, 2001, 2003, 2004). Imitating movement based on 1st-person visual perspective is congruent to “self” perspective, while the 3rd-person visual perspective is closely related to the “others”. Increased activation of the somatosensory cortex, when the participant is simply observing actions performed by others, may be a neural correlate of the fact that the visual stimulus is being registered as “Like-Me” and activating self-action (Gallagher and Meltzoff, 1996; Meltzoff, 2005; Meltzoff and Decety, 2003).
Direct comparison between imitation of the action seen from the 1st-person versus 3rd-person perspective yielded hemodynamic changes in different regions of the occipital lobe, the frontal pole, medial orbitofrontal cortex (BA 25), and also in the left pre and postcentral gyrus. While frontal pole activation has been reported in different studies involving self/other distinction, its precise role remains unclear. For instance, Ruby and Decety (2001) reported frontopolar activation in a condition where participants had to imagine the actions performed by another individual versus themselves, suggesting a more prominent role for this region in 3rd-person perspective. This interpretation was later extended to 3rd-person perspective-taking in an emotional context (Ruby and Decety, 2004). Generally, the ventromedial cortex, including frontopolar regions, has been thought to regulate perspective-taking and social cognition (Decety and Jackson, 2004), and the frontopolar cortex plays an important role in inhibitory processes during behavior. However, our experiment did not involve social interaction with a live model nor a high degree of imagination or mentalizing effort. Yet, perhaps another interpretation related to the role of this region in decision making (Bechara et al., 2000) and response selection based on reward value (Elliott et al., 2000) may explain why we found frontopolar cortex activation in our study, given that the participants were trying to comply with the instructions. Elliott et al. (2000) proposed that the reward value of a response could be related to its familiarity or rightness, hence broadening the meaning of “reward” traditionally associated with physiological and psychological incentives. This familiarity interpretation is supported by the results of a positron emission tomography study showing modulation of the cerebral blood flow in the medial ventral prefrontal cortex after extensive motor imagery practice of a sequence of movements (Jackson et al., 2003). Although still conjectural at this point since these results are based on the findings from simple effects, the explanation based on familiarity could also account for the activation of the frontopolar cortex for 1st-person perspective compared to 3rd-person perspective.
3rd-person-perspective, the other
Lesions that include the right inferior frontal gyrus have been shown to be impaired perspective-taking abilities (Samson et al., 2005). Involvement of this region during 3rd-person perspective imitation suggests that some mechanisms related to perspective-taking or spatial transformation (or transposition) are at play during this condition. Such activation could also reflect greater executive function load associated with spatial transformation in this perspective.
Signal change was also found in the temporo-occipital region bilaterally. This finding is consistent with a series of fMRI experiments conducted by Zacks et al. (1999, 2002), who reported specific activation in that region during egocentric body transformation. More activation of this region during 3rd-person perspective is thus consistent with the interpretation that some spatial transformation (which is not necessary during 1st-person perspective) is at play in this condition.
Concerning the hemodynamic change in the extrastriate cortex, we did not replicate the results of Chan et al. (2004) and Saxe et al. (2005) who found higher hemodynamic signal changes for 3rd-person (allocentric) than for 1st-person (egocentric) perspectives in the right but not the left EBA when viewing people or limbs. Chan et al. (2004) nevertheless acknowledged that this effect is of small amplitude compared to the difference that can be produced in this region by different types of stimuli. In line with this, in the experiment, the effect of task (Imitation vs. Observation) on the hemodynamic signal change in this region is much higher than what we found for the effect of perspective. Finally, the absence of localizer session and the restricted size of EBA may mean that the reported coordinates do not fall exactly in this region and may correspond to MT or the lateral occipital complex that is known to partially overlap with EBA. These two latter regions have recently between shown not to differentiate between the two visual perspectives (Saxe et al., 2005).
One final but important difference between the two perspective lies within the visual cortex. While 1st-person perspective was mainly associated with primary and secondary visual cortex activation, 3rd-person perspectives yielded more hemodynamic changes within associative visual cortex, mainly, the lingual gyrus and superior occipital gyrus. This again suggests that actions seen from the 1st-person perspective are more directly mapped, without further visuospatial transformation, to its corresponding motor representations.
Finally, the lack of strong interaction between Task (Imitation vs. Observation) and Perspective (1st vs. 3rd-person) suggests that 1st-person perspective recruits the motor system to a greater extent than 3rd-person perspective regardless of whether the movements are actually produced or not.
Together, these findings may have some important implications as to how the brain performs imitation for simple body movements, i.e., intransitive actions. This is interesting because they are the first type of actions that newborns imitate (e.g., Kugiumutzakis, 1999; Meltzoff and Moore, 1977, 1997). The coding of such action takes place in the posterior parietal cortex and possibly in the lateral occipital EBA. Multiple areas in the posterior parietal cortex play different roles related to body schema and egocentric body transformation. The parietal cortex plays a fundamental role in imitation as shown in a number of functional neuroimaging studies (e.g., Chaminade and Decety, 2002; Chaminade et al., 2005; Decety et al., 2002; Iacoboni et al., 1999; Tanaka and Inui, 2002), as well as from the observation of neurological patients with apraxia (e.g., Buxbaum, 2001, 2005; Halsband et al., 2001). The properties of some cells in the parietal cortex are multimodal and may well account for the kinesthetic-visual matching that allows solving of the problem of correspondence in imitation, as proposed by Meltzoff and Moore (1997). This problem would reduced somewhat when the model is presented from a 1st-person perspective, and thus the visual input of the model is more similar to what the participant would see if looking as his or her own actions.
The main finding of this study that 1st-person perspective recruits the motor production systems more extensively (i.e., motor priming) than 3rd-person perspective, whether the movements are produced or not, is fully compatible with ideomotor theories (e.g., Greenwald, 1970). These theories state that the parallel between observed (or imagined) and executed actions stems from an integration of a motor program and its corresponding sensory feedback. This sensory feedback (or the idea of this feedback) would be more readily available through 1st-person perspective models. Theories based on the mirror neurons and mirror, as classically described, on the other hand, might be interpreted to predict that 3rd-person perspective models would provide the best representation since they evolved to help individual understand others. However, as previously discussed, mirror systems are more specifically involved when a clear goal is intended with the observed action. This was not the case in the present study, which may explain the lack of ventral premotor cortex activation during observation of action.
Conclusion
The observation of simple intransitive body actions does not seem to tap the ventral premotor cortex. Such a finding is consistent with electrophysiological recordings in monkeys that have shown that mirror cells respond to goal-directed actions, goal only, but not to meaningless actions, or to hand movements. Moreover, it was shown that both the observation and imitation of intransitive actions seen from a 1st-person perspective yielded increased activity of the contralateral sensory-motor cortex compared to the same actions perceived from a 3rd-person perspective. Finally, we found that the EBA region has interesting ‘mirror properties’ for simple body movements inasmuch as it shows increased activity during both observation and imitation. Together, these findings have implications for the neural underpinnings of both perspective-taking and imitation, and they also suggest interesting refinements in our conception of the mechanisms underlying imitation. One could hypothesize that the rote repetition of known movements is faster and more efficient (in terms of recruiting the relevant motor representation/motor program) from the 1st-person perspective, while the learning of new actions might be more robust and generalize further if seen from the 3rd-person perspective (which requires some transformation) because this would lend itself to a more effortful and better understanding of the spatial configuration of the action. The answer to the question of which perspective a teacher should take in modeling an action likely depends on the prior expertise of the student and the expected generalization and use of the learned response by the learner.
Acknowledgments
We thank the personnel at the Lewis Center for Neuroimaging, Eugene, Oregon for their help during fMRI data acquisition and Craig Harris at the University of Washington for the behavioral data coding. We gratefully acknowledge support by grants from NSF (SBE-0354453) and NIH (HD22514). Philip L. Jackson was supported by a fellowship from the Canadian Institute for Health Research.
References
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat. Neurosci. 2004;7:542–547. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Craighero L, Fadiga L. Modulation of spinal excitability during observation of hand actions in humans. Eur. J. Neurosci. 2001;13:190–194. doi: 10.1046/j.0953-816x.2000.01368.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berlucchi G, Aglioti S. The body in the brain: neural bases of corporeal awareness. Trends Neurosci. 1997;20:560–564. doi: 10.1016/s0166-2236(97)01136-3. [DOI] [PubMed] [Google Scholar]
- Brass M, Heyes C. Imitation: is cognitive neuroscience solving the correspondence problem. TICS. 2005;9:489–495. doi: 10.1016/j.tics.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W. Movement observation affects movement execution in a simple response task. Acta Psychol. 2001;106:3–22. doi: 10.1016/s0001-6918(00)00024-x. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzi A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: a call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Cogn. Brain Res. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Castiello U. Understanding other people’s actions: intention and attention. J. Exp. Psychol. Hum. Percept. Perform. 2003;29:416–430. doi: 10.1037/0096-1523.29.2.416. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Decety J. Leader or follower? Involvement of the inferior parietal lobule in agency. NeuroReport. 2002;15:1975–1978. doi: 10.1097/00001756-200210280-00029. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J. Does the end justify the means? A PET exploration of the mechanisms involved in human imitation. NeuroImage. 2002;15:318–328. doi: 10.1006/nimg.2001.0981. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Meltzoff AN, Decety J. An fMRI study of imitation: action representation and body schema. Neuropsychologia. 2005;43:115–127. doi: 10.1016/j.neuropsychologia.2004.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AW, Peelen MV, Downing PE. The effect of viewpoint on body representation in the extrastriate body area. NeuroReport. 2004;15:2407–2410. doi: 10.1097/00001756-200410250-00021. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. NeuroImage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Clark S, Tremblay F, St.-Marie D. Differential modulation of the corticospinal excitability during observation, mental imagery and imitation of hand actions. Neuropsychologia. 2003;42:105–112. doi: 10.1016/s0028-3932(03)00144-1. [DOI] [PubMed] [Google Scholar]
- Craighero L, Bello A, Fadiga L, Rizzolatti G. Hand action preparation influences the responses to hand pictures. Neuropsychologia. 2002;40:492–502. doi: 10.1016/s0028-3932(01)00134-8. [DOI] [PubMed] [Google Scholar]
- Decety J. A cognitive neuroscience view of imitation. Imitation and the Development of the Social Mind. In: Rogers S, Williams J, editors. Guilford Publications; New York: 2006. [Google Scholar]
- Decety J, Chaminade T. The neurophysiology of imitation and intersubjectivity. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Cognitive Neuroscience to Social Science. Vol. 1. MIT Press; Cambridge: 2005. pp. 119–140. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyck E, Fazio F. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Decety J, Chaminade T, Grèzes J, Meltzoff AN. A PET exploration of the neural mechanisms involved in reciprocal imitation. NeuroImage. 2002;15:265–272. doi: 10.1006/nimg.2001.0938. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb. Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J. Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Gallagher S, Meltzoff AN. The earliest sense of self and others: Merleau-Ponty and recent developmental studies. Philos. Psychol. 1996;9:211–233. doi: 10.1080/09515089608573181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. Exp. Brain Res. 1996;112:103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Greenwald AG. Sensory feedback mechanisms in performance control: with special reference to the ideo-motor mechanism. Psychol. Rev. 1970;77:73–99. doi: 10.1037/h0028689. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J. Does visual perception of objects afford action? Evidence from a neuroimaging study. Neuropsychologia. 2001a;40:212–222. doi: 10.1016/s0028-3932(01)00089-6. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation and verb generation of actions: a meta-analysis. Hum. Brain Mapp. 2001b;12:1–19. doi: 10.1002/1097-0193(200101)12:1<1::AID-HBM10>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grèzes J, Costes N, Decety J. The effects of learning and intention on the neural network involved in the perception of meaningless actions. Brain. 1999;122:1875–1887. doi: 10.1093/brain/122.10.1875. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to mirror and canonical neurons in the human brain: an fMRI study. NeuroImage. 2003a;18:928–937. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Tucker M, Armony J, Ellis R, Passingham RE. Objects automatically potentiate action: an fMRI study of implicit processing. Eur. J. Neurosci. 2003b;17:2735–2740. doi: 10.1046/j.1460-9568.2003.02695.x. [DOI] [PubMed] [Google Scholar]
- Halsband U, Schmitt J, Weyers M, Binkofski F, Grutzner G, Freund HJ. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: a perspective on apraxia. Neuropsychologia. 2001;39:200–216. doi: 10.1016/s0028-3932(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Rijntjes M, Dettmers C, Glauche V, Weiller C, Büchel C. The human action recognition system and its relationship to Broca’s area: an fMRI study. NeuroImage. 2003;19:637–644. doi: 10.1016/s1053-8119(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Hari R, Fross N, Avikainen E, Kirveskari E, Salenius S, Rizzolatti G. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes C, Bird G, Johnson H, Haggard P. Experience modulates automatic imitation. Brain Res. Cogn. Brain Res. 2005;22:233–240. doi: 10.1016/j.cogbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Understanding others: imitation, language, and empathy. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Cognitive Neuroscience to Social Science. Vol. 1. MIT Press; Cambridge: 2005. pp. 77–99. [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Decety J. Motor cognition: a new paradigm to study self other interactions. Curr. Opin. Neurobiol. 2004;14:259–263. doi: 10.1016/j.conb.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. NeuroImage. 2003;20:1171–1180. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The mechanism of self-recognition in humans. Behav. Brain Res. 2003;142:1–15. doi: 10.1016/s0166-4328(02)00384-4. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Paulignan Y, Blakemore SJ. An interference effect of observed biological movement on action. Curr. Biol. 2003;13:522–525. doi: 10.1016/s0960-9822(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297:846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Kollias SS, Alkadhi H, Jaermann T, Crelier G, Hepp-Reymond MC. Identification of multiple nonprimary motor cortical areas with simple movements. Brain Res. Rev. 2001;36:185–195. doi: 10.1016/s0165-0173(01)00094-7. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC. Modulation of cortical activity during different imitative behaviors. J. Neurophysiol. 2003;89:460–471. doi: 10.1152/jn.00248.2002. [DOI] [PubMed] [Google Scholar]
- Kugiumutzakis G. Genesis and development of early infant mimesis to facial and vocal models. In: Nadel J, Butterworth G, editors. Imitation in Infancy. Cambridge Univ. Press; Cambridge: 1999. pp. 36–59. [Google Scholar]
- Maeda F, Kleiner-Fishman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of the observer orientation. J. Neurophysiol. 2001;187:1329–1335. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- Makuuchi M. Is Broca’s area crucial for imitation. Cereb. Cortex. 2005;15:563–570. doi: 10.1093/cercor/bhh157. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Imitation and other minds: the “Like Me” hypothesis. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Cognitive Neuroscience to Social Science. Vol. 2. MIT Press; Cambridge: 2005. pp. 55–77. [Google Scholar]
- Meltzoff AN, Decety J. What imitation tells us about social cognition: a rapprochement between developmental psychology and cognitive neuroscience. Philos. Trans. R. Soc. London, Ser. B Biol. Sci. 2003;358:491–500. doi: 10.1098/rstb.2002.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977a;198:75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Moore MK. Explaining facial imitation: a theoretical model. Early Dev. Parent. 1997b;6:179–192. doi: 10.1002/(SICI)1099-0917(199709/12)6:3/4<179::AID-EDP157>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Prinz W. The Imitative Mind: Development, Evolution and Brain Bases. Cambridge Univ. Press; Cambridge: 2002. [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc. Natl. Acad. Sci. U. S. A. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz W. An ideomotor approach to imitation. In: Hurley S, Chater N, editors. Perspectives on Imitation: From Cognitive Neuroscience to Social Science. Vol. 1. MIT Press; Cambridge: 2005. pp. 141–156. [Google Scholar]
- Rijntjes M, Dettmers C, Buchel C, Kiebel S, Frackowiak RS, Weiller C. A blueprint for movement: functional and anatomical representations in the human motor system. J. Neurosci. 1999;19:8043–8048. doi: 10.1523/JNEUROSCI.19-18-08043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu. Rev. Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev., Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of the subjective perspective taking during simulation of action: a PET investigation of agency. Nat. Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective taking. Eur. J. Neurosci. 2003;17:2475–2480. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective taking with social emotions. J. Cogn. Neurosci. 2004;16:988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Bekkering H. To imitate or not to imitate? How the brain can do it, that is the question. Brain Cogn. 2003;53:479–482. doi: 10.1016/s0278-2626(03)00208-2. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Johansen-Berg H, Göbel SM, Devlin JT. The left parietal and premotor cortices: motor attention and selection. NeuroImage. 2003;20:S89–S100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain. 2005;128:1102–1111. doi: 10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- Saxe R, Jamal N, Powell L. My body or yours? The effect of visual perspective on cortical body representations. Cereb. Cortex 27. 2005 doi: 10.1093/cercor/bhi095. doi:10.1093/cercor/bhi095. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. Academic Press; USA: 2000. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Chen EE, Small SL. Fine modulation in network activation during motor execution and motor imagery. Cereb. Cortex. 2004;14:1246–1255. doi: 10.1093/cercor/bhh086. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Inui T. Cortical involvement for action imitation of hand/arm postures versus finger configurations: an fMRI study. Neuro-Report. 2002;13:1560–1599. doi: 10.1097/00001756-200209160-00005. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Inui T, Konishi J, Nakai T. Neural substrates involved in imitating finger configurations: an fMRI study. NeuroReport. 2001;12:1171–1174. doi: 10.1097/00001756-200105080-00024. [DOI] [PubMed] [Google Scholar]
- Thomas R, Press C, Haggard P. Shared representations in body perception. Acta Psychol. doi: 10.1016/j.actpsy.2005.08.002. in press. [DOI] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing: a neurophysiological study. Neuron. 2001;32:91–101. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 2004;16:817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vogt S. Visuomotor priming by pictures of hand postures: perspective matters. Neuropsychologia. 2003;41:941–951. doi: 10.1016/s0028-3932(02)00319-6. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci. Behav. Rev. 2001;25:287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Williams JHG, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2005.06.010. doi:10. 1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Rypma B, Gabrielli J, Tversky B, Glover G. Imagined transformations of bodies: an fMRI study. Neuropsychologia. 1999;37:1029–1040. doi: 10.1016/s0028-3932(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Ollinger JM, Sheridan MA, Tversky B. A parametric study of mental spatial transformation of bodies. Neuro-Image. 2002;16:857–872. doi: 10.1006/nimg.2002.1129. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Feng CM, Fox PT, Gao JH, Tan LH. Is left inferior frontal gyrus a general mechanism for selection. NeuroImage. 2004;23:281–287. doi: 10.1016/j.neuroimage.2004.06.006. [DOI] [PubMed] [Google Scholar]


