Abstract
Voiding dysfunction is common, and patients with urge incontinence, frequency/urgency syndromes, and chronic urinary retention are challenging to treat once conservative therapies (such as pharmacologic agents, pelvic floor rehabilitation, and intermittent catheterization) have been exhausted. Sacral nerve stimulation (SNS) is a new, minimally invasive, reversible therapy for the management of refractory voiding dysfunction and provides an attractive therapeutic alternative for patients with this condition. In this review, the role of SNS in the management of voiding dysfunction is examined critically, and the efficacy, risks, and benefits of this new modality are evaluated.
Key words: Sacral nerve stimulation; Incontinence, urinary; Frequency/urgency syndromes; Urinary retention; Neuromodulation
Voiding dysfunction is common and may be idiopathic or may have a distinct cause, such as neurologic disease or local genitourinary factors. Common types of voiding dysfunction include urge incontinence, frequency/urgency syndromes, and urinary retention. In many cases, there is a clear etiology for the voiding dysfunction, such as detrusor hyperreflexia causing urgency and urge incontinence in a patient with multiple sclerosis, or urinary retention caused by bladder outlet obstruction in a patient with benign prostatic hyperplasia. However, in some cases, there is no clear etiology for the voiding problem, and the voiding dysfunction does not respond to the usual therapeutic regimens. Urinary urge incontinence and frequency/urgency syndromes are difficult to manage once conservative pharmaceutical treatments with anticholinergics and/or tricyclic antidepressants and pelvic floor rehabilitation have been exhausted. Similarly, few options besides clean intermittent catheterization are available to the patient who has urinary retention once obstruction has been ruled out.
Sacral nerve stimulation (SNS) offers an alternative state-of-the-art, minimally invasive treatment for patients with voiding dysfunction for whom conservative therapies have failed and who are being considered for irreversible major surgery, such as augmentation enterocystoplasty or urinary diversion. Although the exact mechanisms of sacral neuromodulation have yet Co be elucidated, its principles are based on the fact that the S2–S4 nerve roots provide the primary autonomic and somatic innervation to the lower urinary tract, including the pelvic floor, urethra, and bladder. In this article, we review the principles, current techniques, and expected results of sacral neuromodulation in the management of refractory voiding dysfunction.
History
Although vaginal, perineal, and anal electrical stimulation have been used frequently for the management of incontinence, Caldwell1 reported (in 1963) the use of electrical stimulation of the pelvic floor by implanted electrodes to control stress incontinence. The instruments in that era were rudimentary, and it was only with advances in cardiac pacing and pain management that research into neuromodulation of bladder function was again undertaken. Most of the credit for laying the foundation of neuromodulation as it is performed today goes to Drs Schmidt and Tanagho,2,3 who did many of the early studies in the 1960s, at the University of California, San Francisco. Further research in understanding the mechanism of neuromodulation was done by Craggs and Fowler4 in the mid-1990s using mag-roots. Much of the recent clinical information has been gained through a multicenter clinical trial that led to the approval by the FDA of SNS for the management of refractory urge incontinence in September 1997 and the management of chronic urinary retention and frequency/urgency syndrome in April 1999.
Mechanism of Action
The mechanism of action of SNS has not been fully elucidated. Neuromodulation works on the principle that activity in one neural pathway can influence activity in another neural pathway. Chancellor and DeGroat5,6 have suggested that SNS causes somatic afferent inhibition of sensory processing in the spinal cord. The S2–S4 nerve roots provide the primary autonomic and somatic innervation to the bladder, urethra, and pelvic floor. Thus, sacral neuromodulation somehow helps in dysfunctional voiding syndromes by stimulating or inhibiting these nerve roots.
Whether this process is primarily efferent and mediated through the pudendal nerve to stabilize the pelvic floor or whether it has an important afferent component that may be mediated through the pontine or higher cortical centers is a matter of current debate. It is possible that in some patients, the mechanism is primarily efferent, while in others, afferent pathways are of significance. It is known that contractions of the pelvic floor and voluntary external sphincter can abolish unstable bladder contractions. This may represent one of the mechanisms whereby stimulation of the S3 or S4 nerve root results in strengthening of the pelvic floor and sphincter, resulting in less “inappropriate relaxation” of the pelvic floor and urethra and less instability. Experimental evidence in animal models7 would support this thesis, but it is unclear how well animal models can explain the pathophysiology of human urge incontinence. Based on a cat model, Schultz-Lampel and colleagues8 have reported that a “rebound” phenomenon may be responsible for improving patients who have urinary retention. Recent data from Fowler and associates9 indicate that afferent pathways certainly play a role in neuromodulation, probably mediated via the spino-bulbo-spinal pathways to the pontine micturition center.
Suffice it to say that modulation of the sacral nerve toots restores a more normal relationship between the excitatory and inhibitory urinary control systems, helping the two extremes of voiding dysfunction-urinary retention and urge incontinence.2 Electrical stimulation for urinary incontinence is discussed in a thorough review by Fall and Lind-strom.10 A recent review by Bemelmans and colleagues11 discusses the mechanism of action of SNS in more detail.
Indications
SNS is indicated for the management of refractory urge incontinence, refractory frequency/urgency syndromes with or without a pain component (including interstitial cystitis and prostatodynia), and urinary retention in the absence of obstruction. In practice, since most of these patients represent a referred population, they have generally tried a variety of therapies, including pharma-cotherapy, pelvic floor rehabilitation through biofeedback and/or vaginal or anal electrical stimulation, clean intermittent catheterization, and other therapies. In the multicenter clinical trial, which provided for the largest number of patients studied on a well-designed specific protocol, patients with neurologic disease were excluded. However, there are data available in smaller trials showing a beneficial effect of sacral neuromodulation for patients with neurologic disease, such as multiple sclerosis.12 Furthermore, the clinical trial excluded patients with pain as their primary component, although patients with frequency/urgency and pain were included if pain was not their primary complaint.
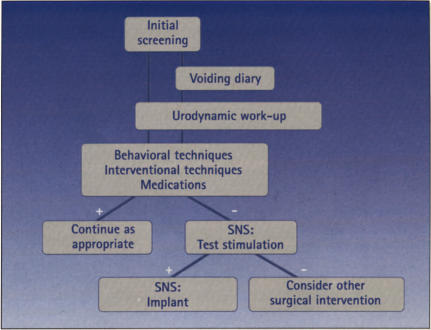
All patients being considered for SNS should have a complete history and physical examination, with an emphasis on the genitourinary and neurologic examinations, voiding diaries, and urodynamic studies. Treatment algorithms are presented for patients with urge incontinence and frequency/urgency (Figure 1) and for urinary retention (Figure 2). Currently, there is no way to determine with reasonable certainty which patients are unlikely to be helped by more conservative therapies, such as medications and pelvic floor rehabilitation; otherwise, for these patients, one could consider SNS as a primary therapy.
Figure 1.
Treatment algorithm: Urge incontinence and frequency/urgency.
Figure 2.
Treatment algorithm: Urinary retention.
Test Procedure
The test procedure is designed to provide a short-term trial of 3 to 7 days to determine which patients would benefit from implantation of the neurostimulator. The patient completes a voiding diary before the test procedure. Test stimulation is generally performed in the office or outpatient setting under local anesthesia. The patient is placed in the prone position, with one or two pillows under the lower abdomen. The sacral area is prepped with povidone-iodine, and the appropriate landmarks are identified by palpation. Local anesthesia is then achieved with 1% plain lidocaine. An insulated needle is placed percutaneously in the S3 or S4 foramen (Figure 3), and sensory and motor nerve root responses are identified. Sensory responses typically include a vibratory feeling or a pulling sensation in the vagina or rectum, and motor responses include a tightening of the levators (“bellows” response) as well as plantar flexion of the great toe. Once the appropriate responses have been achieved, an insulated wire (a temporary electrode) is inserted through the needle, and the needle is removed. The wire is securely taped in place and attached to an external neurostimulator, and the patient is sent home. The patient maintains the sensory stimulation at a comfortable level and keeps additional voiding diaries for 3 to 7 days. Sacral radiographs are used to document the position of the wire electrode immediately after the procedure. The wire is then removed at 3 to 7 days, and a voiding diary is repeated a week later to make certain the patient returns to baseline voiding. The diaries are compared with the patient’s pretest diary and, if there is both significant (greater than 50%) objective improvement in the parameter being evaluated (such as number of urge incontinence episodes) and significant subjective improvement, the patient can then be considered for permanent implantation.
Figure 3.
Schematic of test stimulation for SNS.
Implant Procedure
Patients who have undergone a successful test stimulation may be considered for implantation of the currently available neurostimulator, the Inter-Stim device, manufactured by Medtronic, Inc. The patient should understand fully the benefits and risks of the procedure and that the battery life will range from 6 to 10 years, depending on the settings. Patients who may become pregnant should consider alternative therapies, since the effect of neurostimulation on the fetus is unknown. Foreseeable need for MRI is also a relative contraindication to neurostimulator implantation.
The procedure is performed under general anesthesia using short-acting or nonparalytic anesthetic agents. During the surgery, prophylactic broad-spectrum antibiotics are provided and strict antiseptic precautions are maintained. While lying in the prone position, the patient is prepped with multiple layers of povidone-iodine, and a midline sacral incision is made. This is carried down to the level of the lumbodorsal fascia, which is opened sharply about 1.5 cm from the midline. The underlying paravertebral muscles are separated or divided, and the sacral periosteum is identified. The sacral foramen can be palpated as dimples or a marbling effect on the sacral surface. An insulated needle is placed into the appropriate foramen, and the motor responses are evaluated until the appropriate foramen is located (Figure 4).
Figure 4.
Insulated needle in S3 sacral foramen during the implant procedure.
A quadripolar lead is placed in the foramen, and the fixed collar on the lead is sutured to the periosteum to prevent lead migration. The lead is again tested to make certain that excellent motor responses (levator bellows and big toe) are obtained on at least three of the four leads. Another incision is now made over the upper buttock 3 to 5 cm below the superior posterior iliac crest. A tunneling tool is used to transfer the free end of the lead to the buttock incision. Using a short 10-cm connecting lead, the appropriate connections are now made between the connecting lead and the stimulating quadripolar lead and between the connecting lead and the InterStim neurostimulator (Figure 5). The incisions are closed in layers and, generally, no drains are left. A confirmatory radiograph is obtained before patient discharge (Figure 6). Alternatively, the neurostimulator can be placed in the anterior abdomen subcutaneously or under the rectus in very thin patients. The procedure is similar except that a longer, 50-cm connecting lead is used. The procedure has been described in detail by Siegel.13 The patient is generally discharged home within 23 hours and returns 1 week later for activation of neurostimulation.
Figure 5.
Neurostimulator being ploced in subcutaneous pocket in upper buttock region. The midline incision is also seen.
Figure 6.
Anterior-posterior radiograph showing quodripolor lead electrode in S4 foramen and neurostimulator in upper buttock.
All controls on the neurostimulator are accessible using an external programmer, and the patient can control the amplitude of the stimulation within a set range to adjust the sensory stimulation to a comfortable level. Periodic adjustments to the neurostimulation can then be performed as needed.
Some investigators have placed bilateral neurostimulators; although this is theoretically appealing, there are few data to suggest that bilateral neurostimulation is significantly better than unilateral stimulation. Other investigators have performed a sacral laminectomy and placed bilateral guarded cuff electrodes around the sacral nerve roots as a precaution against any movement and to maintain a constant distance from the nerve root14 These cuff electrodes are not available in the United States, and (overall) this procedure is more complicated because of the necessity for a sacral laminectomy.
Another variation has been a two-stage implant in patients who responded to percutaneous test stimulation but still did not improve enough to merit implantation.15 In this technique, the patient undergoes implantation of the quadripolar stimulating electrode, which is attached to a modified connecting lead that is tunneled subcutaneously and then brought through the skin. This connecting lead is then attached to an external temporary neurostimulator. If the trial is successful, the connecting lead is replaced by a new connecting lead and the permanent InterStim neurostimulator. The main advantage of this procedure is the lack of electrode migration, compared with the percutaneous test stimulation procedure. Furthermore, there is, perhaps, better stimulation because of electrode differences.
Efficacy
We reviewed published studies in the peer-reviewed literature as well as data from the multicenter study, which had a well-defined protocol and included a larger number of patients. While some of these data have been published in the peer-reviewed literature, some data have been submitted or are pending publication. Because of changes in both equipment and procedure during the last 10 years, some of the results may not be comparable. For example, the early neurostimulators, which had very limited programming capabilities, were used in the late 1980s, while neurostimulators used today have far better capabilities for programming and also have certain patient-controlled parameters. Other advances in technique, such as upper buttock placement of the neurostimulator, have significantly decreased operative time, as the patient no longer needs to be repositioned.
Dijkema and co-workers16 reported on 23 patients who underwent implantation after successful test stimulation. Sixteen patients had urge incontinence, while the remainder represented a variety of other voiding dysfunctions. Fourteen of the 23 patients had a “good” result (more than 90% improvement in main symptom), 5 patients had a “partial” result (more than 50% improvement), and the remaining 4 patients had a “bad” result (no improvement). Thus, the overall success rate for these patients was 83%.
Elabbady and associates17 reported on 50 patients with dysfunctional voiding who were screened via percutaneous test stimulation. Seventeen responded with significant (more than 70%) improvement in their symptoms and underwent neurostimulator implantation. In 8 patients with chronic retention, there was statistically significant improvement, with the number of self-catheterizations decreasing from 4.2 to 1.3 (P < .05) per day. In the remaining patients with urinary frequency/urgency, urge incontinence, or pain, frequency improved by 73% and urgency by 42%, and diaper use decreased by 50%. Follow-up ranged from 3 to 52 months.
Thon and colleagues18 demonstrated 60% significant improvement (75% to 100%) and 25% moderate improvement (50% to 75%) in 20 patients with urge incontinence. In 33 patients with voiding dysfunction, 51% had significant improvement and 18% had moderate improvement.
Bosch and Groen19,20 as well as Van Kerrebroeck and co-workers,21 who also evaluated and saw improvement in urodynamic parameters in patients with urge incontinence, have presented similar results.
More recently, Shaker and Has-souna22 reported on 20 patients with urinary retention who showed significant improvement (postvoid residual decreased from 78% to 10% of total urinary output), with a mean follow-up of 15 months. The authors also reported a marked reduction in urge incontinence episodes in a series of 18 patients.23 Leaking episodes decreased from 6.49 to 1.98, and the number of voids per 24 hours decreased from 16 to 9.
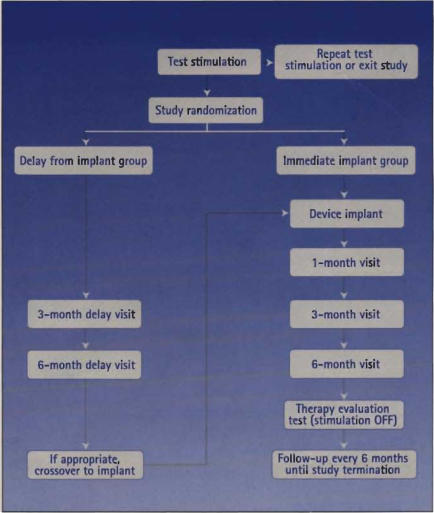
These studies show SNS to be a viable option for this difficult group of patients. However, the studies are limited in the number of patients enrolled, length of follow-up, and standardized criteria for defining a successful outcome. Some of these shortcomings have been addressed in a prospective, randomized, multicenter study (Figure 7), whose results are presented in Figure 8 to Figure 11. After 6 months, 77% of patients with urge incontinence who were randomized to receive implantation had no heavy leaking episodes, compared with 8% in the group without implantation (P < .0001) (Figure 8). Clinical efficacy was sustained in this group for up to 18 months; 52% of the patients with implants were completely dry at 18 months, and another 24% had more than 50% improvement (Figure 9). The average number of voids per day was significantly reduced 6 months after implantation in patients with refractory frequency/urgency syndrome (Figure 10). Neurostimulation was also effective in patients with urinary retention; the catheter volume per catheterization was significantly reduced 6 months after implantation, compared with patients without implantation (Figure 11). The data for urge incontinence have been published recently,24 and the data for frequency/urgency and for urinary retention have been submitted for publication.
Figure 7.
Design of the prospective, randomized, MDT-103 multicenter clinical trial.
Figure 8.
Leaking episodes at 6 months in patients with urge incontinence enrolled in the randomized MDT-103 clinical trial of SNS implantation. Seventy-seven percent of the implanted patients had no heavy leaking episodes (P <.0001, compared with the delay group). Five patients in the delay group and eight in the implant group did not have any heavy leaks of baseline. (Adopted with permission from Schmidt et at24)
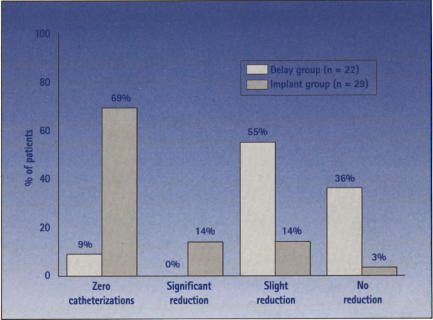
Figure 11.
Average volume per catheterization at 6 months for 51 patients in urinary retention enrolled in the randomized MDT-103 clinical trial of SNS implantation. A significant improvement was seen in implanted patients versus the control group (P < .0001). Zero catheterizations: no catheterization at 6 months; significant reduction: 50% or more reduction in catheter volume per catheterization at 6 months; slight reduction: less than 50% reduction in catheter volume per catheterization at 6 months; no reduction: no change or slight increase in catheter volume per catheterization at 6 months. (Data from Medtronic, Inc, and the MDT-103 study group.)
Figure 9.
Results from the randomized MDT-103 clinical trial of SNS implantation showing sustained clinical efficacy in patients with urge incontinence. At 18 months, 52% of the patients were completely dry, and another 24% had more than 50% improvement (Adopted with permission from Schmidt et al.24)
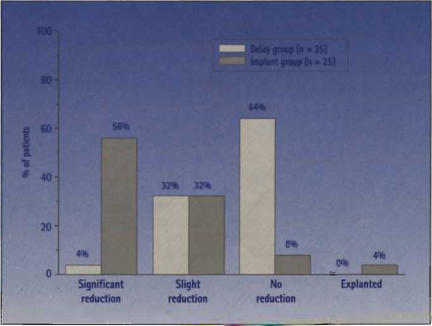
Figure 10.
Average number of voids per day at 6S months for 51 patients with refractory frequency/urgency syndrome enrolled in the randomized MDT-103 clinical trial of SNS implantation, A significant improvement was seen in implanted patients vs the control group (P < .0001). Significant reduction: 50% or more reduction in number of voids per day of 6 months and/or normal range of lour to seven voids per day: slight reduction: less than 50% reduction in number of voids per day at 6 months; no reduction: no change or slight increase in reduction in number of voids per day at 6 months; explanted: patient explanted before 6 months. (Data from Medtronic, Inc, and the MOT-103 study group.)
Complications
Percutaneous test stimulations have been exceedingly safe, with no reported nerve injuries. Lead migration, which occurs in about 15% of cases, has been problematic, although this cannot be considered a true complication. Because the per-cutaneously placed wire electrode is only taped to the skin, it has a tendency to move over time, especially with patient movement; once it moves away from the nerve, the patient no longer has the sensory stimulation and loses its beneficial effects. If this occurs early, the patient should have another test stimulation performed. A recently designed wire lead electrode with a coiling mechanism appears to decrease this problem somewhat, although there are no reported data to support such a statement.
SNS implantation has been hindered by a modestly high rate of complications and need for surgical revision of the neurostimulator device (Table). Approximately 33% (51 of 157) of patients enrolled in the clinical trial required revision surgery, including permanent or temporary device explanation, device exchange, or repositioning of either the lead electrode or pulse generator device components.24 The probability for surgical revision decreased from 29% during the first 6-month interval to 12.1% during the second 6-month interval of the study. The potential for surgical revision seems to decline with time and may partly represent a learning curve. More importantly, the need for surgical revision does not seem to preclude a successful clinical response to neuromodulation. Other adverse effects noted included pain at the stimulator site in 15.9% of patients, pain at the implant site in 19.1%, and lead migration in 7.0%. The rate of infection or skin irritation that led to explantation was 5.7%. Most adverse events or complications were corrected. There were no reports of permanent injury or significant nerve damage reported with the implant components. Because anterior abdominal placement can lead to wedging of the neurostimulator device between the lower rib cage and the anterior superior iliac spine, some of the complications related to neurostimulator placement may be alleviated by positioning the device in the upper buttock region.
Table 1.
Most Common Complications in the First Year After SNS Implantation
|
Note: Additional events occurred-each less than 2.0%
Discussion and Future Direction
Sacral neuromodulation is an exciting, minimally invasive alternative available for the treatment of patients with voiding dysfunction refractory to conventional therapy. For patients with urge incontinence or frequency/urgency syndrome who have not responded to pharmacotherapy, pelvic floor rehabilitation, and timed voiding, treatment options are limited. In the past, these patients were offered irreversible procedures, such as denervation or augmentation cystoplasty, that are associated with significant risks and the potential need for long-term intermittent catheterization, a situation not particularly appealing to this patient group. When these patients have a successful test stimulation, they seem to have an intuitive feeling that something in their system is “working better.” In patients with chronic urinary retention, clean intermittent catheterization certainly remains an option, although urinary tract infections sometimes occur, and the patient must carry supplies that may limit lifestyle and activity level. There is no other truly viable option for this group of patients, many of whom are young. If test stimulation results in a significant decrease in the postvoid residual, most of these patients are ecstatic and want to proceed with permanent implantation of the neurostimulator.
Currently, the main obstacles to neurostimulation relate to the procedure being in its infancy. The equipment was originally designed for other purposes and has been adapted to sacral neuromodulation. This may be part of the reason why only about 40% of all patients respond to the test stimulation. It is also unknown whether bilateral stimulation would lead to a higher success rate. In the early series, revision rates have been relatively high. This relates to several factors, including a difficult group of patients for whom most other therapies have failed, learning curves related to a new implant procedure, and the limitation of current neuromodulation devices and equipment. As the number of specialists performing the procedure has increased, innovations in both technique and equipment have been made, and these changes should lower the rate of complications. JT Benson, MD, a urogynecologist at Methodist Hospital in Indianapolis, has suggested that simultaneous recording of levator and urethral muscle activity using an anal plug electrode and a urethral electrode is helpful in quantifying the response noted and that these recordings result in more accurate test stimulation and implant procedures (unpublished data). This concept seems exciting, but it needs to be validated in a mul-ticenter, prospective, randomized study to make certain it does not represent a selection bias.
For SNS to be applicable to a larger group of patients, the costs related to the procedure need to be decreased significantly and the procedure should be easier to perform. If the size of the neurostimulator can be significantly decreased, and if the permanent electrode can be placed percutaneously in such a manner that migration is not a problem, then even permanent neurostimulator implantation could be performed easily, as an outpatient procedure under local anesthesia.
Management of voiding dysfunction is evolving in ways similar to the manner in which the management of benign prostatic hyperplasia evolved during the last decade-into pharmacologic treatment, minimally invasive surgery (transurethral microwave thermal therapy, transurethral needle ablation of the prostate), and more invasive surgery (transurethral resection of the prostate, open prostatectomy). As our understanding of voiding dysfunction and the mechanism of action of SNS increases, focused, improved techniques should help an even larger group of patients.
Editorial Comment: A New Option for Urologists.
Michael B. Chancellor, MD
Associate Professor of Urology
University of Pittsburgh School of Medicine
With the introduction of sacral nerve stimulation (SNS), a truly new surgical technique developed by urologists for urologists is now available. SNS placement is minimally invasive and nondestructive. Moreover, this procedure is reversible, and it does not preclude the patient or urologist from using other modes of therapy. SNS should be reserved for the patient with intractable urgency and urge incontinence or idlopathic urinary retention who has not responded to other methods of treatment.
Dr Das and associates from Albany, NY, have written a terrific summary of the SNS state of the art. The most up-to-date references and clinical data are discussed without bias. Readers will appreciate the balance between the advantages of this unique treatment modality and the negatives: revision rate of 33% and hardware cost of approximately $11,000 per patient in the United States.
SNS manages overactive bladder by somatic afferent inhibition of sensory processing in the spinal cord. It blunts the overactivity that the patient with an overactive bladder experiences and relieves symptoms. SNS stimulates pudendal afferent input to the sacral spinal cord, as well as inhibits supraspinally mediated, hyperactive voiding by blocking ascending sensory mechanisms. Interestingly, SNS input can also restore micturition in patients with idiopathic urinary retention, which is thought to be caused by overactivity of the guarding reflexes, with the clinical manifestation of pelvic floor spasticity and time tional detrusor sphincter dyssynergia. Sacral inhibition by SNS allows pelvic floor relaxation and subsequent micturition in these patients.
The skills and backgrounds of urologists make them excellent candidates for performing SNS. Training via a focused course is needed, however. In addition, hands-on mentoring is recommended for the first few cases. SNS provides a unique and exciting treatment option that the urologist can offer to patients for whom conventional treatment options have failed. We encourage urologists with special interests in voiding dysfunction and urinary incontinence to consider and learn SNS.
Main Points.
Urge incontinence, frequency/urgency syndromes, and urinary retention are the common types of voiding dysfunction.
When there is no clear etiology for the voiding problem, the voiding dysfunction is often unresponsive to the usual therapeutic strategies.
For patients with a voiding dysfunction for whom conservative therapies have failed, sacral nerve stimulation (SNS) can be a minimally invasive treatment option.
SNS is indicated for management of refractory urge incontinence and for frequency/urgency syndromes and chronic urinary retention.
Because SNS is still in its infancy, the complication rate is modestly high.
The complication rate should decrease as the number of specialists performing the implantation procedure Increases and there are innovations in both technique and equipment.
It is unknown whether bilateral stimulation will Increase the success rate for the procedure.
Acknowledgments
We thank Medtronic, Inc. and the MDT-103 study group for use of data from the clinical trial entitled “Sacral nerve stimulation for chronic voiding dysfunction.”
References
- 1.Caldwell KPS. The electrical control of sphincter incompetence. Lancet. 1963;2:174–175. doi: 10.1016/s0140-6736(63)92807-1. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt RA. Advances in genitourinary neuro-stimulation. Neurosurgery. 1986;19:1041–1044. doi: 10.1227/00006123-198612000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Tanagho EA, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331–1339. doi: 10.1016/s0022-5347(17)42038-6. [DOI] [PubMed] [Google Scholar]
- 4.Sheriff MK, Shah PJ, Fowler C, et al. Neuromodulation of detrusor hyperreflexia by functional magnetic stimulation of the sacral roots. Br J Urol. 1996;78:39–46. doi: 10.1046/j.1464-410x.1996.00358.x. [DOI] [PubMed] [Google Scholar]
- 5.Lavelle JP, Teahan S, Kim D-Y, Chancellor MB. Medical and minimally invasive treatment of incontinence. Rev Urol. 1999;1:111–120. [PMC free article] [PubMed] [Google Scholar]
- 6.de Groat WC. Neuroanatomy and neurophysiology: innervation of the lower urinary tract. In: Raz S, editor. Female Urology. Philadelphia: WB Saunders Company; 1996. pp. 28–42. [Google Scholar]
- 7.Mersdorf A, Schmidt RA, Tanagho EA. Urodynamic evaluation and electrical and pharmacologic neurostimulation: the rat model. Urol Res. 1993;21:199–209. doi: 10.1007/BF00590037. [DOI] [PubMed] [Google Scholar]
- 8.Schultz-Lampel D, Jiang C, Lindstrom S, Thuroff JW. Experimental results on mechanisms of action of electrical neuromodulation in chronic urinary retention. World J Urol. 1998;16:301–304. doi: 10.1007/s003450050071. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin RJ, Swinn MJ, Fowler CJ. The neurophysiology of urinary retention in young women and its treatment by neuromodulation. World J Urol. 1998;16:305–307. doi: 10.1007/s003450050072. [DOI] [PubMed] [Google Scholar]
- 10.Fall M, Lindstrom S. Electrical stimulation: a physiologic approach to the treatment of urinary incontinence. Urol Clin North Am. 1991;13:393–407. [PubMed] [Google Scholar]
- 11.Bemelmans BL, Mundy AR, Craggs MD. Neuromodulation by implant for treating lower urinary tract symptoms and dysfunction. Eur Urol. 1999;36:81–91. doi: 10.1159/000067976. [DOI] [PubMed] [Google Scholar]
- 12.Bosch JLHR, Croen J. Treatment of refractory urge urinary incontinence with sacral spinal nerve stimulation in multiple sclerosis patients. Lancet. 1996;348:717–719. doi: 10.1016/s0140-6736(96)04437-6. [DOI] [PubMed] [Google Scholar]
- 13.Siegel SW. Management of voiding dysfunction with an implantable neuroprosthesis. Urol Clin North Am. 1992;19:163–170. [PubMed] [Google Scholar]
- 14.Hohenfellner M, Schultz-Lampel D, Dahms S, et al. Bilateral chronic sacral neuromodulation for treatment of lower urinary tract dysfunction. J Urol. 1998;160:821–824. doi: 10.1016/S0022-5347(01)62795-2. [DOI] [PubMed] [Google Scholar]
- 15.Janknegt RA, Well EH, Eerdmans PH. Improving neuromodulation technique for refractory voiding dysfunctions: two-stage Implant. Urology. 1997;49:358–362. doi: 10.1016/S0090-4295(96)00506-7. [DOI] [PubMed] [Google Scholar]
- 16.Dijkema HE, Weil EH, Mijs FT, Janknegt RA. Neuromodulation of sacral nerves for incontinence and voiding dysfunctions: clinical results and complications. Ear Urol. 1993;24:72–76. doi: 10.1159/000474266. [DOI] [PubMed] [Google Scholar]
- 17.Elabbady AA, Hassouna MM, Elhilali MM. Neural stimulation for chronic voiding dysfunctions. J Urol. 1994;152:2076–2080. doi: 10.1016/s0022-5347(17)32312-1. [DOI] [PubMed] [Google Scholar]
- 18.Thon W, Baskin L, Jonas U, et al. Neuromodulation of voiding dysfunction and pelvic pain. World J Urol. 1991;9:138–141. [Google Scholar]
- 19.Bosch JL, Groen J. Neuromodulation: urodynamic effects of sacral (S3) spinal nerve stimulation in patients with detrusor instability or detrusor hyperreflexia. Bebav Brain Res. 1998;92:141–150. doi: 10.1016/s0166-4328(97)00186-1. [DOI] [PubMed] [Google Scholar]
- 20.Bosch JL, Groen J. Sacral (S3) segmental nerve stimulation as a treatment for urge incontinence in patients with detrusor instability: results of chronic electrical stimulation using an implantable neural prosthesis. J Urol. 1995;154:504–507. doi: 10.1097/00005392-199508000-00045. [DOI] [PubMed] [Google Scholar]
- 21.Koldewijn EL, Rosier PF, Meuleman EJ, et al. Predictors of success with neuromodulation in lower urinary tract dysfunction: results of trial stimulation in 100 patients. J Urol. 1994;152:2071–2075. doi: 10.1016/s0022-5347(17)32311-x. [DOI] [PubMed] [Google Scholar]
- 22.Shaker HS, Hassouna M. Sacral root neuromodulation in idiopathic nonobstructive chronic urinary retention. J Urol. 1998;159:1476–1478. doi: 10.1097/00005392-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Shaker HS, Hassouna M. Sacral nerve root neuromodulation: an effective treatment for refractory urge incontinence. J Urol. 1998;159:1516–1519. doi: 10.1097/00005392-199805000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt RA, Jonas U, Oleson KA, et al. Sacral nerve stimulation for treatment of refractory urinary urge incontinence. J Urol. 1999;162:352–357. [PubMed] [Google Scholar]