Abstract
The p24 family consists of type I transmembrane proteins that are present abundantly in transport vesicles, may play a role in endoplasmic reticulum-to-Golgi cargo transport, and have been classified into subfamilies named p24α, -β, -γ, and -δ. We previously identified a member of the p24δ subfamily that is coordinately expressed with the prohormone proopiomelanocortin (POMC) in the melanotrope cells of the intermediate pituitary during black background adaptation of the amphibian Xenopus laevis (∼30-fold increase in POMC mRNA). In this study, we report on the characterization of this p24δ member (Xp24δ2) and on the identification and characterization of a second member (Xp24δ1) that is also expressed in the melanotrope cells and that has 66% amino acid sequence identity to Xp24δ2. The two p24δ members are ubiquitously expressed, but Xp24δ2 is neuroendocrine enriched. During black background adaptation, the amount of the Xp24δ2 protein in the intermediate pituitary was increased ∼25 times, whereas Xp24δ1 protein expression was increased only 2.5 times. Furthermore, the level of Xp24δ2 mRNA was ∼5-fold higher in the melanotrope cells of black-adapted animals than in those of white-adapted animals, whereas Xp24δ1 mRNA expression was not induced. Therefore, the expression of Xp24δ2 specifically correlates with the expression of POMC. Together, our findings suggest that p24δ proteins have a role in selective protein transport in the secretory pathway.
INTRODUCTION
Once secretory proteins are correctly folded and assembled in the endoplasmic reticulum (ER), they become segregated from ER-resident proteins by their selective incorporation into transport vesicles. Formation of these transport vesicles is driven by the coat protein (COP) complex COPII (Barlowe et al., 1994; Aridor et al., 1995, 1998) and is restricted to specialized regions of the ER, called ER exit sites (Bannykh et al., 1996; Bannykh and Balch, 1997). Budded vesicles accumulate in a vesicular tubular cluster (Balch et al., 1994; Scales et al., 1997), also referred to as the ER-to-Golgi intermediate compartment (Schweizer et al., 1990), which is transported as a whole along microtubules to the Golgi complex (Presley et al., 1997). During this transport, retrograde vesicles coated by another protein complex (COPI) recycle ER-resident proteins; within the Golgi complex, a similar mechanism recycles Golgi-resident components (Cosson and Letourneur, 1994; Letourneur et al., 1994; Aridor et al., 1995; Scales et al., 1997). The involvement of COPI in anterograde transport has also been proposed (Pepperkok et al., 1993; Bednarek et al., 1995; Orci et al., 1997; Lavoie et al., 1999).
A group of related 24-kDa type I transmembrane proteins, referred to as the p24 family, has been found to be a major constituent of both COPI- and COPII-coated vesicles (Schimmöller et al., 1995; Stamnes et al., 1995; Belden and Barlowe, 1996; Dominguez et al., 1998). These p24 proteins display a low degree of amino acid sequence identity, but they share certain structural characteristics, such as a short cytoplasmic C tail containing coat-binding motifs and a lumenal domain with two cysteine residues that enable the formation of a loop structure (Stamnes et al., 1995). Structurally, the p24 family can be subdivided into four subfamilies that have been designated p24α, -β, -γ, and -δ (Dominguez et al., 1998). It has been suggested that p24 proteins operate as cargo receptors that sort subsets of secretory proteins into transport vesicles through interaction with their luminal domains, whereas their cytoplasmic domains provide the transport information for the vesicles by binding to specific coat proteins (Schimmöller et al., 1995). Alternatively, p24 proteins may act as coatomer receptors during the formation of retrograde transport vesicles (Sohn et al., 1996; Nickel et al., 1997; Nickel and Wieland, 1997; Majoul et al., 1998) or may have a role in the quality control of newly synthesized cargo proteins in the early secretory pathway (Wen and Greenwald, 1999).
Using a differential screening approach, we recently identified a member of the p24 family (X1262) in a highly specialized secretory cell, the melanotrope cell of the intermediate pituitary of the amphibian Xenopus laevis (Holthuis et al., 1995b). We use this cell type as a model system to explore the pathway of peptide hormone secretion in neuroendocrine cells. The melanotrope cells have a well-defined physiological function, namely, the production and release of the α-melanophore–stimulating hormone (αMSH) during adaptation of the animal to a black background (Jenks et al., 1977). αMSH is proteolytically cleaved from the prohormone proopiomelanocortin (POMC) and causes pigment dispersion in the skin of the animal. In the melanotrope cells of animals adapted to a black background, the POMC gene is highly expressed and the level of POMC mRNA is up to 30-fold higher than in those of white-adapted animals (Martens et al., 1987). Although p24 proteins are thought to be recycled in the secretory pathway and thus likely have a much lower turnover than POMC, the expression of X1262 mRNA is also strongly induced in black-adapted animals (Holthuis et al., 1995b). Here we describe the characterization of X1262 and the isolation and characterization of a second member of the p24 family that is related to X1262. Like X1262, this second member is expressed in the melanotrope cells, but not coordinately with POMC. Our findings suggest that only X1262 and not the novel p24 member is a component involved in the transport of POMC through the early stages of the secretory pathway.
MATERIALS AND METHODS
Animals
South African clawed toads (Xenopus laevis) were adapted to their background by keeping them in either white or black buckets under constant illumination for at least 3 wk at 22°C.
Antibodies
Two polyclonal antisera were raised against synthetic peptides. One peptide comprised the carboxyl-terminal 14 amino acids of the X1262 protein (CYLRHFFKAKKLIE), and the second comprised a stretch of 14 amino acids in the lumenal part of the novel p24 member RH6 (CFDSKLPAGAGRVP). Both peptides were coupled to keyhole limpet hemocyanin (Pierce, Rockford, IL) and used for immunization. The antisera were named anti-1262C and anti-RH6, respectively. A third antiserum, anti-1262N, was raised against a recombinant protein that constituted part of the lumenal domain of the X1262 protein. For this purpose, we cloned a PCR-amplified fragment, encoding amino acids 72–150 of the X1262 protein, in the Qiagen (Chatsworth, CA) expression vector pQE30. Next, recombinant protein was produced in Escherichia coli, isolated by Ni2+ nitrilotriacetic acid agarose affinity chromatography, and used for immunization. Rabbits were immunized with 500 μg of coupled peptide or recombinant protein in Freund's complete adjuvant. Four weeks later, and at 3-wk intervals thereafter, rabbits were boosted with 250 μg of antigen in Freund's incomplete adjuvant. The production of specific antibody was monitored by ELISA. Both anti-RH6 and anti-1262N were purified by affinity chromatography with immobilized recombinant RH6 and X1262 protein, respectively. The antisera against α/γ-COP (Gerich et al., 1995) and ε-COP (Hara-Kuge et al., 1994) were kindly provided by Dr. F. Wieland (Institut für Biochemie, University of Heidelberg, Germany). The actin antibody was obtained from Zymed (San Francisco, CA).
Cell Culture and DNA Transfections
Mouse anterior pituitary-derived AtT20 cells were grown in DMEM (Life Technologies-BRL, Grand Island, NY) supplemented with 10% (vol/vol) FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in an atmosphere of 5% CO2. For X1262 expression in AtT20 cells, the complete coding region of clone X1262 was subcloned downstream of the cytomegalovirus promoter into the eukaryotic expression vector pcDNA3 (Invitrogen, San Diego, CA). X1262-pcDNA3 DNA was isolated with the use of the Qiagen plasmid kit and transfected by the calcium phosphate precipitation method (Graham and van der Eb, 1973). After 48 h, the cells were selected for stable expression of X1262 in medium containing 700 μg/ml neomycin (Life Technologies-BRL).
Metabolic Labeling and Immunoprecipitation
For metabolic cell labeling, neurointermediate lobes (NILs) of black-adapted Xenopus toads were rapidly dissected and preincubated in incubation medium (112 mM NaCl, 2 mM KCl, 2 mM CaCl2, 15 mM HEPES, pH 7.4, 0.3 mg/ml BSA, 2 mg/ml glucose, pH 7.4) at 22°C for 30 min. Radioactive labeling of newly synthesized proteins was performed by incubating the NILs in incubation medium containing 5 mCi/ml ProMix 35S label (Amersham, Arlington Heights, IL) for 5 h at 22°C. Where indicated, 10 μg/ml tunicamycin was added during a preincubation period of 2 h and remained present during the subsequent labeling period. After the labeling, NILs were rinsed in incubation medium and homogenized on ice in lysis buffer (50 mM HEPES, pH 7.2, 140 mM NaCl, 1% Tween-20, 0.1% Triton X-100, 0.1% deoxycholate, 0.1% SDS, 1 mM PMSF, 0.1 mg/ml soybean trypsin inhibitor). Lysates were cleared by centrifugation, supplemented with 0.1 volume of 10% SDS, and diluted 10-fold in lysis buffer before addition of the antiserum (1:500 dilution). For metabolic labeling of AtT20 cells, 10-cm2 dishes with 80% confluent monolayers were rinsed once with medium, preincubated for 30 min in DME-labeling medium (90% Met-/Cys-free DMEM [ICN Biomedical, Costa Mesa, CA], 10% dialyzed FBS, 1 mM sodium pyruvate, 2 mM glutamine), and then labeled for 5 h in DME-labeling medium with 350 μCi/ml Promix. Subsequently, cells were rinsed once with PBS, lysed in lysis buffer, and prepared for immunoprecipitation as described above. Immune complexes were precipitated with protein-A–Sepharose (Pharmacia Biotech, Uppsala, Sweden), washed four times with lysis buffer containing 0.075% SDS, and analyzed on a 15% SDS-polyacrylamide gel.
Construction of the NIL cDNA Library and Low-Stringency Screening
For cDNA library construction, cytoplasmic RNA was isolated from NILs of 50 black-adapted Xenopus toads with the use of the Trizol isolation method (Life Technologies-BRL). After DNase I treatment (40 U/ml, 20 min, 37°C; FPLC-pure, Pharmacia Biotech), cDNA was synthesized with the use of the commercial cDNA synthesis kit (Stratagene, La Jolla, CA), size fractionated on CL-2B Sepharose, and ligated into the HybriZAP vector (Stratagene). The insert sizes varied between 0.7 and 2.2 kilobase pairs (average of 1.0 kilobase pairs). At least 50% of the amplified NIL cDNA library was found to consist of POMC cDNA clones. About 600,000 plaques were replicated on duplicate nitrocellulose filters with a density of 400 plaques/cm2 by standard procedures (Sambrook et al., 1989). Filters were prehybridized for 2 h at 42°C in hybridization mixture (25% [vol/vol] formamide, 1% [wt/vol] nonfat dry milk, 1% [vol/vol] Nonidet P40, 6× SSPE) and hybridized overnight at 42°C in the presence of an α-[32P]dATP randomly labeled PCR product that corresponded to the complete coding sequence of X1262 (signal sequence excluded). Filters were washed twice in 2× SSC/0.1% SDS for 1 h at 50°C and exposed to x-ray films between two intensifying screens for 16 h at −70°C. Subsequently, filters were rewashed with increasing stringency up to 0.1× SSC/0.1% SDS at 60°C and exposed to x-ray films. A second screening to identify X1262 cDNAs was performed with an α-[32P]dATP randomly labeled PCR product corresponding to the 3′-untranslated region of X1262 (nucleotides 820-1070) under high-stringency hybridization conditions (50% formamide at 42°C). Filters were washed twice in 0.1× SSC/0.1% SDS for 1 h at 65°C and exposed to x-ray films.
DNA Sequence Analysis
Sequencing of cDNA clones on both strands was performed with single-stranded DNA by automatic sequencing with the use of the ABI-PRISM DNA sequencing kit and the ABI-PRISM310 automatic sequencer (Perkin Elmer-Cetus Applied Biosystems, Foster City, CA).
Reverse Transcription PCR
For expression studies, total RNA was isolated from different tissues with the use of the Trizol isolation method (Life Technologies-BRL). After treatment with 2.5 U of DNase I, the RNA was quantified by spectrophotometry and its integrity was checked by running samples on denaturating agarose gels followed by ethidium bromide staining. Subsequently, 2 μg of total RNA was reverse transcribed with 200 U of Superscript (Life Technologies-BRL) under standard conditions according to the manufacturer's instructions. Because the expression of ornithine decarboxylase (ODC) mRNA is not linked to POMC, we used ODC to correct for cDNA input in the PCR. The following primers were used: XODC (385 base pairs [bp]): 5′-GTC AAT GAT GGA GTG TAT GGA TC-3′, 5′-TCC ATT CCG CTC TCC TGA GCA C-3′; RH6 (456 bp): 5′-CAC AAT CAG GGC CAA GTG CGG-3′, 5′-TTT GGC CTT AAA GAA ACG GCG-3′; X1262 (307 bp): 5′-CTA GAA TTC ATG ATG TGG CTC CTG CTT TTC-3′, 5′-GGG CCA GAT CTC GAG AAG CTT AGC AGA CTT CAT ACA CAT C-3′. A total of 12.5 pmol of each primer was used in a 25-μl reaction volume containing PCR buffer (Life Technologies-BRL), 2.5 mM MgCl2, and 0.5 U of Taq polymerase (Life Technologies-BRL). To prevent saturation problems during the PCR reactions, three dilutions of cDNA (1:25, 1:125, and 1:625) were used, such that the two most diluted cDNAs gave a smaller amount of PCR product than the least diluted cDNA. Twenty-five cycles were performed (1 min at 92°C, 30 s at 55°C, and 1 min at 72°C). Amplified PCR products were separated on a 2% agarose gel and quantified with a densitometer.
Northern Blot Analysis
RNA was isolated from NILs and anterior lobes (ALs) from both black- and white-adapted animals with the use of the Trizol isolation method. To load approximately equal amounts of RNA on the gel, 5 NILs and 10 ALs of black-adapted animals and 15 NILs and 10 ALs of white-adapted animals were used in the isolation procedure. RNA was separated by electrophoresis on a 2.2 M formaldehyde–containing 1.2% agarose gel in MOPS buffer and blotted onto Hybond filters as described by Ausubel et al. (1989). Hybridization was overnight at 42°C in 6× SSPE, 50% formamide, 3× Denhardt's solution, 0.5% SDS, 40 mM sodium phosphate, pH 7.0, 0.1% sodium pyrophosphate, 0.1 mg/ml salmon sperm DNA. Probes (1 × 106 cpm/ml) were prepared by random prime labeling of 3′-untranslated region PCR fragments of either Xp24δ1 or Xp24δ2.
Western Blot Analysis
For Western blot analysis, tissues were homogenized in 50 mM HEPES, pH 7.2, 140 mM NaCl, 1% Tween-20, 0.1% Triton X-100, 0.1% deoxycholate, 0.1% SDS, 1 mM PMSF, 0.1 mg/ml soybean trypsin inhibitor. After the lysates were cleared by centrifugation, they were resolved on 15% SDS-PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). Immunostaining was performed with the use of Lumi-Light detection (Boehringer Mannheim, Mannheim, Germany). All antisera were used in a 1:5000 dilution, except for the actin antibody, which was used in a 1:1000 dilution. The amount of protein detected was quantified with the use of a Lumi-Imager (Boehringer Mannheim).
Immunocytochemistry
Xenopus brains with pituitary glands attached were fixed for 24 h in Bouin-Hollande solution, dehydrated, and embedded in paraffin. Serial sagittal 5-μm sections were mounted on gelatin-coated glass slides. After deparaffinization and rehydration, sections were blocked with 1% BSA in PBS for 1 h and then incubated with either the affinity-purified anti-1262N antiserum (1:1500) or the affinity-purified anti-RH6 antiserum (1:50) for 16 h, with goat anti-rabbit immunoglobulin G (1:100; Nordic Immunology, Tilburg, The Netherlands) for 1 h, and finally with rabbit peroxidase-antiperoxidase (1:100; Nordic Immunology) for 1 h. After washing in PBS, sections were treated with 0.025% 3,3′-diaminobenzidine tetrahydrochloride, 0.25% nickel ammonium sulfate, and 0.01% hydrogen peroxide in 0.05 M Tris-HCl, pH 7.6, to reveal peroxidase activity. To check the specificity of staining, preimmune serum was used in control experiments.
RESULTS
Biosynthesis of the X1262 Protein in Xenopus Intermediate Pituitary
In a previous study, we reported on the cloning of a 1.2-kilobase pair cDNA (X1262) from the melanotrope cells of the NIL of X. laevis. The protein encoded by X1262 was found to be related to gp25L (Holthuis et al., 1995b), a protein originally described as a constituent of a translocon-associated protein (TRAP) complex (Wada et al., 1991). However, subsequently, gp25L was found to be a member of the p24 family of 24-kDa type I transmembrane proteins (Stamnes et al., 1995) that is enriched in COP-coated transport vesicles (Blum et al., 1996; Fiedler et al., 1996; Sohn et al., 1996). Therefore, the X1262 protein also belongs to the p24 family and, based on amino acid sequence alignments, represents a member of the p24δ subfamily.
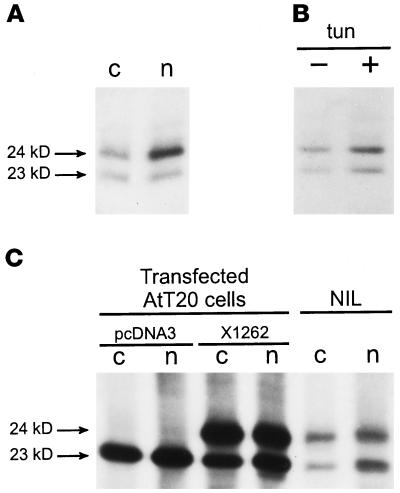
To investigate the biosynthesis of the X1262 protein, we raised a polyclonal antiserum against recombinant X1262 comprising amino acid residues 72–150 (anti-1262N). A second polyclonal antiserum was raised against a synthetic peptide comprising the carboxyl-terminal 14 amino acids of the protein (anti-1262C). Immunoprecipitation analysis of newly synthesized proteins produced by the NIL revealed that both the anti-1262N and the anti-1262C antibodies recognized two radiolabeled proteins of 23 and 24 kDa, whereas the anti-1262N antibody showed a higher affinity for the 24-kDa product (Figure 1A). When loaded on a nonreducing gel, both immunoprecipitated proteins migrated faster in the gel, indicating that each harbors a disulfide bridge (our unpublished results). The X1262 protein has one potential N-linked glycosylation site, namely, Asn-147. When NILs were preincubated and radiolabeled in the presence of tunicamycin, which is a blocker of N-linked glycosylation, the migration of the two immunoprecipitated proteins was not affected (Figure 1B). In contrast, the migration of a number of other newly synthesized proteins (e.g., the N-linked glycosylated POMC) was affected, indicating that N-linked glycosylation was indeed blocked. Therefore, we conclude that neither of the two X1262-like proteins is N-linked glycosylated.
Figure 1.
Two X1262-related proteins in the NIL of Xenopus. (A) NILs of black-adapted toads were radiolabeled, and homogenates were immunoprecipitated with either anti-1262C (c) or anti-1262N (n). (B) NILs were radiolabeled in the absence (−) or presence (+) of 10 μg/ml tunicamycin (tun), and homogenates were immunoprecipitated with the anti-1262C antibody. (C) AtT20 cells were stably transfected with pcDNA3 vector or X1262/pcDNA3 and radiolabeled, and homogenates were immunoprecipitated with either anti-1262C (c) or anti-1262N (n). For comparison, radiolabeled NIL proteins were also immunoprecipitated with these antibodies.
To characterize the two immunoprecipitated NIL products, we transfected X1262 cDNA into the mouse anterior pituitary-derived cell line AtT20 and compared on SDS-PAGE the migration of the overexpressed protein with that of the two immunoprecipitated Xenopus NIL proteins. In mock-transfected AtT20 cells, a single protein of 23 kDa was immunoprecipitated that comigrated with the 23-kDa product produced by the NIL (Figure 1C) and most likely corresponds to endogenous p23, as described for hamster, rat, and human (Sohn et al., 1996; Nickel et al., 1997; Rojo et al., 1997; Dominguez et al., 1998). In AtT20 cells stably transfected with the X1262 cDNA, an additional immunoprecipitated protein of 24 kDa was detected that comigrated with the 24-kDa NIL protein (Figure 1C). Therefore, we conclude that the immunoreactive 24-kDa protein of the NIL represents the X1262 protein. Because the overexpression of X1262 did not increase the level of expression of the 23-kDa product, we hypothesized that the corresponding product in the Xenopus NIL may be an additional X1262-related protein.
Identification of an X1262-related Protein
To search for an X1262-related protein that is expressed in the Xenopus melanotrope cells, we used the coding region of X1262 cDNA as a probe to screen a NIL cDNA library from black-adapted toads under low-stringency hybridization conditions. From a total amount of ∼600,000 plaques that were used in the screening, 161 hybridization-positive clones were obtained after washing under low-stringency conditions (2× SSC/0.1% SDS; 50°C). The signals of 55 of these clones were found to be removed after a more stringent washing step (0.5× SSC/0.1% SDS; 55°C), and sequencing of 10 of these clones revealed that they code for a novel Xenopus member of the p24 family. The largest of these clones, clone RH6, contained a cDNA insert of 1070 bp [excluding the poly(A) tail] with an ORF of 621 nucleotides. Within the protein-encoding region, the degree of nucleotide sequence identity between clones X1262 and RH6 is 68%. The 106 clones that remained positive after more stringent washing were again positive in a screening under high-stringency conditions with a probe directed against the 3′-untranslated region of X1262. Sequence information obtained from 20 of these 106 positive clones revealed that they all originated from the X1262 gene. The numbers of positive clones suggest that the level of expression of X1262 is about two times higher than that of clone RH6.
An alignment of the amino acid sequence deduced from cDNA clone X1262 with the RH6 sequence revealed that the two proteins are highly related. They are similar in length (205 and 207 amino acids, respectively), and both have a signal sequence, a transmembrane domain, and a short C tail (Figure 2). They share an overall amino acid sequence identity of 66% (78% similarity), which is much greater than the degree of identity between p24 subfamilies (<30%). The sequence conservation is highest in the carboxyl-terminal half of the lumenal domain, the transmembrane region, and the cytoplasmic C tail. Hence, the X1262 and RH6 proteins are much more closely related to each other than to other p24 proteins, implying that they both belong to the p24δ subfamily. A database search for p24δ protein sequences revealed that the various species examined each contain one p24δ sequence; the previously reported sequence of a second human p24δ protein (Blum et al., 1996) appears to be derived from a pseudogene (Hörer et al., 1999). Because the RH6 protein is more related to vertebrate p24δ proteins than the X1262 protein, we named the RH6 protein Xp24δ1 (δ1) and the X1262 protein Xp24δ2 (δ2).
Figure 2.
Multiple alignment of the p24δ subfamily. Aligned are the deduced amino acid sequences from the Xenopus p24δ clones X1262 (Xp24δ2) and RH6 (Xp24δ1), the p24δ sequence of human (hp24δ), mouse (mp24δ), puffer fish (Pf p24δ), C. elegans (Ce p24δ), and yeast (Sc Erv25p). Residues that are conserved in at least three sequences are boxed. Indicated are the putative signal peptidase cleavage site (arrow), the cysteine residues that are conserved among the p24 family members (asterisks), and the predicted transmembrane domain (TM; underlined).
Expression of δ1 and δ2 in Xenopus Pituitary
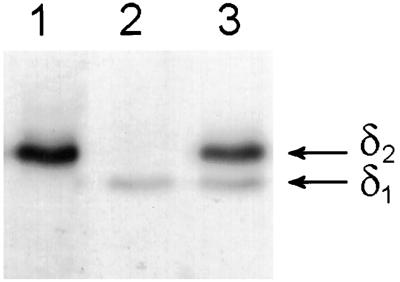
We generated a δ1-specific polyclonal antiserum (anti-RH6) against a synthetic peptide comprising amino acids 72–85 of δ1. Immunoblot analysis of recombinant δ1 and δ2 confirmed that this antiserum does not cross-react with the δ2 protein. A similar analysis established that the anti-1262C antibody reacts with both Xp24δ proteins with comparable affinities, whereas the anti-1262N antibody recognizes δ2 ∼10 times better than δ1 (our unpublished results). Next, we used the three antibodies on immunoblot analysis to characterize the p24δ proteins in the Xenopus NIL. As was the case for radiolabeled proteins, at steady-state levels, two Xenopus NIL proteins of 23 and 24 kDa were recognized by the anti-1262C antibody (Figure 3, lane 3). Immunoblotting with the anti-1262N antibody showed that the 24-kDa protein is δ2, confirming the results of the transfection experiments with AtT20 cells (Figures 1B and 3, lane 1). With the anti-RH6 antibody, we could establish that the 23-kDa band indeed represents the δ1 protein (Figure 3, lane 2).
Figure 3.
Specificity of the three Xenopus p24δ antibodies. NIL lysates were subjected to SDS-PAGE and immunoblotted with the affinity-purified antibodies anti-1262N (lane 1), anti-RH6 (lane 2), or anti-1262C (lane 3).
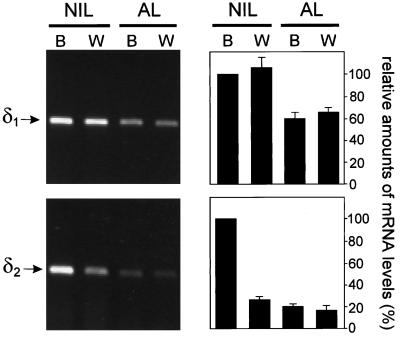
The δ2 gene is ubiquitously expressed, but in the NIL its expression is linked to POMC (Holthuis et al., 1995b), which means that the level of δ2 transcripts is increased when the animal is adapting to a black background. We investigated whether the expression of δ1 is also linked to that of POMC. For this purpose, we performed reverse transcription PCR analysis on cDNAs synthesized from NIL and AL mRNAs of both black- and white-adapted animals. With respect to δ2, we could confirm the results obtained previously with RNase protection analysis (Holthuis et al., 1995b), namely, that δ2 transcripts are induced approximately fivefold in the NIL during adaptation to a black background, whereas transcript levels in the AL remain unchanged (Figure 4). Interestingly, δ1 transcripts were not increased in the NIL of black-adapted animals, which suggests that the expression of δ1 is not coregulated with that of POMC (Figure 4). Similar results were obtained with Northern blot analysis, showing that the levels of δ2 transcripts in the NIL increased at least four- to fivefold during adaptation of the animal to a black background, whereas δ1 transcript levels were not significantly different (our unpublished results).
Figure 4.
Semiquantitative reverse transcription PCR analysis of δ1 and δ2 mRNA expression in Xenopus pituitary tissues. Primers specific for δ1 and δ2 were used for PCR on cDNA generated from NIL and AL mRNAs from black (B)- and white (W)-adapted Xenopus. Left panels show typical examples of the results that were obtained. Right panels show means (±SEM) of three independent experiments with NIL of black-adapted Xenopus as 100%.
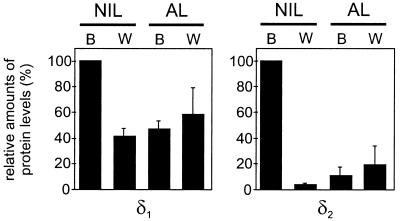
To investigate whether this differential regulation of δ1 and δ2 mRNA levels also occurs at the protein level, we performed quantitative immunoblot analysis on pituitary glands of black- and white-adapted animals. The expression of the δ2 protein was ∼25 times higher in the NIL of black-adapted animals than in that of white-adapted animals, whereas the level of the δ1 protein was induced only 2.5-fold. In the AL, no significant differences in the levels of either δ1 or δ2 were observed between black- and white-adapted animals (Figure 5).
Figure 5.
Western blot analysis of δ1 and δ2 protein expression in Xenopus pituitary. Similar amounts of protein from NILs and ALs of black (B)- and white (W)-adapted Xenopus were resolved by SDS-PAGE and immunoblotted with either anti-RH6 (δ1) or anti-1262N (δ2). To correct for loading, actin protein levels were also determined. Data shown are the means (±SEM) of three independent experiments with NIL of black-adapted Xenopus as 100%.
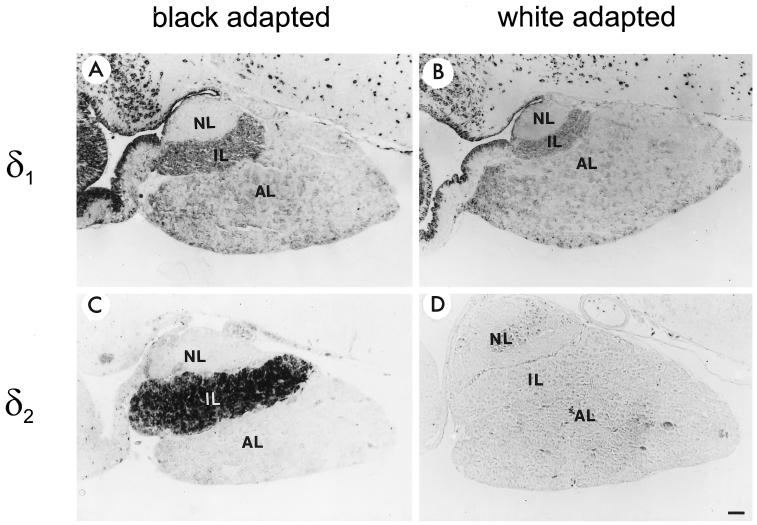
To study the distribution of the δ1 and δ2 proteins in Xenopus pituitary, immunocytochemical analysis was performed on pituitary sections of both black- and white-adapted animals. Based on the results obtained with Western blot analysis (Figure 3), we considered the anti-RH6 and anti-1262N antibodies at steady-state levels to be specific for δ1 and δ2, respectively. The most intense staining of δ1 was observed in cells throughout the brain in both black- and white-adapted toads. Within the pituitary, there was a homogeneous expression of δ1 in the intermediate lobe (IL) and the AL, although the degree of expression was low (Figure 6, A and B). Only a minor difference between the expression levels of the δ1 protein in the IL of black- and white-adapted animals was observed, whereas the expression of δ2 was clearly much higher in the IL of black-adapted animals than in that of white-adapted animals (Figure 6, C and D). These immunocytochemical data confirmed the results obtained with Western blot analysis (Figure 5). We also observed a low level of expression of δ2 in the AL and the brain, but only when higher concentrations of antibody were used, illustrating the high level of δ2 expression in the IL. The homogeneous staining of the entire IL indicates that both δ1 and δ2 are expressed in all intermediate pituitary cells. Because the intermediate pituitary essentially consists of a homogeneous population of a single cell type, namely, the melanotrope cells (Jenks et al., 1977), our results clearly suggest that δ1 and δ2 are expressed in the same cell.
Figure 6.
Immunocytochemical analysis of δ1 and δ2 protein expression in the pituitary gland of X. laevis. Paraffin sections of pituitaries of either black- or white-adapted animals were incubated with affinity-purified anti-RH6 (1:50 dilution; A and B) or anti-1262N (1:1500 dilution; C and D) antibodies. NL, neural lobe. Bar, 100 μm.
Expression of δ1 and δ2 in Xenopus Tissues
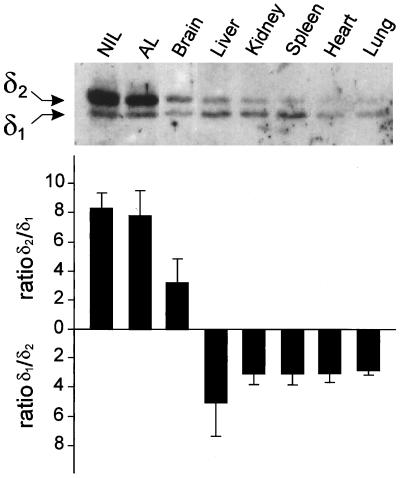
The tissue distribution of the p24δ proteins in X. laevis was studied by Western blot analysis with the anti-1262C antibody. Both δ1 and δ2 could be detected in pituitary, brain, liver, kidney, spleen, heart, and lung, but the relative expression levels of the two proteins differed among the various tissues (Figure 7). In the NIL and the AL of black-adapted Xenopus, the expression of δ2 is ∼10 times higher than that of δ1; the AL contains a number of hormone-producing cells, among which are the POMC-producing corticotropes. Also in brain, δ2 is the most abundant p24δ member (∼3 times more δ2 than δ1 expression). In all other tissues examined, δ1 was the predominant form, with expression levels between 3 and 5 times higher than those of δ2 (Figure 7). We conclude that, despite the fact that they are ubiquitously expressed, the expression levels of δ1 and δ2 are tissue dependent, with relatively high levels of δ2 in the pituitary and the brain and with δ1 as the major p24δ protein in the nonneuroendocrine tissues.
Figure 7.
Western blot analysis and quantification of relative amounts of δ1 and δ2 protein expression in a number of Xenopus tissues. Tissues were immunoblotted with the anti-1262C antiserum, which recognizes both Xp24δ proteins. Similar amounts of total protein were loaded in each lane. Proteins were visualized by chemiluminescence and quantified with a luminescence detector.
DISCUSSION
The p24 proteins belong to a family of small type I transmembrane proteins that form the major constituents of COP-coated vesicles and have a crucial role in the transport of proteins between the ER and the Golgi complex (Schimmöller et al., 1995; Stamnes et al., 1995; Elrod-Erickson and Kaiser, 1996; Rojo et al., 1997). Based on the degree of amino acid sequence identity, the members of the p24 family that have been described thus far can be classified into a number of subfamilies, referred to as p24α, -β, -γ, and -δ (Dominguez et al., 1998; Füllekrug et al., 1999). Two of these subfamilies, p24γ and p24δ, have been reported to contain more than one subfamily member (Blum et al., 1996; Dominguez et al., 1998); in a subsequent study, however, the second δ member appeared to be derived from a pseudogene (Hörer et al. 1999). During database searches, we noticed that, in addition to p24γ, the p24α subfamily also contains two members, whereas no additional members were found for p24β and p24δ. Thus, until now, multiple members have been known only for the p24α and p24γ subfamilies, and no data have been presented concerning the relative levels and sites of expression of these subfamily members. In this study, we report on the characterization of two p24 proteins that belong to the p24δ subfamily (δ1 and δ2) and that are both expressed in one cell type, namely, the melanotrope cell of the Xenopus pituitary gland.
The Xenopus melanotrope cells are primarily devoted to the production of the prohormone POMC. When the background of the animal is changed from white to black, the melanotrope cells become highly active and the level of POMC mRNA is increased ∼30-fold. Approximately 75% of all transcripts produced in the active cells represent POMC mRNA (Holthuis et al., 1995a). We have found that the expression of only δ2, and not that of δ1, is regulated coordinately with POMC. Activation of the melanotropes resulted in an ∼5-fold increase in δ2 transcripts, whereas δ1 mRNA levels remained unchanged. In addition to δ2, several other transcripts in the melanotrope cells are coordinately expressed with POMC. Transcripts encoding the transmembrane proteins TRAPδ and the vacuolar H+-ATPase subunit Ac45, as well as transcripts encoding secretory proteins such as the prohormone convertase PC2, its molecular chaperone 7B2, the secretogranins II and III (SgII and SgIII), and carboxypeptidase E, have been found to be increased during black background adaptation (up to 35-fold; Holthuis et al., 1995a). All of these proteins play a role in the biosynthesis and processing of POMC in the melanotrope cells and therefore are produced in higher amounts when the melanotrope cells are activated. Interestingly, also at the protein level we found an impressive increase (∼25-fold) in the amount of δ2 in the melanotrope cells of black-adapted toads. Thus far, upon black background adaptation, the steady-state levels of proteins coordinately expressed with POMC have been found to be increased much less than that of δ2. For instance, the protein levels of PC2, 7B2, SgII, and αMSH (the hormone produced by POMC processing) are all similar in the NILs of black- and white-adapted animals (Dotman et al., 1998; Van Horssen and Martens, 1999; Kuiper and Martens, unpublished observations). In addition, only a twofold higher protein level was observed for Ac45 (Holthuis et al., 1999). These minor differences in protein levels upon activation of the melanotropes can be explained by the fact that these proteins are all located in the later stages of the secretory pathway and thus are stored in the secretory granules of inactive melanotropes of white-adapted animals. Moreover, the lumenal proteins PC2, 7B2, SgII, and αMSH are rapidly secreted from active melanotropes. In contrast, as was described for p24δ proteins in a number of species (Sohn et al., 1996; Rojo et al., 1997; Nickel et al., 1997; Dominguez et al., 1998; Blum et al., 1999), δ2 is most likely located in the ER-Golgi region of the cell, where it is continuously recycled. The enormous increase in the level of δ2 during black background adaptation indicates that the vesicular machinery in the ER-Golgi region is highly induced. This notion is in line with our observation that the levels of three subunits of the COPI coatomer complex (α-, γ-, and ε-COP) also are induced at least ∼5-fold (our unpublished results) and with previous results at the ultrastructural level that show an extensive elaboration of ER and Golgi membranes in the activated Xenopus melanotrope cells (Hopkins, 1970; De Rijk et al., 1990). The fact that at both the mRNA and protein levels the degree of induction of δ1 and δ2 differs ∼5- to 10-fold suggests that not all components of the ER and Golgi membranes are increased, but only that portion of the machinery involved in the efficient transport of POMC.
The question arises concerning the significance of our findings with respect to a possible role of the p24δ proteins in the melanotrope cells. It is unlikely that δ1 and δ2 function sequentially in the secretory pathway because in such a case one would expect that both would be coordinately expressed with POMC. Moreover, the sequence motifs that are known to influence the intracellular distribution of p24 proteins, namely, the double phenylalanine and the K(X)KXX-like retrieval motif (Fiedler et al., 1996; Fiedler and Rothman, 1997; Dominguez et al., 1998), are identical in the two Xenopus proteins, suggesting that they have a similar subcellular localization. Studies with other species revealed that p24δ is mainly localized to the intermediate compartment and cis-Golgi and to a lesser extent the ER (Rojo et al., 1997; Dominguez et al., 1998; Blum et al., 1999). The high abundance of p24 proteins in the early secretory pathway led to the hypothesis that they are involved in the formation and maintenance of the membrane structure of transport vesicles (Rojo et al., 1997), possibly functioning as a scaffold for the binding of coat proteins (Stamnes et al., 1995; Sohn et al., 1996; Nickel et al., 1997; Nickel and Wieland, 1997). However, the differential regulation of δ1 and δ2 in the melanotrope cells strongly suggests that these proteins have a role in cargo-selective transport rather than function as a nonspecific structural membrane component. Several models with p24 being involved in cargo-selective transport have been proposed. First, p24 proteins could function in a quality control mechanism. This model was proposed by Wen and Greenwald (1999), who showed that in Caenorhabditis elegans p24 proteins behave as negative regulators of protein transport. In this model, p24 proteins act as cargo selectors, preventing the inclusion of misfolded and mutated proteins into newly formed transport vesicles. The differential regulation of δ1 and δ2 in the melanotrope cell would suggest that the δ2 protein is specifically involved in the exclusion of misfolded POMC molecules. Second, p24 proteins could act as cargo receptors, selectively sorting a certain subset of secretory proteins into COPII-coated vesicles for anterograde transport, thereby excluding other cargo proteins and ER-resident proteins (Schimmöller et al., 1995; Belden and Barlowe, 1996; Elrod-Erickson and Kaiser, 1996). In such a model, δ2 would be involved in the inclusion of POMC into transport vesicles, explaining its coordinate expression with this prohormone, whereas δ1 would facilitate the transport of another subset of secretory proteins. In the third model, the p24 proteins function as COPI-binding receptors (Sohn et al., 1996; Nickel et al., 1997) involved in retrograde transport from the Golgi to the ER, as was described for human p24δ (p23; Majoul et al., 1998). Because δ1 and δ2 are differentially regulated in the melanotropes, this would implicate cargo-selective retrograde transport. In this model, δ2 would be increased in the active melanotropes because, through cargo-selective, retrograde Golgi-to-ER transport, it retrieves protein(s) involved specifically in the early stages of POMC biosynthesis. Unfortunately, extensive cross-linking, coimmunoprecipitation, and in vitro binding experiments have not allowed us to establish a specific physical interaction between δ2 and POMC or any other cargo molecule. Thus, at present, we cannot distinguish between the various models.
Both δ1 and δ2 were found to be ubiquitously expressed, and the expression of δ2 is thus not limited to POMC-producing cells. However, δ2 seems to be neuroendocrine enriched, whereas δ1 is the major p24δ member in nonneuroendocrine tissues. Our data, therefore, suggest that δ1 and δ2 are functional in transport routes that coexist in most, if not all, Xenopus cell types, with δ2 being particularly important for the transport of proteins that are predominantly expressed in neuroendocrine tissues and in the melanotrope cells being linked to POMC transport. Because the p24α, -γ, and -δ subfamilies each contain at least two members and may form different heteromeric complexes (Dominguez et al., 1998; Füllekrug et al., 1999; Marzioch et al., 1999), a multiplicity of p24 systems could be generated, providing the possibility for selective transport of secretory proteins. In addition, the abundance of p24 proteins in the early secretory pathway, and their continuous COPI-mediated recycling from the Golgi to the ER, provides a mechanism for the membrane removal and subsequent concentration of anterograde cargo in the vesicular tubular clusters, as was reported recently (Martínez-Menárguez et al., 1999).
In conclusion, we have identified two members of the p24δ subfamily and demonstrated that these forms are expressed in one cell type, the melanotrope cell of the Xenopus pituitary gland. Of these, only δ2 is coordinately expressed with POMC, suggesting a function for this p24 protein in selective protein transport.
ACKNOWLEDGMENTS
We thank A.J.M. Coenen and R. Wieggers for technical assistance, R. Engels for animal care, and Dr. F. Wieland for kindly supplying antibodies to α-/γ-COP and ε-COP. This work was supported by grant 805-33-150 from the Netherlands Organization for Scientific Research-Earth and Life Sciences and by European Union–Training and Mobility of Researchers network ERB-FMRX-CT960023.
Abbreviations used:
- AL
anterior lobe
- COPI and COPII
coat proteins I and II
- IL
intermediate lobe
- αMSH
α-melanophore–stimulating hormone
- NIL
neurointermediate lobe
- POMC
proopiomelanocortin
REFERENCES
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum-to-Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Weissman J, Bannykh S, Nuoffer C, Balch WE. Cargo selection by the COPII budding machinery during export from the ER. J Cell Biol. 1998;141:61–70. doi: 10.1083/jcb.141.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1989. [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Balch WE. Membrane dynamics at the endoplasmic reticulum-Golgi interface. J Cell Biol. 1997;138:1–4. doi: 10.1083/jcb.138.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. The organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Barlowe C. Erv25p, a component of COPII-coated vesicles, forms a complex with Emp24p that is required for efficient endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- Blum R, Feick P, Puype M, Vandekerckhove J, Klengel R, Nastainczyk W, Schulz I. Tmp21 and p24A, two type I proteins enriched in pancreatic microsomal membranes, are members of a protein family involved in vesicular trafficking. J Biol Chem. 1996;271:17183–17189. doi: 10.1074/jbc.271.29.17183. [DOI] [PubMed] [Google Scholar]
- Blum R, Pfeiffer F, Feick P, Nastainczyk W, Kohler B, Schäfer KH, Schulz I. Intracellular localization and in vivo trafficking of p24A and p23. J Cell Sci. 1999;112:537–548. doi: 10.1242/jcs.112.4.537. [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- De Rijk EP, Jenks BG, Wendelaar Bonga SE. Morphology of the pars intermedia and the melanophore-stimulating cells in Xenopus laevis in relation to background adaptation. Gen Comp Endocrinol. 1990;79:74–82. doi: 10.1016/0016-6480(90)90089-5. [DOI] [PubMed] [Google Scholar]
- Dominguez M, Dejgaard K, Füllekrug J, Dahan S, Fazel A, Paccaud JP, Thomas DY, Bergeron JJM, Nilsson T. gp25L/emp24/p24 protein family members of the cis-Golgi network bind both COP I and II coatomer. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotman CH, van Herp F, Martens GJM, Jenks BG, Roubos EW. Dynamics of proopiomelanocortin and prohormone convertase 2 gene expression in Xenopus melanotrope cells during long-term background adaptation. J Endocrinol. 1998;159:281–286. doi: 10.1677/joe.0.1590281. [DOI] [PubMed] [Google Scholar]
- Elrod-Erickson MJ, Kaiser CA. Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Rothman JE. Sorting determinants in the transmembrane domain of p24 proteins. J Biol Chem. 1997;272:24739–24742. doi: 10.1074/jbc.272.40.24739. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Füllekrug J, Suganuma T, Tang BL, Hong W, Storrie B, Nilsson T. Localization and recycling of gp27 (hp24γ3): complex formation with other p24 family members. Mol Biol Cell. 1999;10:1939–1955. doi: 10.1091/mbc.10.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich B, Orci L, Tschochner H, Lottspeich F, Ravazzola M, Amherdt M, Wieland F, Harter C. Nonclathrin-coat protein α is a conserved subunit of coatomer and in Saccharomyces cerevisiae is essential for growth. Proc Natl Acad Sci USA. 1995;92:3229–3233. doi: 10.1073/pnas.92.8.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hara-Kuge S, Kuge O, Orci L, Amherdt M, Ravazzola M, Wieland FT, Rothman JE. En bloc incorporation of coatomer subunits during the assembly of COP-coated vesicles. J Cell Biol. 1994;124:883–892. doi: 10.1083/jcb.124.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JCM, Jansen EJR, Schoonderwoert VTC, Burbach JPH, Martens GJM. Biosynthesis of the vacuolar H+-ATPase accessory subunit Ac45 in Xenopus pituitary. Eur J Biochem. 1999;262:1–9. doi: 10.1046/j.1432-1327.1999.00396.x. [DOI] [PubMed] [Google Scholar]
- Holthuis JCM, Jansen EJR, van Riel MC, Martens GJM. Molecular probing of the secretory pathway in peptide hormone-producing cells. J Cell Sci. 1995a;108:3295–3305. doi: 10.1242/jcs.108.10.3295. [DOI] [PubMed] [Google Scholar]
- Holthuis JCM, van Riel MC, Martens GJM. Translocon-associated protein TRAPδ and a novel TRAP-like protein are coordinately expressed with proopiomelanocortin in Xenopus intermediate pituitary. Biochem J. 1995b;312:205–213. doi: 10.1042/bj3120205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CR. Studies on secretory activity in the pars intermedia of Xenopus laevis. 1. Fine structural changes related to the onset of secretory activity in vivo. Tissue Cell. 1970;2:59–70. doi: 10.1016/s0040-8166(70)80007-6. [DOI] [PubMed] [Google Scholar]
- Hörer J, Blum R, Feick P, Nastainczyk W, Schulz I. A comparative study of rat and human Tmp21 (p23) reveals the pseudogene-like features of human Tmp21-II. DNA Seq. 1999;10:121–126. doi: 10.3109/10425179909008429. [DOI] [PubMed] [Google Scholar]
- Jenks BG, Overbeeke AP, McStay BF. Synthesis, storage and release of MSH in the pars intermedia of the pituitary gland of Xenopus laevis during background adaptation. Can J Zool. 1977;55:922–927. [Google Scholar]
- Lavoie C, Paiement J, Dominguez M, Roy L, Dahan S, Gushue JN, Bergeron JJM. Roles for α2p24 and COPI in endoplasmic reticulum cargo exit site formation. J Cell Biol. 1999;146:285–299. doi: 10.1083/jcb.146.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Démollière C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Majoul I, Sohn K, Wieland FT, Pepperkok R, Pizza M, Hillemann J, Soling HD. KDEL receptor (Erd2p)-mediated retrograde transport of the cholera toxin A subunit from the Golgi involves COPI, p23, and the COOH terminus of Erd2p. J Cell Biol. 1998;143:601–612. doi: 10.1083/jcb.143.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens GJM, Weterings KAP, van Zoest ID, Jenks BG. Physiologically-induced changes in proopiomelanocortin mRNA levels in the pituitary gland of the amphibian Xenopus laevis. Biochem Biophys Res Commun. 1987;143:678–684. doi: 10.1016/0006-291x(87)91407-0. [DOI] [PubMed] [Google Scholar]
- Martínez-Menárguez JA, Geuze HJ, Slot JW, Klumperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COP-I-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- Marzioch M, Henthorn DC, Herrmann JM, Wilson R, Thomas DY, Bergeron JJM, Solari RCE, Rowley A. Erp1p and Erp2p, partners for Emp24p and Erv25p in a yeast p24 complex. Mol Biol Cell. 1999;10:1923–1938. doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Sohn K, Bünning C, Wieland FT. p23, a major COPI-vesicle membrane protein, constitutively cycles through the early secretory pathway. Proc Natl Acad Sci USA. 1997;94:11393–11398. doi: 10.1073/pnas.94.21.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Wieland FT. Biogenesis of COPI-coated transport vesicles. FEBS Lett. 1997;413:395–400. doi: 10.1016/s0014-5793(97)00939-3. [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Scheel J, Horstmann H, Hauri HP, Griffiths G, Kreis TE. β-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993;74:71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJM, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Rojo M, Pepperkok R, Emery G, Kellner R, Stang E, Parton RG, Gruenberg J. Involvement of the transmembrane protein p23 in biosynthetic protein transport. J Cell Biol. 1997;139:1119–1135. doi: 10.1083/jcb.139.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schimmöller F, Singer-Krüger B, Schröder S, Krüger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Matter K, Kreis TE, Ginsel L, Hauri HP. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horssen AM, Martens GJM. Biosynthesis of secretogranin II in Xenopus intermediate pituitary. Mol Cell Endocrinol. 1999;147:57–64. doi: 10.1016/s0303-7207(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Wada I, Rindress D, Cameron PH, Ou WJD, Doherty JJ, Louvard D, Bell AW, Dignard D, Thomas DY, Bergeron JJM. SSRα and associated calnexin are major calcium binding proteins of the endoplasmic reticulum membrane. J Biol Chem. 1991;266:19599–19610. [PubMed] [Google Scholar]
- Wen C, Greenwald I. p24 proteins and quality control of LIN-12 and GLP-1 trafficking in Caenorhabditis elegans. J Cell Biol. 1999;145:1165–1175. doi: 10.1083/jcb.145.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]