Abstract
Anticholinergic drugs act on efferent nerves to counteract overactive bladder (OAB) after it occurs. To prevent the occurrence of OAB, therapies should be directed at blocking the afferent nerves that control the bladder. Tachykinin-receptor antagonists hold great promise in this regard, since they can be administered orally and do not increase the risk of urinary retention that occurs with anticholinergics. Advanced drug delivery systems, such as controlled-release oral oxybutynin (oxybutynin-XL) can reduce the incidence of anticholinergic side effects. In a similar manner intravesical therapy for OAB is site specific, and thus also reduces the occurrence of adverse events. Moreover, the difficulties of intravesical therapy may now be overcome with advanced delivery techniques such as an implantable, long-acting drug-delivery pump. Another intravesical therapy that has met with great acceptance and success is the administration of chili pepper extracts, especially resiniferitoxin, which may be effective for up to 3 months with one application. Finally, gene therapy holds great promise for OAB, because it is possible to access all of the genitourinary organs via endoscopy and other minimally invasive techniques that are ideally suited for gene therapy.
Key words: Afferent blockade, Anticholinergic, Capsaicin, Chili pepper, Gene therapy, Neurokinin Overactive bladder, Resiniferitoxin, Urinary incontinence
The new medications available today for treating urinary incontinence are clearly better than those we have had over the past 20 years. But, the new and improved anticholinergic drugs, such as tolterodine and controlled-release oxybutynin, are nearly at the limit of what can be expected of antimuscarinic agents. The foundation for improving overactive bladder (OAB) therapy requires a total revolution based on targeting the afferent nerves that control the bladder—afferent blockade.
In addition to special emphasis on afferent neuron activity, attention needs to be directed toward gene therapy and delivery of medication directly into the bladder with advanced techniques. The former has great urologic potential because it is possible to access all of the genitourinary organs via endoscopy and other minimally invasive techniques that are ideally suited to gene therapy. It is quite possible that, in the not too distant future, molecular medicine will be successfully applied to conditions ranging from benign prostatic hypertrophy, to OAB, and interstitial cystitis.
Agents that Prevent the Micturition Reflex
Using anticholinergic agents for the treatment of overactive bladder could be considered “after-the-fact” therapy, because it relies on waiting for the bladder to have a spasm and then trying to calm it down. That is, it focuses on efferent neuronal activity. It would be more logical to prevent the micturition reflex that initiates the overactive bladder. There are a number of afferent blockade drugs now in development that can prevent the bladder from having this involuntary contraction.
Since 1993, we have been experimenting with instilling pepper extracts, such as capsaicin and resiniferatoxin (RTX), into the bladder to deaden the vanilloid receptors of afferent neuron C-fibers.1 This procedure prevents the afferents in the bladder from firing and thus prevents it from having the sensation of urgency or the trigger for involuntary detrusor contraction. If this afferent neural activity that triggers OAB can be prevented, it may be possible to give patients much lower drug doses with fewer side effects and with greater efficacy. This may, therefore, not only help urge incontinence and overactive bladder, but interstitial cystitis as well.
Drugs that work on the afferent nervous pathways do not present the risk of urinary retention that occurs with the use of anticholinergic drugs. In this regard, tachykinin receptor antagonists are very promising drugs because they can be administered as oral agents that can prevent the bladder spasm reflex from tripping. In addition, some of the newly developed bladder-specific potassium channel openers may actually target the nerves themselves, rather than the bladder smooth muscle. So, it is possible that highly selective neuronal ’K’ channel openers can be developed that have no effect on smooth muscle, thereby avoiding all cardiovascular side effects.
Neurokinin Receptor Antagonists
Tachykinins (ie, substance P, neurokinin A, neurokinin B) are peptides that are released from sensory nerve endings. These peptides have been shown to directly cause smooth muscle contractions as well as facilitate transmitter release from nerves. Tachykinins act on three receptors: NK-1, NK-2, and NK-3. NK antagonists, specifically those that affect the NK-1 or NK-2 receptor, appear to have promising therapeutic potential in the treatment of bladder hyperactivity. NK-2 receptors are located on bladder smooth muscle and on afferent nerve terminals. Lecci et al were able to reduce bladder hyperactivity induced by capsaicin through the use of an NK-2-receptor antagonist, although normal voiding was not effected.2 NK-1 receptors located within the spinal cord appear to mediate both nociceptive and non-nociceptive afferent stimuli, given that NK-1 antagonists inhibit the micturition reflex induced by the administration of L-dopa and capsaicin.3–5 In addition, NK-1 receptors are located in blood vessels in the bladder to mediate vasodilation and plasma extravasation. Thus, agents that block NK-1 receptors may also reduce inflammatory conditions that would otherwise have stimulated afferent pathways.6
TAK637
The NK drug that has received the most interest in the United States is TAK637 (TAP, Chicago, IL), which is in Phase II FDA trials. TAK637 is highly specific for the NK-1 receptor. NK-1 receptors that are capsaicin sensitive have been found in the afferent nerve terminals of the bladder. When TAK637 is injected intrathecally it can block the micturition reflexes. This intrathecal effect validates the central site of action of TAK637—the NK-1 receptor in the central nervous system, the sacral micturition center.
The key advantage of tachykinin antagonists is that there is essentially no effect on decreasing detrusor contractibility and no residual urine or retention risk. The drug works on the nerves innervating the bladder and not on the bladder itself. There are recent studies demonstrating that when a tachykinin antagonist is administered after the proximal cut end of the pelvic nerve (afferent) is stimulated, it blocks the bladder micturition reflex, but has no effect on electrical activity when the peripheral end of the pelvic nerve (efferent) that goes to the bladder is stimulated.7 Moreover, TAK637 has no activity in isolated bladder-strip studies and bladder-strip response to electrical field stimulation. This represents a major advance when compared with other drugs currently being used.
In the guinea pig model, TAK637 significantly increased the bladder intercontraction interval by 200%, without effect on bladder pressure or on urethral function. It appears to increase bladder contraction intervals, without dampening detrusor contractility, more effectively than the anticholinergic drugs available today.8
Advanced Drug Delivery Systems
The use of advanced drug delivery for voiding dysfunction holds great potential. There is a strong rationale for the employment of such systems: Medications prescribed today cause 100,000 deaths per year in the United States and lead to 10% of all hospitalizations. In addition, medication related errors and noncompliance cost $136 billion dollars annually.9,10 These are startling statistics, and it has been suggested that advanced drug delivery may decrease some of this morbidity and cost. So, there are advantages in methods of delivery that can achieve long-term therapeutic efficacy with decreased side effects and improved patient compliance. The downside of advanced drug delivery, however, is cost.
Advanced drug delivery now represents an estimated $12 billion-a-year industry across a range of medical specialties,9,10 and is already widely used in urology (Table 1). For example, luteinizing hormone-releasing hormone (LHRH) analog is the gold standard of advanced drug delivery to treat prostate cancer. And the new, controlled-release oxybutynin can be considered the first advanced oral drug delivery system in urology.
Table 1.
Advanced Urologic Drug Delivery Systems
| Type of System | Example |
|---|---|
| Oral | Oxybutynin XL |
| Transdermal | Testosterone |
| Implantable polymeric | LHRH |
| Transmucosal (vaginal) | Estrogen ring |
| Nasal delivery | DDAVP |
| Intravesical | BCG, chemo, OAB |
Drug Absorption and Metabolism
It is commonly accepted that an oral drug is absorbed through the gastrointestinal tract and metabolized by the liver. The first-pass effect determines the number of metabolites in the body. However, studies addressing drug metabolism have uncovered a more complex processing of oral drugs.11 When a drug is swallowed, one of four possible processes can occur:
Transportation directly to the vein unaltered and later metabolism by the liver;
Metabolism by the cells of the proximal gut, with no further action by the liver;
Metabolism both by the proximal gut and the liver; and
Lack of absorption or reprocessing back into the gut lumen (see Figure 1).
Figure 1.
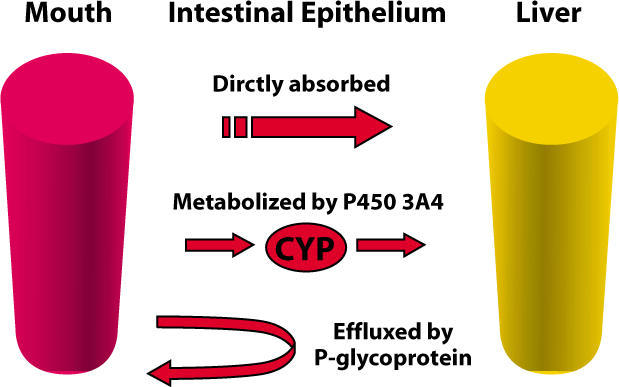
Three models of intestinal drug absorption.
Advanced Delivery Oxybutynin
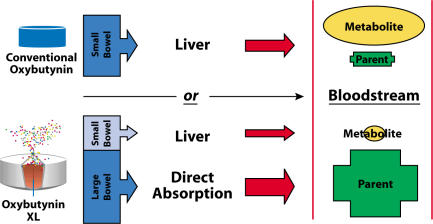
Recent studies12 have demonstrated that oxybutynin is not only metabolized in the liver but also in the proximal small intestine (but not in the large intestine). The metabolite, desethyloxybutynin, is a result of the first-pass metabolism in the liver, as well as direct cytochrome P450 metabolism in the gut wall. There is evidence that the parent compound and its metabolite have similar effects on bladder smooth muscle, but differ significantly with regard to their effects on the salivary gland. The parent compound does not cause as much dry mouth as the metabolite.
With the controlled-release technology of oxybutynin XL, there is significantly less metabolite versus the parent compound—a desirable combination. Oxybutynin XL is delivered at a steady rate for 24 hours, spending only 3 to 5 hours in the upper gut (see Figure 2). Therefore, gut wall metabolism and first-pass metabolism in the liver are proportionally reduced, and subsequently there is a reduced ratio of metabolite to parent compound systemically. Thus, the drug can achieve greater efficacy on the bladder with less dry mouth. Similarly, little metabolite is produced as a result of intravesical administration.
Figure 2.
Metabolism of oxybutynin.
Advanced Delivery Intravesical Therapy
Intravesical therapy of OAB is a clinically proven concept. It utilizes site-specific delivery, which results in fewer side effects compared with conventional oral therapy. It is, however, a difficult procedure, especially when instilling liquid medication into the bladder every day, three to four times a day. In addition, there is no formulation of liquid oxybutynin or any other anticholinergic that is presently approved by the FDA for intravesical use. The clear-cut superiority of the advanced delivery modality for oral oxybutynin just described, suggests that it might be equally advantageous to employ an advanced delivery system to place anticholinergic agents directly into the bladder. Such a system would ensure continuous drug delivery without the peaks and troughs of intermittent catheterization. Moreover, it should provide predictable, reliable therapy over an extended period of time.
One potential solution to the impracticality of intravesical instillation of short acting oxybutynin that meets these requirements is an advanced intravesical delivery technology designed by the Situs Company (San Diego, CA). Situs has developed a long-term drug delivery pump that is essentially a drug-filled reservoir shaped like a horseshoe (see Figure 3).
Figure 3.
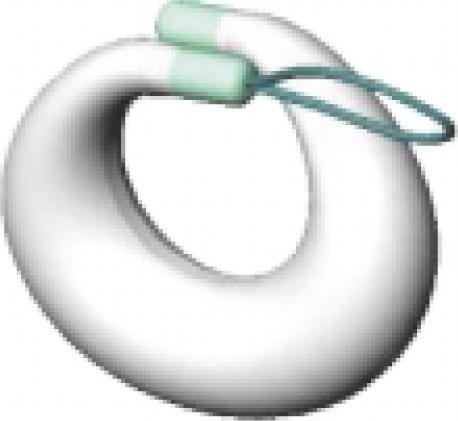
A long-term, intravesical drug delivery system.
The device is inserted into the bladder using a specially designed catheter insertion tool. After the deflated drug pump is placed into the bladder, the urologist fills it with oxybutynin and releases it. The bladder drug pump will then float in the bladder and release the drug at a constant rate for up to 28 days. Because of its unique horseshoe design, the pump can neither obstruct the bladder causing urinary retention or be accidentally eliminated. The downside of the bladder pump is that the physician has to remove and exchange it monthly with a flexible cystoscope and grasper. Dissolvable gels or matrixes that can slowly release drugs are also being investigated for advance intravesical drug delivery.
Chili Pepper Extract Therapy
The approach toward intravesical drug therapy that has probably received the most attention has been the administration of chili pepper extracts into the bladder to act on the afferent C-fiber vanilloid receptors (VR). The use of peppers as medicine is something patients understand. They can go to a health food store and buy capsaicin capsules. Many cultures have used peppers as medicine for over a thousand years.
Normal Bladder Activity
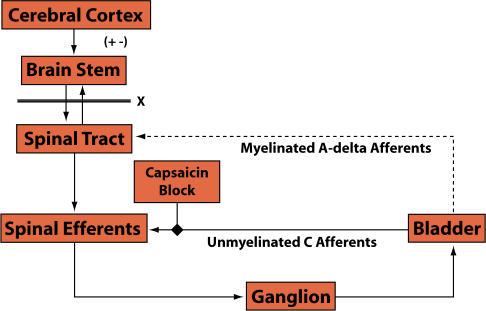
Chili pepper extracts have a specific mechanism of action that offers therapeutic potential in OAB. A brief review of the physiology of normal micturition and involuntary detrusor contraction will explain why. Tension receptors in the bladder normally dormant are triggered when the bladder is full. These sensors then send impulses to the central micturition center in the cerebral cortex through myelinated afferent A-delta fibers (see Figure 4). The “turning on” of the micturition reflex by the tension receptor is processed by the brain, which signals an individual with a full bladder to urinate.
Figure 4.
Normal control of micturition.
C-Fiber Afferents
Bladder afferent nerves are not solely composed of myelinated A-delta fibers. There are also unmyelinated afferent C-fibers in the bladder that are not normally active but act as sentinels to protect the bladder (see Figure 5). Unmyelinated C-fibers are programmed to sense danger by transmitting pain throughout the body. For example, when someone has a bladder infection, the resultant pain may lead to the urge to urinate every 15 minutes. That signal, or urge to urinate when the bladder is nearly empty is probably transmitted by C-fibers. There has been compelling research over the last 15 years suggesting that neurological diseases and inflammatory conditions, possibly including interstitial cystitis, can chronically activate the C-fibers so that they fire even when there are no noxious stimuli in the bladder.1 The activated C-fibers can cause bladder spasms, urgency, and pain.
Figure 5.
Control of micturition in neurologic and inflammatory disease.
Mechanism of Chili Pepper Activity
The activity of the chili pepper extract capsacin and its ultrapotent analogue resiniferatoxin is highly selective on the vanilloid receptors of C-fibers. Intravesical capsaicin opens the vanilloid receptor, causes depolarization, and C-fiber firing. When the afferent C-fiber nerves fire there is release of neuropeptides such as substance P and CGRP. Initially, this release of neuropeptides causes pain. Although capsaicin and RTX stimulate initial C-fiber excitation, over the long-term, they cause desensitization, because their action ultimately depletes the reservoir of substance P from the C-fibers.
The advantage to instilling chili peppers in the bladder is that a single instillation for 30 minutes may desensitize the nerves that cause bladder spasm and pain for up to 3 months without any systemic side effects or affecting the ability to urinate.
Resiniferitoxin
What is the best drug to use on C-fibers, and how “hot” are these pepper-based drugs? The spice industry uses the Scoville scale, created by an expert panel that assigns a heat value to each variety of pepper. The benchmark is the bell pepper, which has a Scoville heat value of one (see Table 2). The jalapeno pepper is 1000 times hotter than a bell pepper; the Tai pepper is 100,000 times hotter, and the Scoville rating for capsaicin is 6 million times greater than the bell pepper. However, we are moving away from capsaicin to RTX in clinical trials, because RTX causes less initial burning than capsaicin yet has similar efficacy. RTX is essentially a “new and improved” capsaicin, and is 16 billion times hotter than the bell pepper and 1000 times hotter than capsaicin. Although capsaicin was the first vanilloid used in the bladder, around the mid 1990s most researchers switched to RTX because it caused very little initial irritation.
Table 2.
Heat Value of Various Peppers and Extracts*
| Pepper or Extract | Scoville Value |
|---|---|
| Bell, Sweet Italian | 0–1 |
| Jalapeno | 1000 |
| Cayenne | 30,000–50,000 |
| Thai | 50,000–100,000 |
| Capsaicin | 16 million |
| Resiniferatoxin (RTX) | 16 billion |
As a multiple of the bell pepper value.
RTX, sponsored by the Afferon Corp. (Wayne, PA), is not clinically available and has completed only preliminary phase II, FDA trials in the United States. Most of the interesting research on RTX is taking place in Europe.
Clinical Studies with RTX
The author and colleagues recently completed a North American multicenter RTX clinical trial designed as a prospective, dose-escalating, double-blind, placebo-controlled study comparing the effects of seven concentrations of RTX (0.005, 0.025, 0.05, 0.1, 0.2, 0.5, 1.0 μM of RTX 10% ethanol in saline) and placebo (10% ethanol) for treatment of neurogenic bladder.
Each patient received a single dose; safety, tolerance, and bladder function assessments were made at screening, immediately following dosing, and at weeks 1, 3, 6, and 12, and quarterly thereafter, until a return to baseline bladder function was obtained.
The final analysis of the results of this study is currently being compiled, but a few trends were noted.
There were no significant differences between control and RTX for urinary incontinence at the lowest doses of 0.005–0.01 μM.
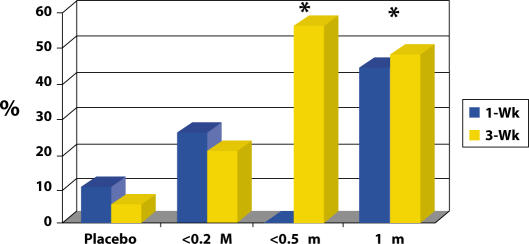
In patients with decreased bladder capacity, the mean change in cystometric capacity was markedly greater than for the placebo dose group (see Figure 6).
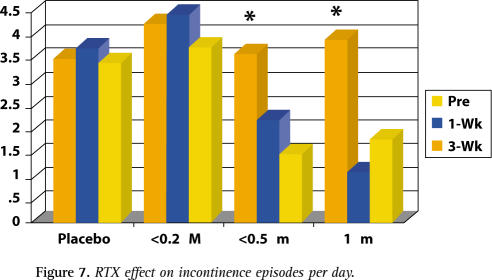
The mean number of incontinence episodes from pretreatment to week 3 of patients treated with 0.5 μM and 1.0 μM decreased by 1 to 2 fewer episodes compared with placebo (see Figure 7).
Figure 6.
RTX effect on cystometric capacity change.
Figure 7.
RTX effect on incontinence episodes per day.
Adverse events, related to RTX, were generally mild to moderate and transient. Because of autonomic dysreflexia (AD), one patient dropped out of the study. In a population involving many spinal cord injury (SCI) patients, the occurrence of AD is an expected event, especially during urologic studies. Cystoscopic evaluation revealed no adverse treatment effect due to RTX, and no signs of a dysreflexia or urinary retention were noted. Clinically significant changes in laboratory abnormalities did not occur.
One patient in the RTX trial was a woman with SCI at the T1 level for three years. Despite high doses of oral oxybutynin and intermittent catheterization, she still had frequent urge incontinence. On urodynamic evaluation, her bladder capacity was only 60 ml. One week after intravesical RTX, her bladder capacity increased to over 320 ml and further increased to 400 ml which was sustained for three months. She had neither side effects nor pain with RTX instillation.
In Europe, RTX is being used for overactive bladder in non-neurogenic bladder patients. In a recently published report in the Journal of Urology, Lazarri et al reported on 18 patients with interstitial cystitis-like symptoms who were randomized between RTX and placebo.13 In this prospective, randomized study it was found that, compared to baseline, RTX achieved a statistically significant decrease in urinary frequency and pain at one month versus placebo (see Figure 8). The improvement gradually returned toward baseline after three months.
Figure 8.
RTX effect on incontinence frequency over 24 hours. Adapted from Lazzeri M et al. J Urol. 2000;164:676-9.
Safety Considerations
There are concerns that the instillation of vanilloid agents may cause retention after many years, because the normal aging process of the bladder may involve a change from A-delta to C-fiber afferent signaling. Thus, safety is the number one concern with intravesical vanilloids. Yet, in over 12 years of clinical experience with capsaicin and RTX on more than three continents, there have not been any long-term complications.
RTX is the first true test of the hypothesis advocating the targeting of the afferent nervous system. Ideally an orally active drug that would desensitize the bladder C-fibers would be even more attractive.
Gene Therapy for OAB
The author has conducted studies of gene therapies that appear to have potential value in two areas of genitourinary disorder-erectile dysfunction and diabetic neurogenic bladder.
When gene therapy is considered for a particular disease, five issues must be addressed:
What is the key pathophysiologic event and is there a way to rectify it through gene therapy?
Is the genetic mechanism to be altered simple, or like some cancers, does it have many genetic events requiring change?
-
Is it possible to create the gene that treats this condition? In the penis, for example, decreased nitric oxide and arterial blood flow causes erectile dysfunction. If a gene can be created that improves arterial blood flow or counteracts the metabolism of nitric oxide, (like Viagra), it is possible to improve erectile function. In diabetic neurogenic bladder, there is strong evidence that a decrease in neurotrophic factors, such as nerve growth factor (NGF), may lead to diabetic neuropathy and, subsequently, a neurogenic bladder dysfunction. Moreover, there is evidence that replacing NGF improves diabetic neuropathy.14 Can a gene be created that replaces NGF in the bladder?
It is possible to construct genes that can alter both these conditions. With regard to erectile dysfunction, there are three types of nitric oxide synthase (NOS) that have been constructed. We used an adenovirus with the construct for inducible NOS (iNOS) to transfect the penis. For diabetic cystopathy, we constructed a herpes simplex virus (HSV) with expression for b-NGF. When injected into the bladder wall, the nonreplicating HSB-NGF is able to travel to the pelvic nerve and express NGF, not only in the bladder but also in the dorsal root ganglion of the pelvic nerve.
Is there an animal model to test the gene therapy? The rat model is commonly used to assess therapeutics for erectile dysfunction as well as diabetic neurogenic bladder.
Are clinical trials of these gene therapies logical and ethical? The answer is not yet available for genetic therapy of erectile dysfunction. In contrast, a clinical trial for diabetic cystopathy may be worthy of consideration. The diabetic bladder is very difficult to treat, patients experience many complications, and currently there is no effective therapy.
Erectile Dysfunction
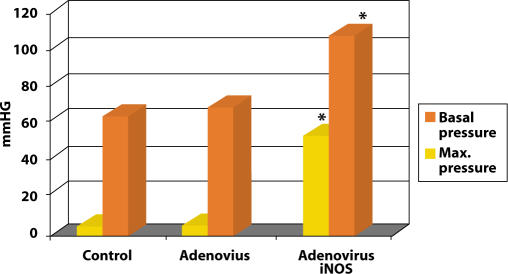
To improve erectile function, we injected the corpora cavernosum with 100 microliters of iNOS in a rat model. Within 4 to 6 hours, it was possible to completely transfect the entire penis and cause a sustained erection. We measured the intracorporal pressure after 4 to 5 days, and found that the basal corporal pressure in the rat’s penis was 6 to 8 mmHg. When we maximally electrically stimulated the penile nerve, the intracorporal pressure would go up to over 50 mmHg. When we just injected the adenovirus without the iNOS there were no change in the penile pressure. However, in the penis injected with iNOS the basal corporal pressure was over 50 mmHg and maximal pressure was over 120 mm with cavernous nerve stimulation (see Figure 9). The experiment was a success, but there was a significant problem—NOS gene therapy can cause a permanent erection. At this time it is a better priapism model then clinically relevant therapy for erectile dysfunction. Perhaps in the future, tailoring of dose and addition of promoters may make this more clinically appealing.
Figure 9.
Effect of iNOS gene therapy in intracorporal pressure.
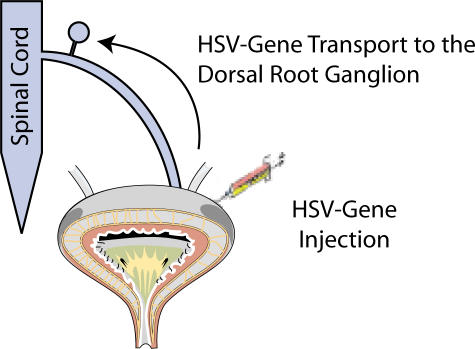
HSV Expressing NGF
HSV is a virus with many genes packaged into it. Its route of spread is critical—after the initial cutaneous infection, HSV travels through the nerve and can reside dormant in the dorsal root ganglion for the life of the host. So, using HSV as the vector to implement gene therapy in treating the nervous system of an organ is efficacious. Over the past two years, we set out to determine whether HSV would infiltrate nerves that innervate the bladder. We had special nonreplicating and latent HSV that can express NGF for at least three months. When we injected NGF intrathecally, the bladder became hyperactive. When we injected the HSV with NGF into a normal animal, the animal developed OAB for up to three months (see Figure 10).
Figure 10.
The use of herpes simplex virus (HSV) as a vector for directed transfection of afferent nerves that innervate the urinary bladder. Such an approach has great potential for the treatment of bladder dysfunction.
In contrast to inducing this reaction, however, neurotrophic factors such as NGF help nerves grow. There is compelling evidence that in certain conditions, such as diabetes, where there is peripheral neuropathy, there is a deficiency of neurotrophic factors. This is the rational for HSV-NGF gene therapy for diabetic neurogenic bladder.
Our results have been quite impressive. A normal rat in our laboratory will urinate at about 1 ml during infusion cystometrogram. Six to 10 weeks after we induced diabetes in the rat, the bladder became over 3 ml in size. When we injected HSV with sham vector but not with NGF, the diabetic bladder capacity did not change. However, 6 weeks after we gave the diabetic rats injections of HSV-NGF, there was a significant decrease in bladder capacity to 1.8 ml. There were no adverse effects in the HSV-NGF treated animals. This is the first report of successful gene therapy with physiological improvement in the autonomic system.
Conclusion
Conventional anticholinergic therapies are approaching the limit of their effectiveness. Therefore, a major transition to afferent nerve intervention to prevent OAB may be the most fruitful therapy for voiding dysfunction and urinary incontinence. In addition, advanced drug delivery systems and gene therapy are highly promising modalities that warrant further study for the treatment of OAB.
Main Points.
The new and improved anticholinergic drugs, such as tolterodine and controlled-releaseoxybutynin, are nearly at the limit of what can be expected of antimuscarinic agents.
Using anticholinergic agents for the treatment of overactive bladder could be considered “after-the-fact” therapy, because it relies on waiting for the bladder to have a spasm.
In the guinea pig model, TAK637 significantly increased the bladder intercontraction interval by 200%, without effect on bladder pressure or on urethral function.
Advanced drug delivery now represents an estimated $12 billion-a-year industry across a range of medical specialties,9,10 and is already widely used in urology.
References
- 1.Chancellor MB, de Groat WC. Intravesical capsaicin and resiniferatoxin therapy: Spicing up the ways to treat the overactive bladder. J Urol. 1999;162:3–11. doi: 10.1097/00005392-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Lecci A, Giuliani S, Tramontana M, et al. MEN 11,420, a peptide tachykinin NK2 receptor antagonist, reduces motor responses induced by the intravesical administration of capsaicin in vivo. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;356:182–188. doi: 10.1007/pl00005039. [DOI] [PubMed] [Google Scholar]
- 3.Ishizuka O, Igawa Y, Lecci A, et al. Role of intrathecal tachykinins for micturition in unanaesthetized rats with and without bladder outlet obstruction. Br J Pharmacol. 1994;113:111–116. doi: 10.1111/j.1476-5381.1994.tb16181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizuka O, Mattiasson A, Andersson KE. Effects of neurokinin receptor antagonists on Ldopa induced bladder hyperactivity in normal conscious rats. J Urol. 1995;154:1548–1551. [PubMed] [Google Scholar]
- 5.Lecci A, Guiliani S, Santicioli P, et al. Involvement of spinal tachykinin NK1 and NK2 receptors in detrusor hyperreflexia during chemical cystitis in anaesthetized rats. Eur J Pharmacol. 1994;259:129–135. doi: 10.1016/0014-2999(94)90501-0. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JFB, Sato A, Sato Y, Yamanishi T. The influence of afferent inputs from skin and viscera on the activity of the bladder and the skeletal muscle surrounding the urethra in the rat. Neurosci Res. 1995;23:195. doi: 10.1016/0168-0102(95)00942-m. [DOI] [PubMed] [Google Scholar]
- 7.Doi T, Kamo I, Imai S, Okanishi S, Ikeura Y, Natsugari H. Effects of TAK-637, a tachykinin receptor antagonist, on the micturition reflex in guinea pigs. Eur J Pharmacol. 2000;395:241–246. doi: 10.1016/s0014-2999(00)00177-1. [DOI] [PubMed] [Google Scholar]
- 8.Kamo I, Imai S, Okanishi S, Doi T. Possible site of action of TAK-637, a tachykinin NK(1) receptor antagonist, on the micturition reflex in guinea pigs. Eur J Pharmacol. 2000;401:235–240. doi: 10.1016/s0014-2999(00)00468-4. [DOI] [PubMed] [Google Scholar]
- 9.Kohn LT, Corrigan JM, Donaldson M. To Err Is Human: Building a Safer Health System. Washington, DC: Institute of Medicine; 1999. [PubMed] [Google Scholar]
- 10.McDonald CJ, Weiner M, Hui SL. Deaths due to medical errors are exaggerated in the Institute of Medicine Report. JAMA. 2000;284:93–95. doi: 10.1001/jama.284.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell J, Marsh M. Metabolism of drugs by the gastrointestinal tract. In: George C, Shand D, editors. Clinical Pharmacology and Therapeutics 1: Presystemic Drug Elimination. London: Butterworth Scientific; 1982. pp. 29–42. [Google Scholar]
- 12.Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once a day controlled versus immediate release oxybutynin chloride for urge incontinence. J Urol. 1999;161:1809. [PubMed] [Google Scholar]
- 13.Lazzeri M, Beneforti P, Spinelli M, Zanollo A, Barbagli G, Turini D. Intravesical resiniferatoxin for the treatment of hypersensitive disorder: A randomized placebo controlled study. J Urol. 2000;164:676–679. doi: 10.1097/00005392-200009010-00014. [DOI] [PubMed] [Google Scholar]
- 14.Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR. Alpha-lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neuroscience Letters. 1997;222:191–194. doi: 10.1016/s0304-3940(97)13383-3. [DOI] [PubMed] [Google Scholar]