Abstract
The management of interstitial cystitis (IC) is predominantly the reduction of the symptoms of frequency, urgency, and pain. Multimodal treatment approaches for IC are helpful in customizing therapy for individual patients. Complementary and alternative therapies are a quintessential addition to the therapeutic armamentarium and frequently include dietary modification, nutraceuticals, bladder training, neuromodulation, stress reduction, and sex therapy. Dietary modification involves elimination of bladder irritants, fluid regulation, and a bowel regimen. Nutraceuticals studied for the treatment of IC include calcium glycerophosphate, L-arginine, mucopolysaccharides, bioflavinoids, and Chinese herbs. Bladder training is effective after pain reduction. The neuromodulation of high-tone pelvic-floor muscle dysfunction is achieved with physical therapy and acupuncture. Stress reduction and sex therapy are best administered by a qualified stress manager and sex therapist. Multimodal, nonconventional management may add efficacy to the treatment of IC.
Key words: Interstitial cystitis, Dietary modification, Nutraceuticals, Neuromodulation, Stress reduction, Sex therapy
Many interstitial cystitis (IC) patients resort to nonconventional therapies after experiencing a lack of perceived efficacy of conventional measures. Unfortunately, little research exists for complementary and alternative therapies in the treatment of IC.
In 1995, complementary and alternative medicine (CAM) was defined by the National Institutes of Health (NIH) Center for Complementary and Alternative Medicine as, “those treatments and healthcare practices not taught widely in medical schools, not generally used in hospitals, and not usually reimbursed by medical insurance companies.”1 CAM therapies are typically multimodal and individualized to each patient. Outcome measures are usually subjective and lack well-defined endpoints, treatment interventions, and patient population.2 When considering CAM therapy, the caregiver is challenged to decide the proper role for such therapy.
Dietary modification has been practiced by most IC patients at some point in their therapy. For example, an elimination diet of bladder irritants, decreasing dietary acid load, and urinary alkalinization with baking soda or potassium citrate has been an effective treatment for many IC patients. Also, a steady intake of water helps to dilute urine and reduce constipation. Fluid and bowel regimens have also been used to assure adequate hydration and normal bowel function.
Nutraceuticals, or chemicals that induce bodily physiological changes, have been popularized in managing IC patients. For example, calcium glycerophosphate reduces titratable acids in foods and has been effective in decreasing the exacerbation of IC symptoms when bladder irritants are ingested. Additional nutraceuticals used by IC patients include L-arginine, mucopolysacchrides (hyaluronic acid, chondroitin sulfate, and aloe vera), bioflavinoids (quercitin), and Chinese herbs.
Increasing the voiding interval after pain has been controlled with bladder training has been effective in decreasing frequency. Pain relief may also be accomplished with anecdotal strategies that include warm baths, ice packs, and the incorporation of numerous other CAM therapies for IC. Further, neuromodulation effects a change in the sustained guarding reflex seen in IC patients with high-tone pelvic floor muscle dysfunction. This is accomplished with CAM therapies, including physical therapy with a home exercise regimen and acupuncture. Stress reduction and sex therapy are often enhanced with a stress manager or sex therapist. Table 1 lists various complementary and alternative therapies used to treat IC.
Table 1.
Complementary and Alternative Medicine Therapies for IC
| Dietary Modification |
| IC diet, fluids, bowel regimen, urinary alkalinization |
| Nutraceuticals |
| Calcium glycerophosphate |
| L-arginine |
| Mucopolysaccharides-hyaluronic acid, chondroitin sulfate, aloe vera |
| Bioflavinoids-quercitin |
| Chinese herbs |
| Bladder Training |
| Neuromodulation |
| Physical therapy: |
| Physical therapy of external pelvis (manual therapy) |
| Physical therapy of internal pelvis (Thiele massage) |
| Biofeedback/electrical stimulation |
| Acupuncture |
| Stress Reduction |
| Sex Therapy |
Dietary Modification
IC Diet
IC patients can identify foods and fluids that will exacerbate their symptoms in 51%–62% of cases.3,4 Three-day food and voiding diaries revealed that the intake of foods and fluids that exacerbate IC symptoms increased painful bladder symptoms within 2–4 hours of ingestion. The symptoms were reduced with the elimination, among others, of the following: alcoholic beverages, carbonated drinks, caffeine, spicy foods, tomatoes, citrus fruits, and vinegar.3 Arylalkylamine-containing foods (tryptophan, tyrosine, tyramine, and phenylalanine) have also been implicated as IC symptom exacerbaters and include the following: bananas, beer, cheese, mayonnaise, aspartame, nuts, onions, raisins, sour cream, wine, and yogurt.5
The mechanism by which foods or fluids exacerbate IC symptoms is unkown and is not supported by evidence-based medicine (EBM). Fisher and colleagues6 found no decrease in urinary pH when IC patients ingested acidic foods; however, the severity of symptoms was not reported. Patients with severe symptoms in our practice tend to respond favorably to dietary modification. Gillespie5 found an exacerbation of symptoms after 250 IC patients ingested a diet high in acid and arylalkylamines; she also found an increase in 24-hour urine metabolites of tryptophan (kynure-nine, xanthurenine, and indicans). In another study, glycosaminoglycan disruption and increased bacterial adherence were reported in the presence of the urinary tryptophan metabolites 3-hydroxykynurenine and hydroxyanthranilic acid.7
IC Dietary Modifications
Dietary modification was reported to be the fourth most common therapy among 581 patients satisfying the entry criteria and consenting to participate in the IC Database, a longitudinal study sponsored by a National Institutes of Health grant.8 Implementing a diet for 2–3 months that eliminates the proposed foods and fluids that exacerbate IC symptoms and then adding them one at a time remains a reasonable therapy until controlled studies are available on the effect of a prescribed diet on IC symptom severity.
In addition to dietary modifications, urinary alkalinization with potassium citrate may be useful in maintaining a urinary pH of 6.0–6.5. A randomized controlled trial to study the effects of potassium citrate on urinary pH and IC symptom severity is ongoing. A scheduled fluid regimen may ensure appropriate urinary dilution. The prevention of constipation with fluid and fiber may also enhance dietary modifications.
Nutraceuticals
Chemicals that affect the body’s physiology are considered nutraceuticals. Several nutraceuticals have been utilized in the treatment of IC symptoms.
Calcium Glycerophosphate
Studies have shown a relationship between acidic, urine-producing foods or liquids and an increase in IC symptoms, as well as an alleviation of these symptoms with the restriction of such foods. The most commonly implicated foods and beverages are coffee, chocolate, alcohol, carbonated drinks, citrus fruits, and tomato-based products.9,10
A prospective, nonrandomized study examining the efficacy of a dietary product, calcium glycerophosphate, showed that calcium glycerophosphate helped reduce IC symptoms among patients who ingested foods that exacerbated their IC symptoms.11 Before taking calcium glycerophosphate, the symptom exacerbaters were identified by means of a food diary that patients kept over a 4-week period. A total of 203 patients completed a voiding diary and Likert scales (range, 0–9) for urgency and pain. Two tablets totaling 0.66 gm of calcium glycerophosphate were taken over a 4-week period immediately before the ingestion of symptom exacerbaters. Table 2 reports the percentage of patients exhibiting an exacerbation to given foods before and after the ingestion of calcium glycerophosphate. Marked reductions in the percentage of the population experiencing flares from such foods were observed across the board. On the Likert scales, pain and discomfort decreased from 5.3 to 3.6 and urgency decreased from 5.3 to 4.1 following the ingestion of calcium glycerophosphate. Calcium glycerophosphate appears to reduce IC symptoms in patients with food- or fluid-related symptom exacerbations.11
Table 2.
Effect of Calcium Glycerophosphate on Exacerbation of Symptoms in 203 IC Patients
| Diet | Before Calcium | After Calcium |
|---|---|---|
| Glycerophosphate | Glycerophosphate | |
| (%) | (%) | |
| Pizza | 45.5 | 14.5 |
| Tomato | 62.5 | 17.0 |
| Spicy food | 55.5 | 9.0 |
| Coffee | 59.5 | 18.0 |
| Acidic juice/fruit | 62.5 | 18.5 |
| Carbonated drink | 57.0 | 18.0 |
| Alcohol | 53.0 | 5.5 |
| Chocolate | 41.0 | 16.0 |
Data from Bologna et al.11
L-Arginine
L-arginine is an essential amino acid that increases the production of nitric oxide (NO) and its precursor nitric oxide synthetase (NOS). NO and NOS have antibacterial, smooth-muscle relaxant, hormone-releasing, and immune-modulating (by increasing T-cell counts) properties. In a randomized, double-blind trial of oral L-arginine in the treatment of IC, NOS production was decreased in IC patients and increased in patients with urinary tract infections when compared to controls. L-arginine (1.5 g/day) was given to 10 IC patients for 6 months. The urinary NOS was then measured. The NOS production in 8 out of the 10 patients reached a mean level similar to that of controls and was accompanied by a decrease in pain, frequency, and nocturia.12 The same authors performed a randomized, double-blind trial using 1.5 g of L-arginine per day for 3 months. At the end of this period, 6 (29%) of the 21 patients in the L-arginine group and 2 (8%) of the 25 patients in the placebo group were clinically improved. When an intent-to-treat analysis was done, no significant difference was found among the L-arginine and placebo groups, although Likert scales showed a significant difference in pain intensity and global scores favoring those taking L-arginine.13 Another randomized, controlled trial with L-arginine found no improvement in IC symptoms scores when compared to controls.14 The efficacy of L-arginine, NO, and NOS regarding IC treatment remains controversial.
Mucopolysaccarides
Mucopolysaccharides containing glycosaminoglycans (GAGs) potentially benefit IC patients by replenishing the defective GAG layer at the mucosal surface of the bladder (Figure 1). Naturally occurring mucopolysaccharides used to treat IC include hyaluronic acid, chondroitin sulfate, and aloe vera.
Figure 1.
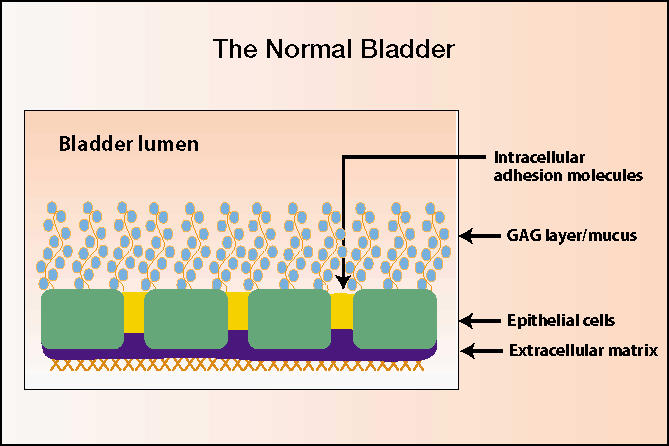
Normal bladder. GAG, glycosaminoglycans. Illustration courtesy of C. Lowell Parsons, MD.
Hyaluronic acid is a nonsulfated mucopolysaccharide that is found in the subepithelial connective tissue and is increased in the urine of IC patients. Morales and colleagues15 found a 56% (13 of 24 patients) response rate (>50% reduction in symptom scores, urgency, and pain) in 25 patients given 4 weekly intravesical instillations of 40 mg hyaluronic acid. Patients were then given monthly instillations. At 12 weeks, the response rate was 71%, and this rate was maintained until week 20. One patient withdrew from the study. A multi-center randomized trial is ongoing.
In an open-label pilot study, 80 mg of an intravesical chondroitin sulfate compound were given to 14 IC patients weekly for 4 weeks and then monthly for 8–38 weeks. Twelve patients responded after 3–12 weeks of therapy.16 A multicenter randomized study has recently begun.
The aloe vera plant, also known as the “burn plant,” has been used extensively to heal and relieve the pain of burns. A small, open-label, double-blind, crossover study utilized a freeze-dried whole-leaf aloe vera concentrate.17 Three 600 mg capsules of aloe vera were given twice a day for 3 months followed or preceded by placebo. Of 12 IC patients, 8 completed the study and 7 had significant symptom relief. A multicenter trial is planned.
Bioflavinoids—Quercitin
The bioflavinoid quercitin is a naturally occurring substance that inhibits histamine release from mast cells, and it has anti-inflammatory and anti-oxidant properties. It is rich in seeds, citrus fruits, olive oil, tea, and red wine. A quercitin-containing compound was studied in 22 IC patients given 500 mg twice a day for 4 weeks. Two patients dropped out of the study. Of the remaining 20 patients, 57% had a significant decrease in O’Leary Sant Symptom and Problem indices and a decreased global assessment of pain (Likert scale; range, 0–10).18 A randomized, placebo-controlled trial is needed to determine the efficacy of quercitin in treating IC.
Chinese Herbs
Herbal tea and pills have been used to treat IC patients who were referred for herbal therapy as offered as a treatment alternative. A pilot study utilized the herbs Cornus, gardenia, curculigo, rhubarb, Psoralea, and Rehmannia in a tea twice a day for 6 days a week for 3 months, then once a day. Of 25 patients, 61% had a significant decrease in pain at 4 weeks, and an additional 22% had a significant response at 3 months.19 Table 3 illustrates a current combination of Chinese herbs in a tea to treat IC.
Table 3.
Chinese Herbal Tea Used to Treat IC Patients
| Rehmannia-Shutihuang |
| Dioscorea-Shanyao |
| Poria-Fuling |
| Cornus-Shanyurou |
| Curculigo-Xiannao |
| Rhubarb-Dahuang |
| Morinda-Bajitian |
| Cuscuta-Tusizi |
| Gardenia-Zhizi |
| Anemarrhea-Zhimu |
Bladder Training
Bladder training involves inhibiting the urge to urinate in an effort to extend the voiding interval. This is best accomplished after severe pain associated with bladder filling is controlled. In one study, progressive increases in the voiding interval of 15–30 minutes every 3–4 weeks resulted in a decrease in frequency, nocturia, and urgency in 15 (71%) of 21 IC patients.20 In another study, Chaiken and colleagues21 reported a significant increase in the voiding interval in IC patients with weekly pelvic floor muscle exercises and relaxation by listening to audio tapes. Of the 42 patients in the study, 98% had a significant decrease in the number of voids per day, and 71% showed a significant increase in functional bladder capacity on their voiding diaries after 3 months of treatment.
Neuromodulation
Physical Therapy
The pelvic floor musculature performs an important role in the tonic support of the pelvic viscera provided by a preponderance of slow-twitch (type I) fibers. In addition, fast-twitch (type II) fibers within the levator ani provide active periurethral muscle contraction with increased intra-abdominal pressure. An increase in pelvic floor muscle tone occurs during bladder filling via a guarding reflex, accompanied by bombardment by unmyelinated C-fiber afferents with an increased somatic efferent stimulation of the pelvic floor muscles during vesical distention. In IC patients, the result is high-tone pelvic floor muscle dysfunction. High-tone pelvic floor muscle dysfunction has also been known as coccygodynia, tension myalgia, levator ani spasm, and levator syndrome. Nociceptive, afferent C-fibers become active in response to bladder inflammation or irritation, resulting in pain and reflex voiding. A sustained guarding reflex manifests as pelvic floor muscle hypertonus (Figure 2 and Figure 3).22
Figure 2.
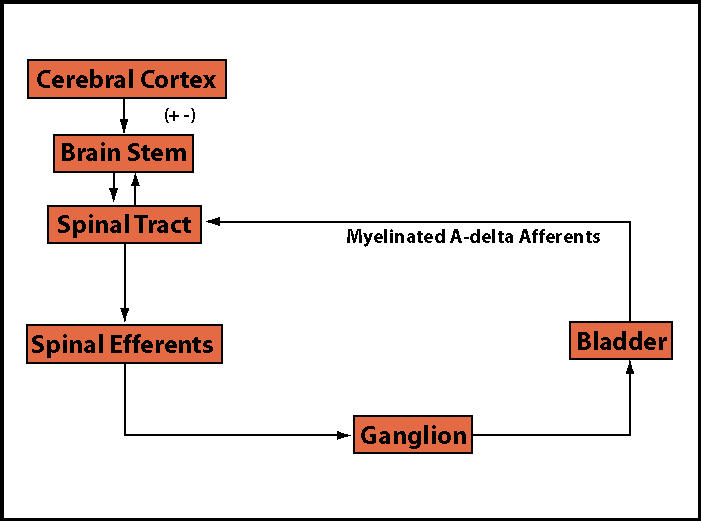
Normal control of micturition.
Figure 3.
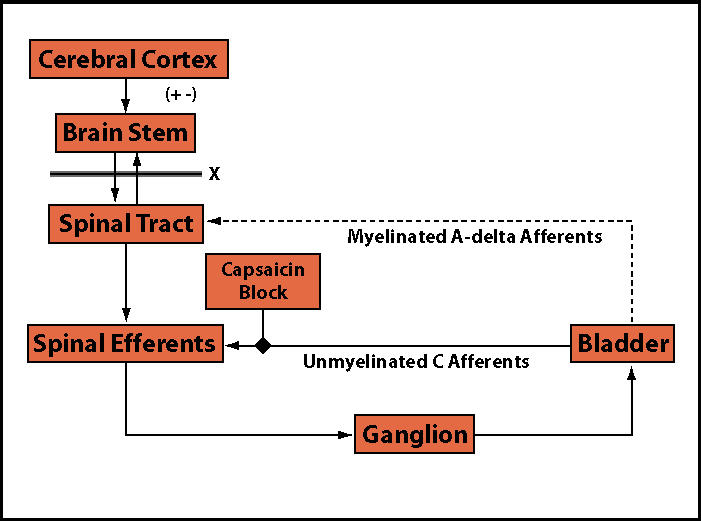
Control of micturition in neurologic and inflammatory disease.
High-tone pelvic floor muscle dysfunction has neurologic and musculoskeletal components. Neuromodulation therapy attempts to ablate the sustained guarding reflex. Treatment of high-tone pelvic floor dysfunction (PFD) involves physical therapy of the outer and inner pelvis followed by biofeedback and functional electrical stimulation of the pelvic floor muscles.
Manual Physical Therapy of the External Pelvis
Realignment of the sacrum and ilium aids in restoring normal tension to the pelvic floor musculature. In a pilot study of 16 IC patients with PFD and sacroiliac dysfunction, 94% had a significant improvement in irritative voiding symptoms and dyspareunia following manual therapy, myofascial massage, and muscle-energy techniques, along with a home exercise regimen that included stretch and strengthening exercises (Table 4).23
Table 4.
Physical Therapies in the Treatment of IC
| Manual Physical Therapy of External Pelvis-6–12 weeks |
| Direct myofascial release |
| Joint mobilization |
| Muscle energy |
| Strengthening and stretching |
| Neuromuscular re-eductation |
| Home exercise |
| Intravaginal Thiele Massage-6 weeks |
| Intravaginal myofascial release |
| Home exercise |
| Biofeedback/Electrical Stimulation-6–12 weeks |
| Neuromuscular re-education |
| Electrical stimulation frequency, 25 and 50 Hertz |
| Home exercise |
Intravaginal Thiele Massage
In a recent study evaluating the use of the Thiele massage in women with IC, twice-weekly intravaginal Thiele myofascial massages for 6 weeks stabilized trigger points in the levator ani, obturator internus, and pyriformis muscles in 9 of 10 IC patients (Figure 4).24 Stabilization of intra-vaginal trigger points qualified patients for pelvic floor muscle rehabilitation with combined biofeedback and functional electrical stimulation.
Figure 4.
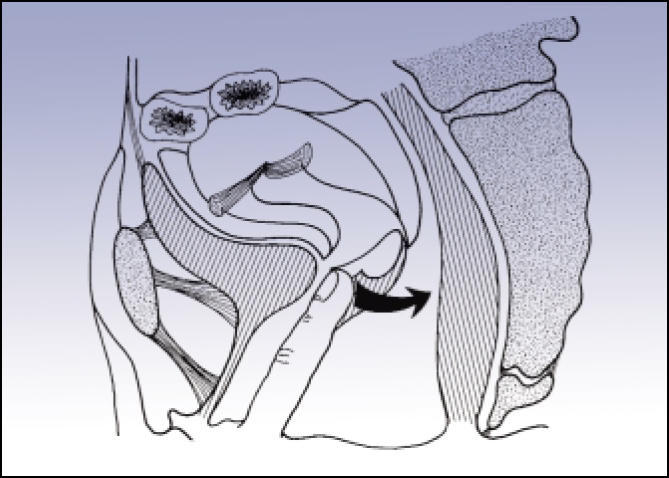
Intravaginal Thiele myofascial massage. Reproduced from Urogenital Manipulation by Jean-Pierre Barral, with permission of Eastland Press, P.O. Box 99749, Seattle, WA 98199. Copyright 1993. All rights reserved.
Biofeedback/Electrical Stimulation
Patients with persistent high-tone PFD following stabilization of the outer pelvis through manual therapy and the inner pelvis through Thiele massage may benefit from biofeedback and/or functional electrical stimulation (transvaginal at a frequency of 50 Hertz). One study used 6 weekly sessions of biofeedback with 14 patients with intractable rectal pain. The treatment resulted in significant relief from pain in 6 of the patients (43%) at a mean follow-up of 15 months.25 A larger study in IC patients with PFD will utilize digital exams, perineometry, voiding diaries, and questionnaires assessing quality-of-life, voiding function, bowel function, pain, and sexual function. Patients completing physical therapy will be treated with weekly biofeedback and functional electrical stimulation for 6 weekly sessions and be re-assessed for pelvic floor dysfunction. A home exercise program will be used for maintenance of pelvic floor function.
Acupuncture
Acupuncture is over 5000 years old. Neuromodulation through acupuncture occurs by re-establishing a balanced flow of energy, termed Yin and Yang, throughout the body through 12 meridians and 1000 acupoints. A rebalance of energy flow is thought to occur by increasing endorphin production and pain ablation by stimulating A delta, large-diameter, myelinated sensory nerve fibers with the inhibition of unmyelinated sensory C-fibers. Rapkin and Kames26 reported the results of a study of 14 patients with pelvic pain who had 6–8 weeks of acupuncture therapy twice a week. Eleven patients had a >50% reduction in pain. Our experience at the Pelvic Floor Institute shows that IC patients need 10–20 acupuncture treatment sessions in order to achieve symptom relief. Further studies are needed to determine the efficacy of acupuncture therapy in patients with IC.
Stress Reduction
Because IC is a chronic illness, effective treatment mandates stable mental health regarding achieving coping mechanisms, psychological adjustment, the development of a high-functioning patient, the physician-patient relationship, and stress reduction.
Coping skills are significantly affected by self-efficacy, which is defined as the extent to which an individual feels confident in his or her ability to manage a task. Patients who cope best are those who take an active role in managing their lives.3 Coping mechanisms include avoiding foods that exacerbate symptoms, keeping busy, seeking emotional support from family and friends, wearing loose-fitting clothing, avoiding stress, and undergoing behavioral therapy.
Depression has been found to be a common outcome of chronic painful illness.27 In one study of 80 female IC patients, the Center for Epidemiologic Studies Depression (CES-D) mean score was 22.04 compared with 9.25 in the general population.28 Supportive psychotherapy and anti-anxiety or antidepressant medications to treat the depression and emotional distress secondary to chronic pain will aid in the problem-solving and healthy psychological adjustment in the IC population.
The high-functioning IC patient feels in control of his or her life and puts up with discomfort in order to resume normal activities leading to increased self-efficacy. This patient achieves self-efficacy by utilizing cognitive and behavioral strategies to control pain and maximize activities. The physician-patient relationship should be emotionally supportive, and the physician should give adequate time to listen to the patient. The physician should not personalize the patient’s frustration and anxiety, but should refer the patient for behavioral counseling and stress management. The management of frustration and anger will give the patient better coping skills and allow the physician to provide effective medical care.
Diaphragmatic breathing, progressive muscle relaxation, exercise, self-visualization, and self-hypnosis have been shown to be effective in reducing stress. In a study of 19 IC patients with pelvic floor dysfunction, treatment consisted of sitz baths, 2 mg of diazepam 3 times a day, and relaxation therapy utilizing diaphragmatic breathing and progressive relaxation techniques. There was a significant decrease in pain and urgency scores, as shown on a visual analog scale (with scores ranging from 0 to 10), after 3 months of therapy.29
Sex Therapy
Most IC patients report that their symptoms affect sexual responsiveness and enjoyment. Self-care strategies commonly used are emptying the bladder before and after sex, avoiding prolonged intercourse, minimizing pressure on the urethra, engaging in outercourse, cleansing after sexual activity, and taking pain killers or antispasmotics before engaging in sexual activity. In a study of 80 women completing a self-efficacy scale and a CES-D scale, 80% reported that the frequency of sex was affected by their IC. As many as 81% of these patients refrained from sex at times when they and their partner wanted to have it, and in 80% IC affected the specific sexual activities engaged in.28 Sexuality in the IC patient is best managed by decreasing pain and by utilizing a sex therapist to identify and treat desire, arousal, and orgasmic disorders.
Conclusions
Complementary and alternative medicine therapy for IC is multimodal and individualized to each patient. Studies have described positive outcomes resulting from the use of dietary modification, nutraceuticals, bladder training, neuromodulation with physical therapy and home exercise programs, acupuncture, stress reduction, and sex therapy. There is a need for randomized, controlled trials utilizing CAM to establish the efficacy and reliability of different IC treatments. Optimal control of IC symptoms may also require stress management with a behavioral therapist to assure stable mental health during the early stages of therapy.
Main Points.
Complementary and alternative medical therapy for interstitial cystitis (IC) is multimodal and individualized.
Dietary modification can decrease symptoms by identification of food and fluid symptom exacerbators.
Nutraceutical therapy for IC includes calcium glycerophosphate, L-arginine, mucopolysaccharides, bioflavinoids, and Chinese herbs.
Neuromodulation with physical therapy and acupuncture inhibits the sustained guarding reflex.
Stress reduction and sex therapy are best accomplished with the help of a stress manager and sex therapist.
References
- 1.Ernst E, Resch KI, Mills S, et al. Complementary medicine: a definition. Br J Gen Prac. 1995;45:506–509. [Google Scholar]
- 2.Hirsch I. Integrative urology: a spectrum of complementary and alternative therapy. Urology. 2000;56:185–189. doi: 10.1016/s0090-4295(00)00610-5. [DOI] [PubMed] [Google Scholar]
- 3.Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121–130. [PubMed] [Google Scholar]
- 4.Koziol JA, Clark DC, Gittes RF, et al. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465–469. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- 5.Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293–297. doi: 10.1111/j.1464-410x.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher BP, Bavendam TG, Roberts BE, et al. Blinded placebo controlled evaluation on the ingestion of acidic foods and their effect on urinary pH and the symptomatology of interstitial cystitis; Presented at the Interstitial Cystitis Association’s Research Symposium on Interstitial Cystitis; October 18–21,1993; Orlando, FL. [Google Scholar]
- 7.Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogensis. Urology. 1987;30:255–258. doi: 10.1016/0090-4295(87)90248-2. [DOI] [PubMed] [Google Scholar]
- 8.Rovner E. Treatments used in women with interstitial cystitis: the interstitial cystitis data base (ICDB) study experience. Urology. 2000;56:940–945. doi: 10.1016/s0090-4295(00)00845-1. [DOI] [PubMed] [Google Scholar]
- 9.Bade JJ, Peeters JMC, Mensink HJA. Is the diet of patients with interstitial cystitis related to their disease? Eur Urol. 1997;32:179–181. [PubMed] [Google Scholar]
- 10.Erickson R, Davies MF. Interstitial cystitis, review article. Int Urogynecol J Pelvic Floor Dysfunct. 1997;9:174–178. doi: 10.1007/BF02001088. [DOI] [PubMed] [Google Scholar]
- 11.Bologna RA, Gomelsky A, Lukban JC, et al. The efficacy of calcium glycerophosphate in the prevention of food-related flares in interstitial cystitis. Urology. 2001;57(6) suppl 1:119–120. doi: 10.1016/s0090-4295(01)01070-6. [DOI] [PubMed] [Google Scholar]
- 12.Smith SD, Wheeler MA, Foster HE, Jr, Weiss RM. Improvement in interstitial cystitis scores during treatment with oral L-arginine. J Urol. 1997;158(3 pt1):703–708. doi: 10.1097/00005392-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Korting GE, Smith SD, Wheeler MA, et al. A randomized double-blind trial of oral L-arginine for treatment of interstitial cystitis. J Urol. 1999;161:558–565. [PubMed] [Google Scholar]
- 14.Cartledge JJ, Davies AM, Eardley I. A randomized double-blind placebo-controlled crossover trial of the efficacy of L-arginine in the treatment of interstitial cystitis. BJU Int. 2000;85:421–426. doi: 10.1046/j.1464-410x.2000.00490.x. [DOI] [PubMed] [Google Scholar]
- 15.Morales A, Enerson L, Nickel JC, et al. Intravesical hyaluronic acid in the treatment of refractory interstitial cystitis. J Urol. 1996;156:45–48. [PubMed] [Google Scholar]
- 16.Steinhoff G. A pilot study to determine the response of patients with IC and a positive potassium sensitivity test to intravesical chondroitin sulfate (Urocyst-S) instillation; Presented at: International Bladder Symposium (sponsored by the National Institutes of Health and the American Bladder Foundation); November 4–7, 1999; Washington, DC. [Google Scholar]
- 17. [Accessed November 2001];Findings reported by Desert Harvest. Available at: www.desertharvest.com. Data on file.
- 18.Katske F, Shoskes DA, Sender M, et al. Treatment of interstitial cystitis with a quercitin supplement. Tech. Urol. 2001;7:44–46. [PubMed] [Google Scholar]
- 19.Interstitial Cystitis Association, authors. ICA Update. 1997;12:3. [Google Scholar]
- 20.Parsons CL. Interstitial cystitis: successful management by increasing urinary voiding intervals. Urology. 1991;37:207–212. doi: 10.1016/0090-4295(91)80286-g. [DOI] [PubMed] [Google Scholar]
- 21.Chaiken DC, Blaivas JG, Blaivas ST. Behavioral therapy for the treatment of refractory interstitial cystitis. J Urol. 1993;149:1445–1448. doi: 10.1016/s0022-5347(17)36411-x. [DOI] [PubMed] [Google Scholar]
- 22.Das AK, White MD, Longhurst PA. Sacral nerve stimulation for the management of voiding dysfunction. Rev Urol. 2000;1:43–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Lukban JC, Whitmore K, Kellogg-Spadt S, et al. The effect of manual physical therapy in patients diagnosed with interstitial cystitis, high-tone pelvic floor dysfunction, and sacroiliac dyfunction. Urology. 2001;57(6) suppl 1:121–122. doi: 10.1016/s0090-4295(01)01074-3. [DOI] [PubMed] [Google Scholar]
- 24.Holzberg AS, Kellogg-Spadt S, Lukban JC, et al. The evaluation of transvaginal Thiele massage as a therapeutic intervention for women with IC; Presented at: The National Institute of Diabetes and Digestive and Kidney Diseases, Interstitial Cystitis and Bladder Research Symposium; October 19–20, 2000; Minneapolis, Minn. [Google Scholar]
- 25.Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139–145. doi: 10.1007/BF02051169. [DOI] [PubMed] [Google Scholar]
- 26.Rapkin AJ, Kames LD. The pain management approach to chronic pelvic pain. J Reprod Med. 1987;32:323–327. [PubMed] [Google Scholar]
- 27.Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain. 1994;56:287–297. doi: 10.1016/0304-3959(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 28.Rabin C, O’Leary A, Neighbors C, Whitmore K. Pain and depression experienced by women with interstitial cystitis. Womens Health. 2000;31:67–81. doi: 10.1300/j013v31n04_05. [DOI] [PubMed] [Google Scholar]
- 29.Mendelowitz F, Moldwin R. Complementary therapies in the management of interstitial cystitis. In: Sant G, editor. Interstitial Cystitis. Philadelphia: Lippincott-Raven; 1997. pp. 235–239. [Google Scholar]


