Abstract
The incidence of prostate cancer in the United States has recently undergone dramatic and unprecedented changes. Exposure to prostate-specific antigen testing has led to a steep increase in reported incidence. The data indicate that the strongest risk factors for prostate cancer are age and African American race/ethnicity. Family history is also an important risk factor for prostate cancer, although only a small proportion of cases will be due to high-penetrance genes such as those at the putative susceptibility loci (eg, ELAC2) recently identified through linkage analysis. International variation in the risk of prostate cancer is profound, as is the frequency with which migrants from low- to high-risk areas adopt the risk pattern of the host country, possibly within a single generation. This article reviews the current state of knowledge regarding risk factors for prostate cancer, including factors related to diet, anthropometrics, hormone profiles, and concomitant medical conditions. The need for unifying, overarching hypotheses is emphasized, along with identification of some of the barriers to future progress in the field.
Key words: Prostatic neoplasms, Risk factors, Epidemiology
Trends in Incidence and Mortality
For men in the United States, the incidence of prostate cancer—which is equivalent to the instantaneous risk of developing the disease—has undergone dramatic and unprecedented changes in recent years. As can be seen in the latest figures from the National Cancer Institute Surveillance, Epidemiology, and End Results (NCI SEER) Program (Figure 1), age-adjusted incidence was increasing steadily from the 1970s until about 1988. Some of this increase was probably due to the popularity of transurethral resection for benign prostatic hyperplasia (BPH) and the resulting incidental diagnosis of prostate cancer. In the late 1980s the introduction of prostate-specific antigen (PSA) testing led to the steepest increase in reported cancer incidence that has ever been observed for any cancer site. In 1991 alone, reported incidence increased by nearly 30% as substantial numbers of men had PSA tests for the first time. First-time and repeat PSA testing continued to increase during the mid-1990s, but since 1992 incidence rates declined steadily for a few years. Although this topic will not be explored in detail here, this decline tells us that PSA testing raised incidence by “pulling forward in time” the date of diagnosis for many prostate cancers, but eventually exhausted the pool of prevalent cancers within reach of its sensitivity. It is noteworthy that since 1996, incidence began to increase again and appears to have resumed the linear trend established before PSA testing. This implies that the risk of “pseudo-disease” (prostate cancer detected by PSA that would otherwise never have been manifested as a diagnosis) is not as large as some have suspected. Since the use of surgery for BPH has declined substantially with the advent of improved drug therapy, the reasons for the persistent upward trend in prostate cancer incidence remain unidentified.
Figure 1.
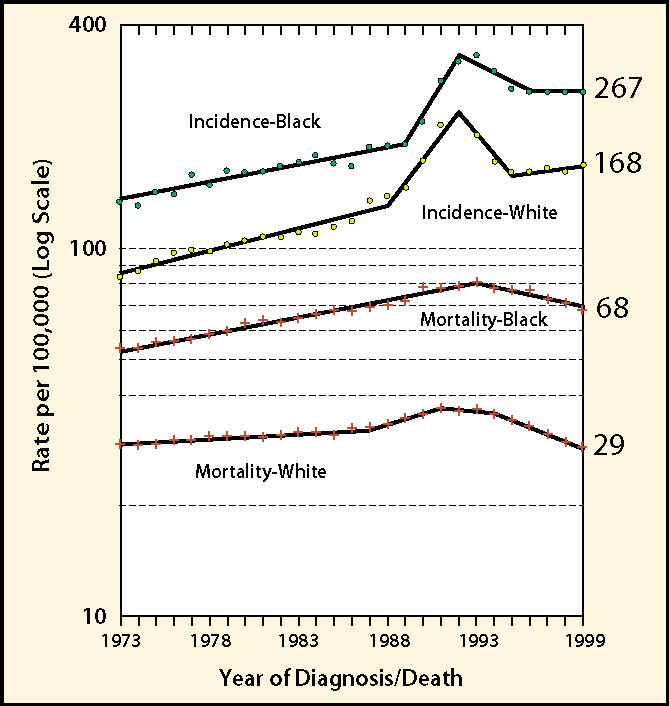
Age-adjusted incidence and mortality of prostate cancer in the United States, 1973–1999 (Surveillance, Epidemiology, and End Results Program data).
In the United States, the risk of dying from prostate cancer began to decline measurably in 1994, for the first time since statistics have been kept, and mortality has continued to decline at an average annual rate of about 2% to 3%. Reasons for this decline are beyond the scope of this article, but a contribution of PSA screening appears to be very likely.1
Established Risk Factors: Age, Race, and Family History
The only risk factors for prostate cancer that can be considered established are age, race/ethnicity, and family history. Study of age-specific incidence curves reveals that prostate cancer risk begins to rise sharply after age 55 years and peaks at age 70–74, declining slightly thereafter. Autopsy studies confirm that prostate cancer has a long induction period, and that many men have incipient lesions in their 20s and 30s.2 As Figure 1 shows, the risk of prostate cancer is approximately 60% higher in African Americans than in whites. Mortality among African Americans is approximately double that of whites. Conflicting data exist as to whether this mortality difference is explained entirely by differences in socioeconomic status variables and stage at diagnosis, or whether an inherent difference exists between these racial/ethnic groups in the underlying biology of prostate cancer. It should be emphasized that biological explanations for these risk differences could involve genetic factors, environmental factors, or, more likely, an interaction between the two.
Epidemiological studies conducted as far back as the 1950s determined that having a first-degree relative (brother or father) with prostate cancer increased risk for an individual by approximately two- to three-fold, on average. Risk is further increased by early age at onset in relatives and multiple relatives with the disease. More recently, segregation studies have identified familial clustering patterns of prostate cancer that are consistent with the presence of highpenetrance genetic mutations that confer a Mendelian pattern of inheritance.3 The number of genes involved, as well as the number of specific sequence changes in these genes, is currently not known, but it can be estimated that altogether these highpenetrance genetic alterations probably explain no more than 10% of all prostate cancer cases.
Linkage mapping studies using whole or partial genome scans within high-risk pedigrees have so far identified at least six potential prostate cancer susceptibility loci. The first of these loci, labeled HPC1, was reported in 1996. However, several attempts to replicate the association observed between this locus and prostate cancer within other families with prostate cancer have not yielded consistent results. The initial hope that heritable prostate cancer would be explained by a defect in a single important gene has given way to the realization that the situation is far more complex and that multiple genes are probably involved. The causal gene(s) at HPC1, as well as those at two other loci (HPCX and HPC20), might be associated with prostate cancer only in subsets of families that exhibit specific patterns of inheritance (eg, male-to-male) or patterns of age at onset. Therefore, unless the prostate cancer families are properly grouped for analysis, linkage for any particular locus will be difficult to demonstrate or confirm. This point was demonstrated again with the potential susceptibility gene HPC2/ELAC2, which was identified recently by linkage studies and positional cloning among high-risk families in Utah.4 ELAC2 is the first actual gene to be proposed as a high-penetrance gene for prostate cancer. While research has already identified amino acid-altering variants in ELAC2, and suggested an association between these variants and prostate cancer, other family studies have failed to demonstrate the HPC2/ELAC2 linkage observed in Utah.5
Risk Factors Under Investigation
International Rates and Migration
Prostate cancer exhibits an extraordinary amount of variation in its occurrence worldwide. For example, the incidence rate for African Americans is approximately 60-fold higher than the rate among men in Shanghai, China.6 Although part of this disparity is due to differences in diagnostic ascertainment and the prevalence of screening, mortality rates, which are less subject to such influences, also vary profoundly. For example, the mortality rate for African Americans from 1988 to 1992 was approximately 12 times higher than the mortality rate in Hong Kong. These differences notwithstanding, mortality is increasing faster in the westernizing parts of Asia than anywhere else in the world. Observation of Asian migrants, moreover, provides the most compelling argument for environmental influences linked to Western lifestyle as causal factors in prostate cancer. Japanese Americans have an incidence rate 43 times higher than their counterparts in Japan, and there are data indicating that migrants develop the high-risk pattern within one generation.6,7 Shimizu and colleagues reported that prostate cancer incidence rates in Los Angeles among migrants from Japan were similar regardless of whether men immigrated early or later in life.7 We interpret this to mean that environmental forces can accelerate the progression of latent tumors even late in life.
Table 1 shows the dietary, anthropometric, and hormonal factors that have been associated with prostate cancer risk and are currently under active investigation.
Table 1.
Potential Environmental/Non-Genetic Risk Factors for Prostate Cancer
| Dietary | ||
| Saturated fat | + | CS |
| Alpha-linolenic acid | + | CS |
| Red meat | + | CS |
| Dairy food (and/or calcium) | + | CS |
| Selenium | − | RCT |
| Lycopene (tomato foods) | − | CS |
| Vitamin E supplements | − | RCT |
| Legumes (incl. soy) | − | CS |
| Anthropometric | ||
| Height | +? | CS |
| Abdominal obesity | +? | CS |
| Hormonal | ||
| Elevated intraprostatic androgens | + | CS |
| Elevated IGF-1 (bioactive fraction) | + | CS |
+ indicates positive association; −, inverse association; CS, prospective cohort study; RCT, randomized clinical trial; IGF-1, insulin-like growth factor.
Diet
One of the most obvious characteristics of the Western diet is a high intake of total calories and fat. Ecological studies performed more than 25 years ago indicated strong correlations between per capita fat consumption across countries and the rate of prostate cancer mortality. These international correlations were strongest for saturated fat, which is largely fat from animal sources. Subsequent case-control and cohort studies have provided mixed results, but taken as a whole, the findings are consistent with a small positive association (relative risks 1.3–2.0) between high-saturated-fat diets and prostate cancer incidence.8 Despite the relatively small increase in risk, this association could explain a substantial fraction of overall prostate cancer cases, due to the ubiquity of high fat intakes. Although calorie restriction is a powerful inhibitor of cancer development in a variety of animal tumor models, epidemiological evidence to date does not suggest that total caloric intake plays an important etiologic role by itself. Meat—especially red meat—is an important component of animal-fat intake, and studies of red-meat intake are relatively consistent in showing risk ratios of 1.5 to 2.0 comparing the highest to lowest categories of intake, independent of fat consumption.8 The biological reasons for this association are unclear, but hypotheses include the effects of meat on hormone profiles, the paucity of anticarcinogenic phytochemicals in high-meat diets, and the potentially carcinogenic effects of compounds generated during hightemperature meat cooking.
Of course, fat can be categorized into subgroupings, beginning with major distinctions such as polyunsaturated versus monounsaturated and progressing on to distinctions among specific fatty acids. When viewed as a whole, studies of these fat subgoups, which include some based on reported dietary intake and others based on measurement of fatty-acid concentrations in blood, have provided weak evidence for a possible protective effect of long-chain ω-3 fatty acids such as eicosapentaenoic acid, and a possible riskenhancing effect of α-linolenic acid. Alpha-linolenic acid, an 18-carbon essential fatty acid commonly consumed in meat and certain vegetable oils, is interesting because it is an important precursor for synthesis of prostaglandins and leukotrienes. Further work is needed to determine whether dietary alteration of fatty acids involved in the prostaglandin synthesis pathways can affect prostate carcinogenesis.
More than 20 epidemiological studies have examined the role of dairy-food intake in prostate cancer. These studies are consistent with a positive association, independent of the contribution of dairy foods to total and saturated fat intake. Research has recently focused on the contribution of dairy foods to dietary calcium intake. One of the most intriguing results obtained so far was from the Health Professionals Follow-up Study, which reported that men who consumed the most calcium (>2,000 mg/day) due to supplements—mostly antacids—had a 4.6-fold increase in prostate cancer risk compared to men with low total calcium intake.9 One hypothesis under investigation is that high calcium intake suppresses levels of the vitamin D metabolite 1,25-dihydroxyvitamin D, which exhibits anticarcinogenic properties in the laboratory.
A trio of dietary antioxidants has been linked to reduced risk of prostate cancer: selenium, vitamin E, and lycopene. In the case of selenium and vitamin E, the associations were observed in clinical trials that were originally designed to address other endpoints. In one study, men at increased risk for a recurrence of skin cancer were randomly assigned to 200 μg/day of selenium or placebo. Although the effects on skin cancer were null, there was a 67% reduction in prostate cancer in the group receiving selenium.10 Similarly, in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study, a trial conducted in Finland among male smokers, the group receiving α-tocopherol (vitamin E) at a dose of only 50 mg/day experienced a 35% reduction in prostate cancer incidence.11 These post hoc findings from trials, together with supportive evidence from some observational studies, led to the development of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) in the United States, which is described in detail elsewhere in this volume.
Although β-carotene intake has not been associated with prostate cancer risk, another major dietary carotenoid compound—lycopene—has become the focus of considerable attention. Lycopene, which enters the body largely from the consumption of tomato foods, is the predominant carotenoid in the diet of most Americans, and exhibits strong singlet oxygen-quenching ability in vitro. In the Physicians’ Health Study, plasma obtained long before diagnosis in 578 cases was compared to plasma in 1294 matched controls.12 The data were analyzed separately for participants assigned to a β-carotene supplement versus those assigned to placebo. The results, summarized in Table 2, showed a strong trend towards lower risk with higher plasma lycopene level among the men not receiving the β-carotene. Interestingly, although higher lycopene was not associated with lower risk among men receiving β-carotene, men with the lowest level of lycopene had a significantly reduced risk if they consumed the β-carotene. These results suggest that a ceiling effect might exist, and that one could reduce prostate cancer risk by about 40% by consuming either a diet high in lycopene or a β-carotene supplement. Phase 2 trials on lycopene are currently under way.
Table 2.
Odds Ratios and 95% Confidence Intervals for Prostate Cancer According to Plasma Lycopene at Baseline and Random Assignment to Active Beta-Carotene Supplements or Placebo (Physicians’ Health Study)
| All Cases (n = 578 sets) | Aggressive Cases (n = 259 sets) | |||||||
|---|---|---|---|---|---|---|---|---|
| Plasma Lycopene | ||||||||
| Quintile | Placebo | Beta-carotene | Placebo | Beta-carotene | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Q1 (low) | 1.00 | - | 0.57 | 0.37–0.88 | 1.00 | - | 0.69 | 0.37–1.27 |
| Q2 | 0.72 | 0.46–1.12 | 0.61 | 0.39–0.97 | 0.72 | 0.38–1.36 | 0.32 | 0.15–0.68 |
| Q3 | 0.70 | 0.45–1.09 | 0.65 | 0.42–1.02 | 0.59 | 0.29–1.16 | 0.56 | 0.29–1.09 |
| Q4 | 0.58 | 0.37–0.89 | 0.75 | 0.49–1.15 | 0.45 | 0.23–0.86 | 0.72 | 0.38–1.37 |
| Q5 | 0.59 | 0.37–0.94 | 0.55 | 0.35–0.87 | 0.40 | 0.19–0.84 | 0.48 | 0.24–0.94 |
| P, trend | 0.01 | 0.006 | ||||||
| P, interaction | 0.04 | 0.05 | ||||||
OR indicates odds ratio; CI, confidence interval.
Legumes—including but not limited to soy—have also been studied in relation to prostate cancer risk. The results are inconclusive thus far. However, there is considerable interest in the anticarcinogenic potential of genistein, a soy isoflavone, and its relatives. Decisions about how to deliver these compounds in trials must be made very carefully, because of the possibility that their action depends on a particular food matrix or interaction with other constituents in whole food. Green tea, which contains numerous polyphenolic compounds with potential anticarcinogenic properties, is another plant food currently under early but active investigation.
Anthropometric Factors and Physical Activity
Measures of obesity and height have been extensively studied in relation to prostate cancer risk. These studies do not indicate any substantial association between either obesity or height and prostate cancer.13 Adult height was hypothesized to be important, in part because it is influenced by nutrition during childhood. However, attained height is not correlated with adult levels of the somatotrophin insulin-like growth factor (IGF)-1, which has been linked to prostate cancer risk. The lack of association between obesity and prostate cancer is vexing because obesity reduces sex hormone-binding globulin (SHBG) levels, which could lead to an increase in bioavailable testosterone, and increases both insulin and bioavailable IGF-1, which are both potentially important prostate mitogens. Relatively little research has been conducted so far on body fat distribution, as opposed to total body fat. Of particular interest is abdominal fat, which has metabolic effects different from those of subcutaneous fat. In addition, investigators have hypothesized that physical activity, apart from its beneficial effect on body fat, could reduce prostate cancer risk. The data thus far, however, are not conclusive, perhaps due to difficulty in measuring physical activity in study populations or assessment of activity during the wrong period of life.
Endogenous Hormones
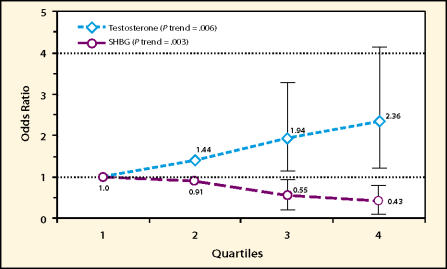
Two major hypotheses regarding endogenous hormones and prostate cancer etiology have emerged. The first states that risk is linked to the intraprostatic concentrations of androgens. The prostate requires androgens for development, and studies of patients receiving either orchiectomy or androgen blockade have clearly shown that androgen deprivation can cause prostate cancer to regress. What remains unclear, however, is whether variations in endogenous androgen levels among men are important. Variation in serum testosterone levels in middle-aged men, for example, is considerable. The importance of such interindividual differences is magnified when one considers the cumulative effects of these levels over decades. Case-control studies, which obtain blood samples from cases after diagnosis, are not especially useful for studying these relationships because of the effects of the disease on androgens. Cohort studies are more relevant, but there have been few relatively large such studies so far. In one, plasma testosterone was positively associated with risk (approximate doubling in risk between the highest and lowest quartile) only after adjustment for SHBG (see Figure 2).14 In this analysis, risk also declined with increasing SHBG concentration, after adjustment for testosterone. Taken together, this result is consistent with the concept that only non-SHBG-bound androgen is biologically important.
Figure 2.
Prostate cancer risk by quartile of plasma testosterone and sex hormone-binding globulin (SHBG): simultaneous adjustment for testosterone, SHBG, and estradiol (Physicians’ Health Study).
Further prospective cohort studies are under way. It is now recognized that these studies must be fairly large, must have archived blood samples that have been carefully stored, must use assay techniques for hormone levels that are highly reliable within and between batches, and must have a plausible approach to handling the question of hormone bioavailability. A problem facing all of these studies, however, is that we know little about the relationship of circulating androgens to androgen levels in the prostate. In particular, the determinants of intraprostatic dihydrotestosterone—the most potent androgen in the prostate—remain unknown. Within the past two years, however, the three-dimensional structures of two major molecules in the androgen system, SHBG and androgen receptor, have been determined. Thus, our understanding of the molecular details of this system is now progressing rapidly and can be expected to inform future population studies.
The second major hormonal hypothesis regarding prostate cancer concerns IGF-1. Levels of this hormone, which mediates the action of growth hormone, have been associated with prostate cancer risk in at least three prospective studies. Moreover, the effect of IGF-1 might be enhanced in the presence of low levels of IGF binding protein (IGFBP)-3, which is the principal binding protein for IGF-1 in the circulation. Both in vitro and in vivo experiments have provided abundant evidence that IGF-1 can promote prostate carcinogenesis, including the observations that IGF-1 administration induces prostate growth in the rat, and that prostate tumor development in transgenic mouse models is accompanied by elevations in IGF-1 expression.15
Concomitant Medical Conditions
There is some evidence that prostate cancer risk is reduced in the presence of cirrhosis, which is characterized by elevated estrogen levels. Men with acromegaly, due to growth-hormone hypersecretion, have an unusual degree of prostatic hyperplasia.16 Diabetes, when longstanding and thus likely to be characterized by low insulin levels, has been associated with lower risk in some studies.17 Certain drugs that have side effects on androgen metabolism, such as spironolactone and cimetidine, form interesting targets for research. Conditions within the prostate, including BPH and prostatitis, have only been studied to a limited extent due to methodological difficulties. BPH is difficult to study as a risk factor for prostate cancer, largely because its presence makes diagnosis of prostate cancer more likely, in the absence of any biological relationship. Prostatitis is an important potential risk factor, but thus far we have no reliable or valid methods to ascertain its presence. One of the most intriguing hypotheses states that prostate cancer risk is increased by sympathetic nervous system activity. It is known that prostate cell growth can be stimulated by neurotrophic growth factors released by adrenergic nerve endings in the prostate. Our group found, in a cohort with long-term follow-up, that resting heart rate—an indicator of basal sympathetic activity—had a positive linear relationship with prostate cancer mortality.18 Interestingly, a study of schizophrenic patients in Denmark found that prostate cancer risk was reduced overall, but that the reduction in risk was confined to men taking antipsychotic drugs with pronounced antiadrenergic effects.19
Markers of Risk in Tissue
Prostate cancer develops as a continuum from normal epithelium through various preneoplastic states to invasive cancer. We now have abundant circumstantial evidence implicating high-grade prostatic intraepithelial neoplasia (HGPIN) as a major premalignant lesion. On a morphological level, PIN is characterized by increasing size and variation in nuclear shape, changes in chromatin texture, and increasingly prominent nucleoli. In one cohort study of men with HGPIN, the relative risk for developing prostate cancer, compared to men without PIN, was 14.9—even after adjustment for PSA level.20 Tissue exhibiting HGPIN shares many chromosomal and molecular abnormalities with prostate cancer. Indeed, for several molecular markers indicating proliferation, loss of differentiation, loss of apoptosis, and abnormal cell regulation, expression in PIN is intermediate between normal epithelium and cancer. As the number of men undergoing PSA screening and subsequent biopsy has increased, so has the recognition of men with isolated PIN, who now form an important subgroup at especially high risk. Studies have shown that HGPIN can be reversed by androgen deprivation, suggesting that PIN could be an informative intermediate marker for the identification of effective chemopreventive agents.
Overarching Hypotheses
Now that previous research has built up a number of possible leads regarding the etiology of prostate cancer, there is an increasing need to tie these leads together in unifying or overarching schemes. Four such schemes include:
oxidant/antioxidant balance in the prostate;
calcium/vitamin D interaction;
IGF-1/androgen system interaction;
gene-environment interaction in hormone synthesis, action, and metabolism.
The evidence regarding selenium, vitamin E, and lycopene—plus other evidence on oxidant damage in the prostate—supports the concept that oxidant/antioxidant balance is crucial. Although reliable data are sparse, we believe that chronic inflammation in the prostate, accompanied by substantial endogenous formation of free radicals, is very common. The oxidant/antioxidant scheme is quite useful because it suggests several molecules and processes involved in antioxidant defense as potential targets for future research. The calcium/vitamin D interaction hypothesis ties together the findings on meat, dairy, and calcium intake, as well as other findings on variations in the vitamin D receptor and the possible inverse relation of prostate cancer risk to ultraviolet light exposure. This hypothesis rests upon the importance of 1,25 vitamin D as a suppressor of tumor development, and, no doubt, further insight will be gained as this and related compounds are studied as potential chemopreventive agents in the laboratory.
The IGF-1 and androgen systems are capable of affecting prostate cancer independently; however, their true impact perhaps can be better understood by considering their interactions and joint effects. Insulin, which is homologous to IGF-1 and can bind to the IGF-1 receptor, may provide an important link between these two systems.21 In addition to increasing bioavailable IGF-1 by strongly downregulating IGFBP-1, insulin strongly downregulates expression of SHBG and increases the activity of key enzymes in the androgen synthesis pathways. Information concerning human genetic polymorphisms in the genetic pathways involved in hormone synthesis, metabolism, and action is now accumulating very rapidly. Most of these minor genetic variants will have little or no functional significance, and even fewer will have effects on cancer risk by themselves. It is more plausible that some of the functionally significant variants will modify risk in conjunction with other genes or environmental factors. Thus, identification of gene-environment or gene-gene interaction in the etiology of prostate cancer is now a major focus of work. Some of the earliest studies in this area reported that short CAG repeat length in the gene for androgen receptor (AR) was associated with higher prostate cancer risk, and could explain some of the differences in risk that are observed between blacks and whites. This hypothesis is supported by in vitro evidence that the short CAG receptor is more transcriptionally active, and by analogy to Kennedy Syndrome, in which a very long CAG repeat length leads to almost totally inactive AR. Future studies will examine how this polymorphism interacts with hormone levels or other determinants of androgen action in the prostate. Studies of genetic polymorphisms in androgen synthesis/metabolism enzymes such as CYP17 and CYP19 and of polymorphisms in the gene for Type II 5α-reductase have not yielded conclusive findings thus far.22
Future Challenges
From the initial phase of research into the risk factors for prostate cancer, we have learned that future progress will require large prospective cohort studies with banked blood and DNA samples, as well as high-quality questionnaire information. Several such cohort studies are currently under way and will be producing results over the next few years. The challenge of incorporating genetic information into these studies will be met by sifting through the vast amount of variation and selecting those genotypes or haplotypes most likely to be biologically important. Moreover, investigators will have to specify statistical models of interaction that correspond to real biological hypotheses, or else the plethora of statistical comparisons that can be made will be overwhelming.
Main Points.
Prostate cancer incidence in the United States has recently undergone dramatic and unprecedented changes.
The introduction of prostate-specific antigen testing in the 1980s led to the steepest increase in reported incidence that has ever been recorded for any cancer site.
The established risk factors are age, African-American race/ethnicity, and family history.
Other potential risk factors include diet, anthropometric factors, hormone profiles, and concomitant medical conditions.
Selenium, vitamin E, and lycopene have been linked to reduced risk.
Current hypotheses propose that risk is linked to insulin-like growth factor-1 levels and intraprostatic concentrations of androgens.
Overarching hypotheses integrating information gained so far about prostate cancer etiology are needed, and include those concerning oxidant/antioxidant balance in the prostate; calcium/vitamin D interaction; IGF-1/androgen system interaction; and gene-environment interaction in hormone synthesis, action, and metabolism.
Acknowledgments
The author wishes to thank Laura Studee for her help with preparation of the tables and figures.
References
- 1.Etzioni R, Legler JM, Feuer EJ, et al. Cancer surveillance series: interpreting trends in prostate cancer-part III: quantifying the link between population prostate-specific antigen testing and recent declines in prostate cancer mortality. J Natl Cancer Inst. 1999;91:1033–1039. doi: 10.1093/jnci/91.12.1033. [DOI] [PubMed] [Google Scholar]
- 2.Yatani R, Chigusa I, Akazaki K, et al. Geographic pathology of latent prostatic carcinoma. Int J Cancer. 1982;29:611–616. doi: 10.1002/ijc.2910290602. [DOI] [PubMed] [Google Scholar]
- 3.Carter BS, Beaty TH, Steinberg GD, et al. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavtigian SV, Simard J, Teng DH, et al. A candidate prostate cancer susceptibility gene at chromosome 17p. Nat Genet. 1001;27:134–135. doi: 10.1038/84808. [DOI] [PubMed] [Google Scholar]
- 5.Stanford JL, Ostrander EA. Familial prostate cancer. Epidemiol Rev. 2001;23:19–23. doi: 10.1093/oxfordjournals.epirev.a000789. [DOI] [PubMed] [Google Scholar]
- 6.Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu H, Ross RK, Bernstein L, et al. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolonel LN. Fat, meat, and prostate cancer. Epidemiol Rev. 2001;23:72–81. doi: 10.1093/oxfordjournals.epirev.a000798. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E, Rimm EB, Wolk A, et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58:442–447. [PubMed] [Google Scholar]
- 10.Clark LC, Combs GF, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. JAMA. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 11.Heinonen OP, Albanes D, Virtamo J, et al. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial [see comments] J Natl Cancer Inst. 1998;90:440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 12.Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 13.Severson RK, Grove JS, Nomura A, Stemmermann GN. Body mass and prostatic cancer: a prospective study. BMJ. 1988;297:713–715. doi: 10.1136/bmj.297.6650.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gann PH, Hennekens CH, Longcope C, et al. A prospective study of plasma hormone levels, non-hormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995;26:40–49. doi: 10.1002/pros.2990260109. [DOI] [PubMed] [Google Scholar]
- 15.Pollak M. Insulin-like growth factors and prostate cancer. Epidemiol Rev. 2001;23:59–66. doi: 10.1093/oxfordjournals.epirev.a000796. [DOI] [PubMed] [Google Scholar]
- 16.Colao A, Marzullo P, Ferone D, et al. Prostatic hyperplasia: an unknown feature of acromegaly. J Clin Endocrinol Metab. 1998;83:775–779. doi: 10.1210/jcem.83.3.4645. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Rimm EB, Stampfer MJ, et al. Diabetes mellitus and risk of prostate cancer (United States) Cancer Causes Control. 1998;9:3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 18.Gann PH, Daviglus ML, Dyer AR, Stamler J. Heart rate and prostate cancer mortality: results of a prospective analysis. Cancer Epid Prev Biomarkers. 1995;4:611–616. [PubMed] [Google Scholar]
- 19.Mortensen PB. Neuroleptic medication and reduced risk of prostate cancer in schizophrenic patients. Acta Psychiatr Scand. 1992;85:390–393. doi: 10.1111/j.1600-0447.1992.tb10325.x. [DOI] [PubMed] [Google Scholar]
- 20.Bostwick DG. Prostatic intraepithelial neoplasia is a risk factor for cancer. Semin Urol Oncol. 1999;17:187–198. [PubMed] [Google Scholar]
- 21.Kaaks R. Nutrition, hormones, and breast cancer: is insulin the missing link? Cancer Causes Control. 1996;7:605–625. doi: 10.1007/BF00051703. [DOI] [PubMed] [Google Scholar]
- 22.Makridakis NM, Reichardt JK. Molecular epidemiology of hormone-metabolic loci in prostate cancer. Epidemiol Rev. 2001;23:24–29. doi: 10.1093/oxfordjournals.epirev.a000791. [DOI] [PubMed] [Google Scholar]