Abstract
In this article the author tries to forecast how urologists will treat the overactive bladder (OAB) in the next decade. He reviews drugs currently under development and also logical and exciting pharmacological targets that would be suitable targets for treating OAB in the future. The author also discusses intravesical therapy and alternative drug delivery methods, such as intravesical capsaicin and botulinum toxin. There are many advantages to advanced drug delivery systems, including the achievement of long-term therapeutic efficacy, decreased incidence and severity of side effects, and improved patient compliance. Special emphasis is placed on approaches to modulating bladder afferent nerve function to prevent OAB. Speculation on future techniques such as gene therapy can also be considered for treating OAB, because they may make it possible to access all of the genitourinary organs via minimally invasive techniques. Traditional anticholinergic therapies are limited in their effectiveness. There is great hope for future research and therapy for OAB and urinary incontinence.
Key words: Duloxetine, Incontinence, Neurogenic bladder, Pharmacology
Traditional anticholinergic drugs are the “gold standard” for treating the overactive bladder (OAB) today. Tremendous advances over the past few years, especially with advanced drug delivery (oxybutynin sustained release), have improved efficacy and decreased side effects when treating OAB. However, most urologists would readily acknowledge that when it comes to treating our patients with OAB, we still have a long way to go in finding drugs that can further improve efficacy and decrease the incidence and severity of side effects.1
I believe that there is great hope for future translational research on voiding dysfunction and urinary incontinence. I believe that the key to the next major advance is to focus on afferent nerve intervention to prevent OAB. Afferent blockade, a revised treatment approach, targets the afferent nerves that control the bladder.2,3 Wouldn’t it be more desirable to prevent urgency and the micturition reflex that initiates OAB? There are a number of afferent blockade drugs now in development that can prevent the bladder from having this involuntary contraction. Treating the patient with OAB using this approach would allow the possibility of giving lower drug doses with fewer side effects, as well as greater efficacy. Drugs that work on the unmyelinated C-fiber afferent limb of the micturition reflex do not present the risk of urinary retention that occurs with the use of anticholinergic drugs. Studies are also underway of advanced drug delivery systems, gene therapy, and tissue engineering as potential therapies further into the future (Table 1).
Table 1.
Alternative Drug Delivery Methods
| Intravesical Therapies: |
|
| Transdermal Delivery of Anticholinergic Drugs |
| Gene Therapies: |
|
Likely Therapies by 2005
Transdermal Oxybutynin
I am excited about the prospect of transcutaneous anticholinergic drug delivery. It is logical that transcutaneous application of anticholinergic drugs can further decrease side effects while still maintaining efficacy. Transdermal delivery of oxybutynin decreases first-pass drug metabolism in the liver and gut wall and allows a lower concentration of oxybutynin metabolites. The metabolites of oxybutynin, rather than the parent component itself, appear to account for the majority of the dry mouth side effects.4 If once- or twice-a-week transcutaneous anticholinergic patches can be developed, then higher-dose regimens as well as increased patient compliance can be expected.
Davila and colleagues recently reported on the outcome of a multicenter, randomized, double-blind study of transdermal versus immediate-release oral oxybutynin.5 Transdermal oxybutynin achieved reductions in urge incontinence episodes similar to those seen with oral immediate-release oxybutynin but with significantly less occurrence of dry mouth.
Bladder-Specific Anticholinergics
Pharmacologically defined subtype-selective drugs have been developed. Darifenacin and vamicamide have recently been demonstrated to be selective for the M3 receptor subtype. Darifenacin, which has 11 times the affinity for M3 that it has for M2 receptors, was similar in potency to atropine in blocking acetylcholine (ACH)-induced contractions of the guinea pig urinary bladder but had one-fifth the affinity of atropine for M3 receptors in the parotid gland.6 Similarly, in the anesthetized dog, darifenacin was 8.6 times as potent in blocking pelvic nerve-evoked bladder contractions as in suppressing trigeminal nerve-evoked salivation.7 There can be substantial differences in the results from one species to another, and these observations may or may not prove to predict the response in humans. Still, darifenacin is a promising M3 subtype-affinity agent under investigation for OAB. If the phase 3 studies confirmed the bladder selectivity, darifenacin would be an attractive agent.
(S)-Oxybutynin is a single-isomer version of racemic oxybutynin. Racemic oxybutynin exhibits two pharmacologies, one related to relaxation of the bladder’s detrusor muscle and the second related to anticholinergic activity. (S)-oxybutynin may possess a superior balance between bladder relaxation and anticholinergic activity. In a phase IIB, 12-week study in over 650 patients, (S)-oxybutynin, at 120 mg three times a day, demonstrated a statistically significant improvement in the reduction of micturition frequency and number of patients achieving complete continence compared with placebo.
Antidiuretic Hormones
Desmopressin is a synthetic vasopressin analog with strong antidiuretic effects. The drug is commonly used in children with nocturnal enuresis. The use of desmopressin at bedtime for bothersome nocturia is becoming more popular. The initial reports in patients with neurogenic bladder dysfunction were encouraging.8 More recently, desmopressin is being considered for treatment of nocturia associated with benign prostatic hypertrophy (BPH) and OAB. Caution must be practiced before considering desmopressin in any patient with cardiovascular risk factors, including angina and congestive heart failure. Dilutional hyponatremia can occur very quickly and pose a health risk to these patients.
Modulation of the Micturition and Continence Reflexes
Selective serotonin and norepinephrine reuptake inhibitors, like duloxetine, that are specific for reflexes that control the bladder and urethra have the promise for treating not only OAB but also stress incontinence. At present, there are no clinically proven pharmacotherapies acting in the central nervous system to treat OAB. However, recent advances in animal studies have revealed potential targets in the brain and spinal cord for the treatment of OAB.
The sympathetic and parasympathetic autonomic nuclei as well as the sphincter motor nuclei receive a prominent serotonergic input from the raphe nuclei in the caudal brain stem. Activity in the serotonergic pathway generally enhances urine storage by facilitating the vesicosympathetic reflex pathway and inhibiting the parasympathetic micturition pathway.9,10 Among the various subtypes of 5-hydroxytryptamine (5-HT) receptors, 5-HT2 and 5-HT3 receptors mediate excitatory effects on sympathetic and somatic reflexes to increase outlet resistance. Moreover, 5-HT2C and 5-HT3 receptors are involved in inhibition of the micturition reflex. Targeting specific subtypes of 5-HT receptors could offer new treatments for lower urinary tract dysfunctions such as OAB or stress incontinence. For example, duloxetine, a selective serotonin and norepinephrine reuptake inhibitor, is most promising presently, and is now in clinical trials (Eli Lilly and Co.) for both urge and stress incontinence.9,10 In animal models, duloxetine has been shown to significantly increase bladder capacity and sphincter tone without interfering with the normal micturition cycle.
Bladder Peppers
Capsaicin and resiniferatoxin (RTX) are drugs derived from plants in the pepper family, commonly referred to as the vanilloids. Capsaicin and RTX activate nociceptive sensory nerve fibers through an ion channel known as vanilloid receptor subtype 1 (VR1).3 This receptor is a nonselective cation channel and is activated by heat and protons, suggesting that it functions as a transducer of painful thermal stimuli and acidity in vivo. Vanilloid receptors are located predominantly on C-fiber bladder afferents, and activation of the receptors initially excites and subsequently desensitizes C fibers.
The bladder afferent pathways consist of myelinated A-delta fibers and unmyelinated C fibers. A-delta fibers transmit signals from mechanoceptors that initiate the normal micturition reflex, where C-fiber bladder afferent neuron signals are not essential for normal voluntary voiding. However, various pathologic conditions, such as spinal cord injury or chronic bladder irritation, induce sensitization and/or recruitment of C-fibers, resulting in an overall increase in the C-fiber contribution to mechanotransduction and bladder overactivity.
RTX is a much more potent sensory antagonist than capsaicin. It is approximately 1000 times “hotter” (Scoville Heat Value) than capsaicin. Like capsaicin, it possesses vanilloid receptor agonist activity, resulting in desensitization. The key advantage of RTX is that it is at least as effective as capsaicin, without many of the local side effects, such as pain and inflammatory neuropeptide release. Recently, there are reports of RTXs being effective for the treatment of non-neurogenic urge incontinence, OAB, and even interstitial cystitis.
Poisoning the OAB
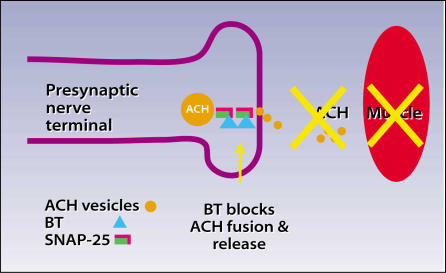
Botulinum toxin, first isolated by Emile van Ermengem in 1897, is the most potent biological toxin known. A single gram of this toxin, if maximally distributed, can kill 1 million people! The toxin acts by inhibiting ACH release at the presynaptic cholinergic junction. Inhibited ACH release results in regionally decreased muscle contractility and muscle atrophy at the site of injection. The chemical denervation that results is a reversible process, as axons resprout in approximately 3 to 6 months11 (Figure 1).
Figure 1.
Botulinum toxin (BT) blocks fusion and release of acetylcholine (ACH) vesicles into the neuromuscular junction. ACH causes the muscle to contract. Botulinum toxin causes long-term inhibition of muscle contraction where the toxin is injected.
Urologically, botulinum toxin has been used to treat spinal cord-injured patients who suffer from detrusor-external sphincter dyssynergia (DESD).12 More recently, Schurch and colleagues reported successful treatment of spinal cord-injured patients with detrusor hyperreflexia using intravesical botulinum toxin injections at up to 30 sites.13 The authors demonstrated a significant increase in mean maximum bladder capacity (from 296 mL to 480 mL, P < .016). The clinical effects begin within 5 to 7 days and last up to 6 months.
Recent animal research demonstrated marked decreases in the release of labeled norepinephrine and ACH in botulinum-injected rat bladder and urethra.11 While the therapeutic effect of inhibiting ACH release is obvious, blockage of norepinephrine release may also provide clinical benefit by inhibiting sympathetic transmission and smooth-muscle dyssynergia. The potential treatment targets of botulinum would therefore include not only DESD but also BPH and OAB. Phelan and coworkers14 have expanded the role of urethral injections to include treatment for women patients: those with urinary retention after pubovaginal sling placement or secondary to pelvic floor spasticity and those with acontractile bladder who wish to void by means of the Valsalva maneuver. With injection localized to the external sphincter, the risk of developing stress urinary incontinence has been minimal in my personal experience over the past 3 years.
Potential Therapies by 2010
Potassium Channel Openers
One promising class of drugs that a number of pharmaceutical companies are considering for the treatment of OAB is potassium channel openers (KCOs). Drugs, such as cromakalim, pinacidil, and ZD6169, that open ATP-sensitive K+ (KATP) channels and produce membrane hyperpolarization are effective in suppressing spontaneous action potentials and isolated contractions of bladder smooth muscle. KATP channel openers are less effective in blocking neurally evoked than spontaneous bladder contractions and therefore should be more active in suppressing unstable bladder contractions during bladder filling and not interfere with normal voiding. Oral administration of ZD6169 reduces voiding frequency in rats and dogs without lowering blood pressure.15 Intravesical administration in rats increases the bladder volume at which a micturition reflex is induced and also decreases the frequency and amplitude of spontaneous bladder contractions and reduces voiding pressure in both normal and outlet-obstructed animals.15,16 It has been suggested that the drug acts not only on bladder smooth muscle but also on capsaicin-sensitive bladder afferents to reduce afferent firing induced by bladder distention or chemical irritation of the mucosa.16
Tachykinin Antagonists and Afferent Peptides
Tachykinins released in the bladder can act on: 1) NK1 receptors in blood vessels to induce plasma extravasation and vasodilation; 2) NK2 receptors to stimulate the bladder contractions; and 3) NK2 receptors on primary afferent terminals to increase excitability during bladder filling or during bladder inflammation.16 Substance P also acts on receptors on urothelial cells to release nitric oxide. Intrathecal administration of NK1 antagonists increased bladder capacity in normal conscious rats without changing voiding pressure, whereas NK2 antagonists were ineffective. Bladder hyperactivity in rats was also suppressed by intrathecal injection of NK1 antagonists. Bladder hyperactivity induced by capsaicin was reduced by an NK2 antagonist (MEN 11,420) that did not influence normal voiding.17 TAK-637, which is a highly specific antagonist for the NK1 receptor, is also reportedly effective to suppress bladder activity in guinea pigs.18 The key advantage of tachykinin antagonists is that there is essentially no decrease in detrusor contractility and no residual urine or retention risk. The drug works on the sensory nerves innervating the bladder and not on the bladder itself. Would it not be lovely to have one drug that can help not only OAB but also irritable symptoms of BPH and interstitial cystitis and yet causes no dry mouth or risk of urinary retention?
Relaxing the Detrusor Without Causing Retention
A fascinating and promising new approach for the treatment of the OAB that the general urology community may not be familiar with is use of β3-adrenergic receptor agonists.
Recent studies demonstrated that the predominant β-adrenergic receptor subtype in the human is β3-receptors, rather than β1- or β2-receptors. Thus, activation of β3-adernergic receptor subtype could be useful for treating OAB by directly relaxing human bladder smooth muscle.19
Advanced Drug Delivery
Intravesical instillation of oxybutynin has been demonstrated to have efficacy in patients with OAB in whom oral oxybutynin failed, proved subtherapeutic, or was not tolerated. Let’s consider an apparent oxybutynin paradox: Why can higher concentrations of oxybutynin be delivered via intravesical instillation, dermal patch, or oral controlled-release technology with less incidence and severity of side effects than oral immediate-release oxybutynin?
The key is that dry mouth, due to anticholinergic effects on the salivary gland, is produced to a much greater extent by oxybutynin’s metabolite desethyloxybutynin than by the parent compound itself. Desethyloxybutynin is produced not only by first-pass metabolism in the liver but also by direct cytochrome P450 metabolism in the proximal gut wall (stomach and duodenum).4
Where oxybutynin is delivered alters the amount of metabolites that enters the systemic circulation. Intravesical delivery of oxybutynin should yield significantly less metabolite formation. If constant therapeutic levels of oxybutynin in the bladder can be achieved without repeated instrumentation, this would provide an extremely effective regimen for controlling OAB. The key to this intravesical regimen is a long-lasting intravesical pump to deliver the desired dose of drugs. This technology is not available today but is currently under development.20
Purine
Contractions of normal human bladders are induced primarily by ACH released from cholinergic nerve terminals in the bladder. It has been reported that nonadrenergic, noncholinergic bladder contractions are induced by increases of ATP levels in the human bladder under pathologic conditions such as denervation, bladder outlet obstruction, or idiopathic urge incontinence.
ATP acts on two families of purinergic receptors: an ion channel family (P2X) and a G protein-coupled receptor family (P2Y). P2X3 receptors have also been detected in the wall of the bladder in a suburothelial plexus of afferent nerves. In P2X3 knockout mice, afferent activity induced by bladder distention was significantly reduced.21
In patients with idiopathic detrusor instability, numbers of detrusor P2X2 receptors were significantly elevated, whereas those of other P2X receptor subtypes were significantly decreased.22 There was no detectable purinergic component of nerve-mediated detrusor muscle contractility in normal women without instability. However, there was a significant (approximately 80%) purinergic contractility component in bladder of women with detrusor instability.
In conclusion, the purinergic pathway may be a novel target for the pharmacological treatment of OAB. P2X-receptor antagonists could modulate efferent/afferent activities to treat OAB if receptor subtype-specific agents become available.
Bladder-Selective PDE Inhibitors
Phosphodiesterase (PDE) is the enzyme that catalyzes the degradation of cyclic adenosine monophosphate (cAMP) to AMP and thus limits the action of cAMP. There are several classes of PDEs that have individual substrate affinities, specific species and tissue distributions, and pharmacologic selectivities.23 Considerable research is currently underway to try to identify the specific isoform of PDE present in the bladder as opposed to that in the penis. Selective inhibition of bladder PDE would result in both an increase in the basal levels of cAMP (and possibly relaxation of the detrusor) and enhancement of the sensitivity and efficacy of β-adrenergic receptor agonists. Thus, a selective β-adrenergic receptor agonist or a selective PDE inhibitor might prove effective alone or in combination for relaxation of the detrusor.
Star Trek Urology: To Boldly Go Where No Urologists Have Gone Before
Pharmacogenomic Medicine
Through microarray gene chip technology, it is hoped that in the future we will be able to determine a patient’s drug metabolism profile, receptor profile, and allergy risk. These factors can be used to screen a list of medications prior to therapy. A urologist will then always be able to choose the best drug for each patient every time without the risk of allergic reaction.
Gene Therapy
The bladder is ideally suited for molecular medicine. Through gene therapy we can replace, supplement, or suppress a protein or cytokine to correct a disease process. I would like to give three examples.
First, let’s consider gene therapy for the treatment of OAB. Large-conductance calcium-sensitive (KCa, or maxi-K) channels play a role in modulating contraction and relaxation responses in smooth muscle cells. Bladder injection of human maxi-K channel DNA blocked detrusor hyperactivity in partially urethral-obstructed female rats.24 It is hypothesized that expression of hSlo in rat bladder functionally antagonizes the increased contractility normally observed in obstructed animals and thereby ameliorates bladder overactivity.
Second, I believe that there may be a way to prevent the inevitable deterioration of diabetic neurogenic bladder through organ-specific gene therapy. In a rat model of diabetic cystopathy, the bladder wall is injected with a specially constructed nonreplicating human herpes simplex virus (HSV) vector. This recombinant herpes vector mediates expression of β-nerve growth factor (β-NGF), a neurotrophic factor that in experimental conditions has been shown to prevent and reverse diabetic neuropathy. Using the safe, nonreplicating, latent HSV vector, expression of β-NGF occurs not only in the bladder but also in the dorsal root ganglia of the pelvic nerve. We have exciting data suggesting that over-expression of β-NGF in the bladder and dorsal root ganglia can decrease diabetic cystopathy.25
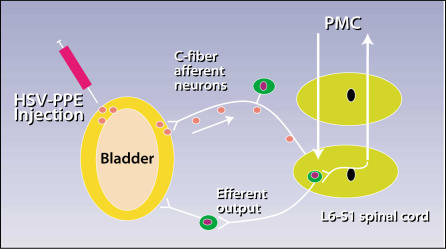
Third, what about the single condition most troubling to just about every urologist, interstitial cystitis (IC)? Can the introduction of a viral vector that is targeted to nerves and that carries a gene for an endogenous opioid peptide, which blocks pain pathways,26 be used to help alleviate pain, regardless of the cause of IC? HSV vector-mediated gene transfer to the bladder and bladder afferent nerves of preproenkephalin (PPE), a precursor of enkephalin, has demonstrated exciting proof-of-concept results. Because opioid peptides such as enkephalin are known to suppress not only pain but also the micturition reflex, HSV-PPE gene therapy can be a potential modality for treating OAB as well as bladder pain (Figure 2).
Figure 2.
The use of herpes simplex virus (HSV) to treat interstitial cystitis. HSV gene transfer to the bladder and bladder afferent nerves of preproenkephalin (PPE), a precursor of enkephalin. HSV-PPE injected into the bladder wall will be taken up and transported up the afferent nerve that innervates the bladder. Because the HSV has a replication defect, there is no risk of clinical herpes infection. Enkephalin is released only in the nerve pathway that innervates the bladder to block pain by suppressing synaptic transmission via local spinal reflex or via the ascending central nervous system at the pontine micturition center (PMC) pathways.
Stem Cell Tissue Engineering
Can we use tissue engineering and stem cell technology to actually rebuild the damaged bladder and urethra?27,28 Because the bladder and urethral smooth muscle generally lack regenerative ability, research has centered on tissue repair by using pluripotent stem cells derived from other lineages.29 Through purifying techniques, muscle-derived stem cells that can differentiate into urinary tract smooth muscle and improve the function of the bladder wall and urethral sphincter may become possible.
Stem cell-based tissue engineering can also be a platform for ex vivo gene therapy. The aim of ex vivo cell therapy is to replace, repair, or enhance the biological function of damaged tissue or organs. An ex vivo process involves harvesting of cells from patients or donors, in vitro manipulation to enhance the therapeutic potential of the harvested cells (ex vivo gene therapy), and subsequent injection or implantation of the cells into the patient. One particular advantage of cell-based ex vivo gene therapy is that the manufactured cells act like bioreactors.28 At any stage of the process, cells can be cryopreserved so that therapy can be scheduled according to the patient’s requirements.
Main Points.
Therapies for urinary incontinence likely to be available by 2005 include transdermal oxybutynin, bladder-specific anticholinergic drugs, antidiuretic hormones, modulation of the micturition and continence reflexes, capsaicin and resiniferatoxin, and botulinum toxin.
Potential therapies and techniques like potassium channel openers, tachykinin antagonists, ways to relax the detrusor without causing urinary retention, advanced drug delivery, purine, and bladder-selective phosphodiesterase inhibitors may be in use by 2010.
Treatments based on pharmacogenomic medicine, gene therapy, and stem cell tissue engineering lie further in the future.
References
- 1.Yoshimura N, Chancellor MB. Pharmacotherapy of the overactive bladder. J Urol. In Press. [Google Scholar]
- 2.Chancellor MB, Yoshimura N. Physiology and pharmacology of the bladder and urethra. In: Walsh PC, Retik AB, Stamey TA, Vaughan ED, editors. Campbell’s Urology. 8th ed. Philadelphia, PA: Harcourt Publishers; 2002. In Press. [Google Scholar]
- 3.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: spicing up the ways that we treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Sathyan G. Pharmacokinetics of an oral once-a-day controlled-release oxybutynin formulation compared with immediate release oxybutynin. J Clin Pharmacol. 1999;39:22–29. [PubMed] [Google Scholar]
- 5.Davila GW, Daugherty CA, Sanders SW. A short-term, multicenter, randomized double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence. J Urol. 2001;166:140–145. [PubMed] [Google Scholar]
- 6.Smith CM, Wallis RM. Characterization of [3H]-darifenacin as a novel radioligand for the studyof M3 receptors. J Recep Sig Trans Res. 1997;17:177–184. doi: 10.3109/10799899709036602. [DOI] [PubMed] [Google Scholar]
- 7.Chapple CR. Muscarinic receptor antagonists in the treatment of overactive bladder. Urology. 2000;55:33–46. doi: 10.1016/s0090-4295(99)00492-6. [DOI] [PubMed] [Google Scholar]
- 8.Hill S, Khullar V, Cardozo L. Desmopressin tablets in the treatment of nocturia in women. Neurourol Urodyn. 1995;14:462–463. [Google Scholar]
- 9.Sharma A, Goldberg MJ, Cerimele BJ. Pharmacokinetics and safety of duloxetine, a dual serotonin and norepinephrine reuptake inhibitor. J Clin Pharmacol. 2000;40:161–167. doi: 10.1177/00912700022008810. [DOI] [PubMed] [Google Scholar]
- 10.Thor KB, Katofiasc MA. Effects of duloxetine, a combined serotonin and norepinephrine reuptake inhibitor, on central neural control of lower urinary tract function in the chloralose-anesthetized female cat. J Pharmacol Exp Ther. 1995;274:1014–1024. [PubMed] [Google Scholar]
- 11.Smith CP, Somogyi GT, Chancellor MB. Botulinum toxin: poisoning the spastic bladder and urethra. Rev Urol. In press. [PMC free article] [PubMed] [Google Scholar]
- 12.Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol. 1988;139:919. doi: 10.1016/s0022-5347(17)42717-0. [DOI] [PubMed] [Google Scholar]
- 13.Schurch B, Stohrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? preliminary results. J Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 14.Phelan MW, Franks M, Somogyi GT, et al. Botulinum toxin urethral sphincter injection to restore bladder emptying in men and women with voiding dysfunction. J Urol. 2001;165:1107–1110. [PubMed] [Google Scholar]
- 15.Hu S, Kim HS. Modulation of ATP-sensitive and large-conductance Ca2+ activated K+ channels by Zeneca ZD6169 in guinea pig bladder smooth muscle cells. J Pharmacol Exp Ther. 1997;280:38–45. [PubMed] [Google Scholar]
- 16.de Groat WC. Basic neurophysiology and neuropharmacology. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth, UK: Health Publications Limited; 1999. pp. 105–154. [Google Scholar]
- 17.Lecci A, Giuliani S, Tramontana M, et al. MEN 11,420, a peptide tachykinin NK2 receptor antagonist, reduces motor responses induced by the intravesical administration of capsaicin in vivo. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:182–188. doi: 10.1007/pl00005039. [DOI] [PubMed] [Google Scholar]
- 18.Kamo I, Imai S, Okanishi S, Doi T. Possible site of action of TAK-637, a tachykinin NK(1) receptorantagonist, on the micturition reflex in guinea pigs. Eur J Pharmacol. 2000;401:235–240. doi: 10.1016/s0014-2999(00)00468-4. [DOI] [PubMed] [Google Scholar]
- 19.Woods M, Carson N, Norton NW, et al. Efficacy of the 3-adrenergic receptor agonist CL-316243 on experimental bladder hyperreflexia and detrusor instability in the rat. J Urol. 2001;166:1142–1147. [PubMed] [Google Scholar]
- 20.Boone TB, Appell RA, Lopez MA, et al. Pharmacokinetic evaluation of intravesical oxybutynin: bolus and continuous delivery. J Urol. 2001;165:252A. [Google Scholar]
- 21.Cockayne DA, Hamilton SG, Zhu QM, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly BA, Kosaka AH, Knight GF, et al. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- 23.Truss MC, Becker AJ, Uckert S, et al. Selective pharmacological manipulation of the smooth muscle tissue of the genitourinary tract: glimpse into the future. BJU Int. 1999;83(suppl 2):36–41. doi: 10.1046/j.1464-410x.83.s2.9.x. [DOI] [PubMed] [Google Scholar]
- 24.Christ GJ, Day NS, Day M, et al. Bladder injection of “naked” hSlo/pcDNA3 ameliorates detrusor hyperactivity in obstructed rats in vivo. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R100–R1709. doi: 10.1152/ajpregu.2001.281.5.R1699. [DOI] [PubMed] [Google Scholar]
- 25.Goins WF, Yoshimura N, Phelan MW, et al. Gene therapy using herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748–1754. [PubMed] [Google Scholar]
- 26.Franks ME, Sasaki K, Yokoyama T, et al. Inhibitory effects of preproenkephalin gene therapy mediated by herpes simplex virus vectors on bladder hyperactivity induced by intravesical capsaicin. J Urol. 2001;165:249A–300A. [Google Scholar]
- 27.Atala A. Creation of bladder tissue in vitro and in vivo: a system for organ replacement. Adv Exp Med Biol. 1999;462:31–42. doi: 10.1007/978-1-4615-4737-2_3. [DOI] [PubMed] [Google Scholar]
- 28.Chancellor MB, Yoshimura N, Pruchnic R, Huard J. Gene therapy strategies for urological dysfunction. Trends in Molecular Medicine. 2001;7:301–306. doi: 10.1016/s1471-4914(01)02088-3. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama T, Yoshimura N, Dhir R, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165:271–276. doi: 10.1097/00005392-200101000-00077. [DOI] [PubMed] [Google Scholar]