Abstract
Clinical reflux was first visualized over 100 years ago. In the 1950s and early 1960s, the assumption was that surgery to relieve bladder neck obstruction would have a positive effect on bladder function and reflux. By the early 1970s it was understood that the underlying structural problems leading to primary reflux were congenitally abnormal distal ureters and orifices. Researchers in the 1960s and 1970s demonstrated the connection between reflux and renal scarring. More recently, reflux nephropathy in the absence of urinary tract infections has been observed, leading researchers to investigate an association between bladder dysfunction and reflux with resulting nephropathy. The cornerstone of management of the child with vesicoureteral reflux is antibiotic prophylaxis; treatment regimens for various grades of reflux are reviewed. Indications for surgical treatment of reflux are also discussed. Controversies regarding vesicoureteral reflux, including duration of prophylactic treatment, remain to be resolved.
Key words: Vesicoureteral reflux, Urinary tract infections, Renal scarring, Bladder dysfunction, Antibiotic prophylaxis, Surgery
Vesicoureteral reflux, the retrograde flow of urine from the bladder into the upper urinary tract, is a well recognized, readily diagnosed, and seemingly clearly understood phenomenon. The current generation of urologists has been trained to understand several “truths” about reflux. We all recognize the distinction between “primary” and “secondary” reflux and realize that reflux, when it is associated with infection, predisposes to renal parenchymal injury. The “Big Bang Theory” of reflux nephropathy postulates that the reflux of infected urine leads to renal parenchymal infection and that as the infection resolves scarring may result.1 We know that reflux is generally managed by the institution of prophylactic antibiotics and that certain subsets of patients with reflux will require surgery. The majority of patients we care for have primary reflux, and we place them on antibiotic prophylaxis until the time when their intrinsically abnormal ureterovesical junction “matures,” becomes competent, and their reflux spontaneously resolves. Most surgeons accept that grade V reflux, febrile breakthrough infections, and patient noncompliance with antibiotic prophylaxis are indications for surgery. Most older girls with reflux are offered ureteral reimplantation as they approach the pubertal age group, because conventional wisdom suggests that the risk of febrile urinary tract infections during pregnancy is best avoided.
Secondary reflux is, as the name suggests, the consequence of some other bladder pathology. The conditions leading to secondary reflux have as a common denominator bladder dysfunction. Posterior urethral valves, ureteroceles, neurogenic bladder dysfunction, and severe non-neurogenic bladder dysfunction, or the Hinman/ Allen syndrome, are conditions typically associated with secondary reflux. The management of reflux in these patients is directed towards the primary etiology with the expectation that as that problem resolves the “secondary” reflux may remit.
In this brief overview, we will review the clinical and experimental data that lead to our current understanding of reflux and highlight some recent clinical data that challenge some of our heretofore immutable perceptions about reflux.
History
Clinical reflux was first visualized over 100 years ago in 1893 when Pozzi observed reflux from the cut end of the distal ureter while he was performing a nephrectomy. Young, working in 1898 with human cadavers, was unable to produce reflux when fluid was instilled into the bladder. It was Hutch’s clinical work in paraplegic patients demonstrating the occurrence of reflux, its association with infections, and an adverse effect on the kidney that lead to more generalized use of the voiding cystourethrogram (VCUG). We learned from his work that infravesical obstruction and neurogenic dysfunction leading to high bladder pressures could cause reflux. Hutch described his results with a “plastic operation” on the ureterovesical junction and documented resolution of difficulties with recurring febrile infections in the patients in whom his operation cured their reflux.2
VCUGs depicting reflux in the 1950s and early 1960s were usually interpreted as demonstrating bladder neck obstruction. The obvious solution was that surgery to relieve the bladder neck obstruction would have a positive effect on bladder function and reflux. During this era Y-V plasties of the bladder neck intending to relieve the obstruction were performed routinely in children with and without reflux who had difficulties with recurrent urinary tract infections. Smith, reviewing the subject in 1969, noted that most authorities of that decade supported the role of bladder neck surgery in patients with reflux.3 From Clinical Pediatric Urology second edition 1985, edited by Kelalis, King, and Belman we learned that “a decade passed before this notion [that the bladder neck was obstructive] was dispelled and the current view of reflux as a primary and congenital abnormality of the ureterovesical junction in most affected children was established.”4
The congenital abnormality of the ureterovesical junction that caused reflux was characterized during the 1960s. We learned that competence of the ureterovesical junction is affected by the length of the submucosal ureter and the muscular support beneath the ureter. As these factors varied, so did the configuration of the ureteral orifice. Lyon et al described the abnormal morphology of the orifice as appearing like a stadium, horseshoe, or golf hole.5 Stephens identified the “lateral pillar defect.” We understood by the early 1970s that the underlying structural problems leading to primary reflux were the congenitally abnormal distal ureters and orifices. There were a variety of surgical techniques available that could reliably correct reflux by the mid-1970s.
Hinman and Allen described an interesting group of patients with severe voiding dysfunction that ultimately lead to structural bladder changes and reflux.6,7 These patients’ bladders appeared similar to patients with neurogenic bladders, but the patients had no apparent neurologic deficit. It was proposed that this form of bladder dysfunction was a learned phenomenon, and the condition became known as the non-neurogenic neurogenic bladder.
During the 1960s and 1970s our understanding of the pathogenesis of reflux nephropathy expanded as a result of both clinical and experimental studies. In 1960 Hodson and Edwards first demonstrated the association between reflux and scarring.8 The condition that had been known as chronic atrophic pyelonephritis began to be recognized as a consequence of reflux. Smellie showed that patients with uncomplicated reflux who maintained a sterile urine while on continuous antibiotic prophylaxis did not develop new renal scars. The only new scars in her series developed in patients who had febrile breakthrough infections.9
Because the pig’s renal papillary morphology is similar to the human it became the animal of choice for researchers working in reflux. Hodson caused reflux in pigs and was able to demonstrate that the reflux caused renal scarring, even though the refluxed urine was sterile.10 Ransley and Risdon could produce renal scars in pigs that were voiding normally only if their urine was infected. These discrepant observations were seemingly resolved by Ransley and Risdon’s experimental observation that sterile reflux could scar the kidney but only in the face of prolonged elevated intravesical pressures that ultimately lead to bladder decompensation.11 They concluded that for the normal child, this clinical situation would be unlikely and therefore renal scarring should only occur if the refluxed urine was infected. These were the clinical and experimental observations that lead to the enunciation of the “Big Bang Theory” of reflux.1 This theory proposes that the reflux of infected urine, especially when there is intrarenal reflux, could lead to renal parenchymal infection and inflammation, which as it healed, might cause the scarring of reflux nephropathy.
The Big Bang Theory summarized our understanding of the pathogenesis of reflux nephropathy by the 1980s. Clinical experience proved that an episode of pyelonephritis was more likely to lead to a renal scar if there was a delay in treatment and/or if the infection occurred in a younger child.12,13 Serial radiologic imaging revealed that it could take from 6 months to some years for the scar caused by a particular episode of pyelonephritis to be visible on an intravenous pyelogram.14 Some children evaluated after an apparent initial urinary tract infection (UTI) were discovered to have reflux with associated nephropathy even though they never had prior urinary tract infections. The consensus opinion was that these children had prior undiagnosed urinary tract infections and that the symptoms of these infections had not been recognized as urinary tract infections but probably attributed to viral illnesses. It was clear to me when I evaluated a child, as recently as 10 years ago, presenting with her “initial UTI” when there was associated nephropathy, that she must have had antecedent undiagnosed infections. I quizzed the parents about “viral illnesses” and problems with “recurrent otitis media” that might have been misconstrued or rather missed urinary tract infections. The clear, although unstated, conclusion might be that their primary-care physician had misdiagnosed these febrile illnesses.
Recent Developments
Over the last 20 years, several areas of clinical practice and research have modified our previously understood “truths” about reflux. Prenatal ultrasonographic screening led to the referral of large numbers of neonates for the evaluation of prenatally diagnosed hydronephrosis. Many of the neonates have obstructive phenomena, but reflux is the cause of the hydronephrosis in about 10% of these children.15 The demographics of reflux in the neonate are different from reflux that presents in the older child. The neonate with reflux will much more frequently be male and have high-grade reflux.16 An interesting feature of the reflux in these boys is that it often improves during the first 2 years of life even if it is initially severe. Dimercaptosuccinic acid (DMSA) renal scanning in these neonates often reveals reflux nephropathy even though they have undeniably never had a UTI.17
Reflux nephropathy in the absence of infections and the frequent resolution of grade V reflux contradict our previously held tenants about reflux. What clinical or experimental data exist that will allow us to reconcile these observations? If we reassess the factors underlying the etiology of reflux, perhaps we can shed some further light on these issues. The first clear association between reflux and abnormal bladder function that was not either neuropathic or obstructive in origin was the Hinman syndrome.6 In the 1970s most people involved in the care of children with reflux recognized that the severely disordered bladder function these children manifest leads to reflux and upper tract changes. McGuire proposed in the early 1980s the association between elevated bladder pressure and vesicoureteral reflux in a population of patients with neurogenic dysfunction due to myelodysplasia.18 Shortly after this it was recognized that less severe bladder dysfunction, manifest in little girls by urgency, frequency, and damp panties had an impact on reflux. Investigators proved that treating children with reflux who had also had symptoms of dysfunctional voiding with anticholinergics in addition to antibiotic prophylaxis improved the rate of reflux resolution compared to antibiotic prophylaxis alone.19 This finding, plus the observation that one of the peak times of reflux detection is around the time of toilet training, when the child is taught to volitionally disturb her natural synergic, reflex voiding, further strengthens the argument that disordered bladder function, with its concomitant elevated intravesical pressures, plays a significant role in the genesis of reflux.
Sillen of Sweden performed urodynamic studies in normal male infants and compared the findings to boys with prenatally diagnosed reflux. The boys with reflux had dramatically elevated voiding pressures compared to normal boys. When these children were followed serially, reflux resolved in the boys whose voiding pressures normalized over time.20,21 Although these boys are conventionally described as having “primary” reflux, as our understanding of their problem expands it seems that the number of patients with “primary” reflux contracts. The reflux nephropathy observed in the boys’ kidneys cannot be explained by infection, and now that we understand the magnitude of these boys’ voiding pressures it is possible to imagine that their renal parenchymal injury may be a consequence of the hydrodynamic so-called “waterhammer effect” of the retrograde flow of sterile urine. It appears that Hodson may have been correct at least in this subset of patients.
A final piece of data that strengthens the argument that “primary” reflux is frequently and perhaps always secondary to bladder dysfunction comes from a review of postoperative results after ureteral reimplantation. Contralateral reflux occurring after unilateral ureteral reimplantation performed for reflux occurs in 5% to 20% of patients regardless of the type of ureteral reimplantation technique employed. Caione et al recorded their results after unilateral reimplantation in patients with vesicoureteral reflux and in a series of patients who had undergone a unilateral ureteral reimplantation because of a primary obstructive megaureter. After ureteral reimplantation performed for reflux they observed contralateral reflux in 11.2% of their patients; on the other hand, when the ureteral reimplantation was performed for primary megaureter, contralateral reflux occurred in only 1.7% of patients.22 The implication is that it is not really the technique of ureteral reimplantation that causes the contralateral reflux but rather that patients with reflux have a primary bladder problem that has enough of an effect to impact on the previously nonrefluxing ureter after surgery. When ureteral reimplantation is performed for obstructive megaureter there is no underlying bladder dysfunction but rather a juxtavesical obstructive problem, and so no contralateral reflux occurs.
Management
The cornerstone of management of the child with vesicoureteral reflux is antibiotic prophylaxis. Prophylactic antibiotics are intended to prevent recurring infections whether the patient is being scheduled for surgery or managed non-operatively. The antibiotics most commonly used for prophylaxis are amoxicillin in the infant and trimethoprim-sulfamethoxazole (TMP-SMX) or nitrofurantoin in children.
There are few absolute indications for surgery. Most authorities suggest surgery for older children who have grade V reflux, and most agree that a febrile breakthrough urinary tract infection mandates surgery. Noncompliance with antibiotic prophylaxis is also an indication for surgery. The vast majority of children with reflux are managed nonoperatively. Relative indications for surgery include persistent reflux of stable, moderate grade over several years and reflux that persists into the peripubertal age group. Some authorities have recommended discontinuing prophylaxis in the older child with lower grade reflux and no voiding dysfunction, and observing the child’s progress. If the child subsequently develops pyelonephritis either prophylaxis is resumed or surgery is performed. This approach is considered safe, because a UTI in a child over the age of 5 years is not as likely to cause a scar compared to a younger child, especially if the infection is promptly treated.
The rationale for non-operative or medical management is predicated on two principles: that sterile reflux does not damage the kidney and that spontaneous resolution of reflux is expected in the majority of children.23 Sterile reflux has not been shown to cause renal damage in both clinical and experimental series in patients with normal bladder function. Years ago it was demonstrated that the use of continuous low-dose prophylactic antibiotics was a superior approach to preventing new renal scars compared to the intermittent administration of antibiotics at the first sign of infection.9,23 The safety of low-dose continuous antibiotic prophylaxis has been clearly established over the last few decades. Although many parents are concerned about the possibility of side effects from long-term prophylactic antibiotics, the risk of a breakthrough infection and subsequent renal scarring seems to outweigh any potential deleterious side effects of the antibiotics.
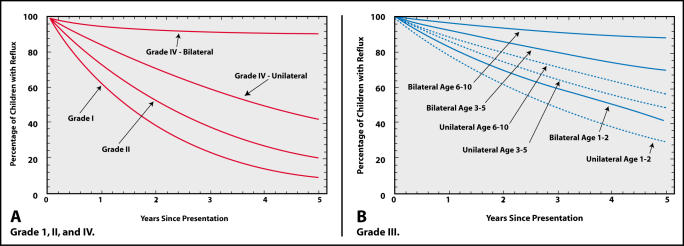
Reflux resolves spontaneously in many children, and the likelihood of reflux resolution is greater with lowgrade reflux compared to moderate or severe reflux, if it is unilateral as opposed to bilateral and if the child’s bladder function is normal.23,24 The American Urological Association Pediatric Vesicoureteral Reflux Guidelines Panel review concluded that the rate of resolution in patients with grades I or II reflux was the same rate regardless of their age at presentation or whether the reflux was unilateral or bilateral. The likelihood of grade III reflux resolving depends on the age at presentation and whether the reflux is unilateral or bilateral (Figure 1).25
Figure 1.
(A) Curves showing rate of reflux resolution for patients with grades I, II and IV reflux are independent of age at presentation. (B) Rates of resolution for patients with grade III reflux depend upon laterality and age at presentation. Reprinted from Elder et al,25 with permission.
Appropriate follow-up of the child on a regimen of medical management includes serial imaging studies to follow the progress of the reflux. At our clinic, we generally perform a radionuclide cystogram on an annual basis. Upper tract imaging by renal ultrasonography or a DMSA renal scan may be performed at less frequent intervals depending on the clinical situation. If the reflux was discovered after a single febrile infection in an older child who has normal kidneys on the initial ultrasound, annual upper tract imaging may not be required. On the other hand, in younger children most authorities suggest evaluation of the upper urinary tract on an annual basis to follow renal growth and detect any evidence of evolving renal scars. It is important to remember that a renal scar resulting as a consequence of a particular infection may take many months or even years to evolve and that the most sensitive technique for detecting renal scars is the DMSA renal scan.26
There are a variety of approaches used to follow the child’s urine and confirm that it remains sterile while on low-dose prophylaxis. Some physicians order urine cultures on a monthly basis, others three or four times a year. If the child develops a febrile illness for which there is no obvious focus, a specimen of urine must be obtained for culture and sensitivity. Data suggest that new scars are almost invariably predated by a febrile urinary tract infection.9 Therefore, our approach has been to obtain a urine culture to rule out the possibility of a breakthrough infection if the child has an unaccounted-for febrile illness. We do not routinely obtain cultures when the child is asymptomatic.
Management of grade I and II reflux is straightforward. The patients are placed on low-dose antibiotic prophylaxis and followed. The reflux resolves in the majority of children over a variable length of time. Virtually all patients with grade V reflux, except neonates, for the reasons mentioned above, are offered surgery at diagnosis. Grade V reflux, especially if it is bilateral, has a low likelihood of spontaneous resolution. Grade III and IV reflux are generally initially managed non-operatively. Non-operative management implies antibiotic prophylaxis, stabilization of bladder function, and appropriate bowel management.
The International Reflux Study group randomized patients with grade III and IV reflux to operative or non-operative management.27 The conclusion of the study after 5 years of follow-up was that there was no difference between the outcomes of the two treatment arms. The conclusions of the International Reflux Study group have had considerable influence, but unfortunately the study did not randomize patients at the time of initial diagnosis. In fact, on the American arm of the study 69% of the children had been placed on medical management prior to consideration of “randomization.” This factor was not as significant on the European arm, where only 18% of the patients had been started on antibiotics prior to randomization.28 The impact of the lack of true randomization will never be known but readers of the “bottom line” are unlikely to understand this design flaw of the study.
The study did reveal interesting differences between the outcomes of the patients undergoing surgery in Europe and North America. The incidence of postoperative ureteral obstruction complicating the reimplantation surgery was 4.2% in Europe.29 Postoperative ureteral obstruction is often complicated by infection and scarring. New scars developed during 5 years of follow-up in six of the ten European kidneys that suffered postoperative ureteral obstruction. No cases of ureteral obstruction occurred in patients operated on in North America, and the incidence of pyelonephritis was significantly lower in the American children undergoing surgery than those placed on medical management.30
The timing of the development of new scars in patients on the European arm was also interesting.31 New scars developed much more frequently within the first 6 months after randomization to surgery than they did in patients placed on medical management. Six of 20 new scars in the surgical arm were associated with postoperative ureteral obstruction. Twelve other new scars were recognized within 6 months of surgery, raising the possibility that these were actually evolutionary parenchymal changes occurring as a consequence of infections that had occurred prior to randomization. On the other hand, in the patients randomized to medical therapy, new scars occurred predominately later than 6 months beyond randomization. One cannot help but wonder if the results of the study might have been dramatically different if the European patients had been operated on by senior American surgeons whose patients suffered no instances of postoperative ureteral obstruction,30 and if modern techniques of renal parenchymal imaging had been available so that “new” scars may have been more accurately characterized.
One of the controversies in reflux management revolves around the duration of antibiotic prophylaxis. If a patient on prophylaxis stops refluxing during follow-up, then the medication is no longer necessary. But what about the child who is older and has normal kidneys but persisting reflux? Most authorities agree that older boys in this situation should discontinue prophylaxis and be followed for infections. If they subsequently have recurrent pyelonephritis, then surgery seems indicated, but if they remain asymptomatic it is generally accepted that they probably can be safely allowed to continue refluxing off prophylaxis. Should older girls with persisting reflux be allowed to come off of prophylaxis? Should they be allowed to go through puberty and become sexually active with persisting reflux? Does reflux during pregnancy predispose to an increased risk of upper tract infections and potential deleterious consequences for the fetus? Does ureteral reimplantation obviate any of these potential risks? The answers to these questions are currently unknown, and although there is no lack of vociferous opinions on either side of these queries, there is no well-designed study that allows us to resolve these issues.
Summary
The evaluation and management of patients with vesicoureteral reflux marked the beginning of pediatric urology as a fledgling subspecialty in urology. Significant advances in our understanding of the pathogenesis of reflux have occurred, and although the surgical management of reflux has largely been perfected, many questions remain. When we review the evolution of our knowledge about reflux, few areas of surgical endeavor more nicely exemplify the adage that “half of what you know today to be absolutely true … isn’t.”
Main Points.
Since its first visualization in the 1890s, understanding vesicoureteral reflux has evolved through many stages. The assumption of the 1950s and early 1960s that reflux resulted from bladder neck obstruction gave way to the current view of reflux as a primary and congenital abnormality of the ureterovesical junction.
In the 1960s, the connection between reflux and renal scarring began to be made. The condition that had been known as chronic atrophic pyelonephritis began to be recognized as a consequence of reflux.
The “Big Bang Theory” of reflux (enunciated in the late 1970s) proposes that the reflux of infected urine, especially when there is intrarenal reflux, can lead to renal parenchymal infection and inflammation, which as it heals, might cause the scarring of reflux nephropathy.
More recent observations have led to the theory that disordered bladder function, with its concomitant elevated intravesical pressures, plays a significant role in the genesis of reflux.
The cornerstone of management of the child with vesicoureteral reflux is continuous antibiotic prophylaxis, with antibiotics most commonly used being amoxicillin in the infant and trimethoprim-sulfamethoxazole (TMP-SMX) or nitrofurantoin in children.
There are few absolute indications for surgery, but most authorities suggest surgery for older children who have grade V reflux, and most agree that a febrile breakthrough urinary tract infection mandates surgery.
Appropriate follow-up of the child on a regimen of medical management includes serial imaging studies to follow the progress of the reflux.
References
- 1.Williams DI. Commentary at International Pediatric Nephrology Association Meeting; 1977; Helsinki, Finland. [Google Scholar]
- 2.Hutch JA. Vesico-ureteral reflux in the paraplegic: cause and correction. J Urol. 1952;68:457–467. doi: 10.1016/S0022-5347(17)68223-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith DR. Critique on the concept of vesical neck obstruction in children. JAMA. 1969;207:1686–1692. [PubMed] [Google Scholar]
- 4.Levitt SB, Weiss RA. Vesicoureteral reflux. In: Kelalis PP, King LR, Belman AB, editors. Clinical Pediatric Urology. 2nd edition. Philadelphia, PA: W.B. Saunders; 1985. p. 356. [Google Scholar]
- 5.Lyon RP, Marshall S, Tanagho EA. The ureteral orifice: its configuration and competency. J Urol. 1969;102:504–509. doi: 10.1016/s0022-5347(17)62184-0. [DOI] [PubMed] [Google Scholar]
- 6.Hinman F, Baumann FW. Vesical and ureteral damage from voiding dysfunction in boys without neurologic or obstructive disease. J Urol. 1973;109:727–732. doi: 10.1016/s0022-5347(17)60526-3. [DOI] [PubMed] [Google Scholar]
- 7.Allen TD. The non-neurogenic neurogenic bladder. J Urol. 1977;117:232–238. doi: 10.1016/s0022-5347(17)58412-8. [DOI] [PubMed] [Google Scholar]
- 8.Hodson CJ, Edwards D. Chronic pyelonephritis and vesicoureteric reflux. Clin Radiol. 1960;11:219. doi: 10.1016/s0009-9260(60)80047-5. [DOI] [PubMed] [Google Scholar]
- 9.Smellie JM, Normand ICS. Reflux nephropathy in childhood. In: Hodson J, Kincaid-Smith P, editors. Reflux Nephropathy. New York: Masson Publishing USA; 1979. pp. 14–20. [Google Scholar]
- 10.Hodson CJ, Maling TMJ, McManamon PJ, Lewis MG. The pathogenesis of reflux nephropathy (chronic atrophic peylonephritis) Br J Radiol. 1975;(suppl 13):1–26. [PubMed] [Google Scholar]
- 11.Ransley PG, Risdon RA. Reflux and renal scarring. Br J Radiol. 1978;(suppl 14) [Google Scholar]
- 12.Ransley PG, Risdon RA. Reflux nephropathy: effects of antimicrobial therapy on the evolution of the early pyelonephritic scar. Kidney Int. 1981;20:733–742. doi: 10.1038/ki.1981.204. [DOI] [PubMed] [Google Scholar]
- 13.Winberg J, Bollgren I, Kallenius G, et al. Clinical pyelonephritis and focal renal scarring. A selected review of pathogenesis, prevention, and prognosis. Pediatr Clin North Am. 1982;29:801–814. doi: 10.1016/s0031-3955(16)34213-4. [DOI] [PubMed] [Google Scholar]
- 14.Hodson CJ. The radiological contribution toward the diagnosis of chronic pyelonephritis. Radiology. 1967;88:857–871. doi: 10.1148/88.5.857. [DOI] [PubMed] [Google Scholar]
- 15.Elder JS. Commentary: importance of antenatal diagnosis of vesicoureteral reflux. J Urol. 1992;148:1750–1754. doi: 10.1016/s0022-5347(17)37020-9. [DOI] [PubMed] [Google Scholar]
- 16.Najmaldin A, Burge DM, Atwell JD. Reflux nephropathy secondary to intrauterine vesicoureteral reflux. J Ped Surg. 1990;25:387–390. doi: 10.1016/0022-3468(90)90376-k. [DOI] [PubMed] [Google Scholar]
- 17.Burge DM, Griffiths MD, Malone PS, Atwell JD. Fetal vesicoureteral reflux: outcome following conservative postnatal management. J Urol. 1992;148:1743–1745. doi: 10.1016/s0022-5347(17)37018-0. [DOI] [PubMed] [Google Scholar]
- 18.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–209. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 19.Koff SA, Murtagh D. The uninhibited bladder in children: effect of treatment on vesicoureteral reflux resolution. Contrib Nephrol. 1984;39:211–220. doi: 10.1159/000409250. [DOI] [PubMed] [Google Scholar]
- 20.Sillen U, Hjalmas K, Aili M, et al. Pronounced detrusor hypercontractility in infants with gross bilateral reflux. J Urol. 1992;148:598–599. doi: 10.1016/s0022-5347(17)36664-8. [DOI] [PubMed] [Google Scholar]
- 21.Sillen U, Bachelard M, Hermanson G, Hjalmas K. Gross bilateral reflux in infants: gradual decrease of initial detrusor hypercontractility. J Urol. 1996;155:668–672. doi: 10.1016/s0022-5347(01)66494-2. [DOI] [PubMed] [Google Scholar]
- 22.Caione P, Asili L, Capozza N, et al. Abstract 53 in the Program for Scientific Sessions, 1999 Annual American Academy of Pediatrics, Section on Urology. Baltimore, MD: American Urological Association; 1999. Is there risk of contralateral reflux after primary obstructive megaureter repair? pp. 109–110. [Google Scholar]
- 23.Lenaghan D, Whitaker JG, Jensen F, Stephens FD. The natural history of reflux and long-term effects of reflux on the kidney. J Urol. 1976;115:728–730. doi: 10.1016/s0022-5347(17)59352-0. [DOI] [PubMed] [Google Scholar]
- 24.Tamminen-Mobius T, Brunier E, Ebel KD, et al. Cessation of vesicoureteral reflux for 5 years in infants and children allocated to medical treatment. The International Reflux Study in Children. J Urol. 1992;148:1662–1666. doi: 10.1016/s0022-5347(17)36997-5. [DOI] [PubMed] [Google Scholar]
- 25.Elder JS, Peters CA, Arant BS, Jr, et al. Pediatric Vesicoureteral Reflux Guidelines Panel summary report on the management of primary vesicoureteral reflux in children. J Urol. 1997;157:1846–1851. [PubMed] [Google Scholar]
- 26.Rushton HG, Majd M. Dimercaptosuccinic acid renal scintigraphy for the evaluation of pyelonephritis and scarring: a review of experimental and clinical studies. J Urol. 1992;148:1726–1732. doi: 10.1016/s0022-5347(17)37014-3. 1992. [DOI] [PubMed] [Google Scholar]
- 27.International Reflux Study Committee, authors. Medical versus surgical treatment of primary vesicoureteral reflux: a prospective international reflux study in children. J Urol. 1981;125:277–283. doi: 10.1016/s0022-5347(17)55009-0. [DOI] [PubMed] [Google Scholar]
- 28.Weiss R, Tamminen-Mobius T, Koskimies O, et al. Characteristics at entry of children with severe primary vesicoureteral reflux recruited for a multicenter, international therapeutic trial comparing medical and surgical management. The International Reflux Study in Children. J Urol. 1992;148:1644–1649. doi: 10.1016/s0022-5347(17)36993-8. [DOI] [PubMed] [Google Scholar]
- 29.Hjalmas K, Lohr G, Tamminen-Mobius T, et al. Surgical results in the International Reflux Study in Children (Europe) J Urol. 1992;148:1657–1661. doi: 10.1016/s0022-5347(17)36996-3. [DOI] [PubMed] [Google Scholar]
- 30.Duckett JW, Walker RD, Weiss R. Surgical results: International Reflux Study in Children-United States branch. J Urol. 1992;148:1674–1675. doi: 10.1016/s0022-5347(17)36999-9. [DOI] [PubMed] [Google Scholar]
- 31.Olbing H, Claesson I, Ebel KD, et al. Renal scars and parenchymal thinning in children with vesicoureteral reflux: a 5-year report of the International Reflux Study in Children (European branch) J Urol. 1992;148:1653–1656. doi: 10.1016/s0022-5347(17)36995-1. [DOI] [PubMed] [Google Scholar]