Abstract
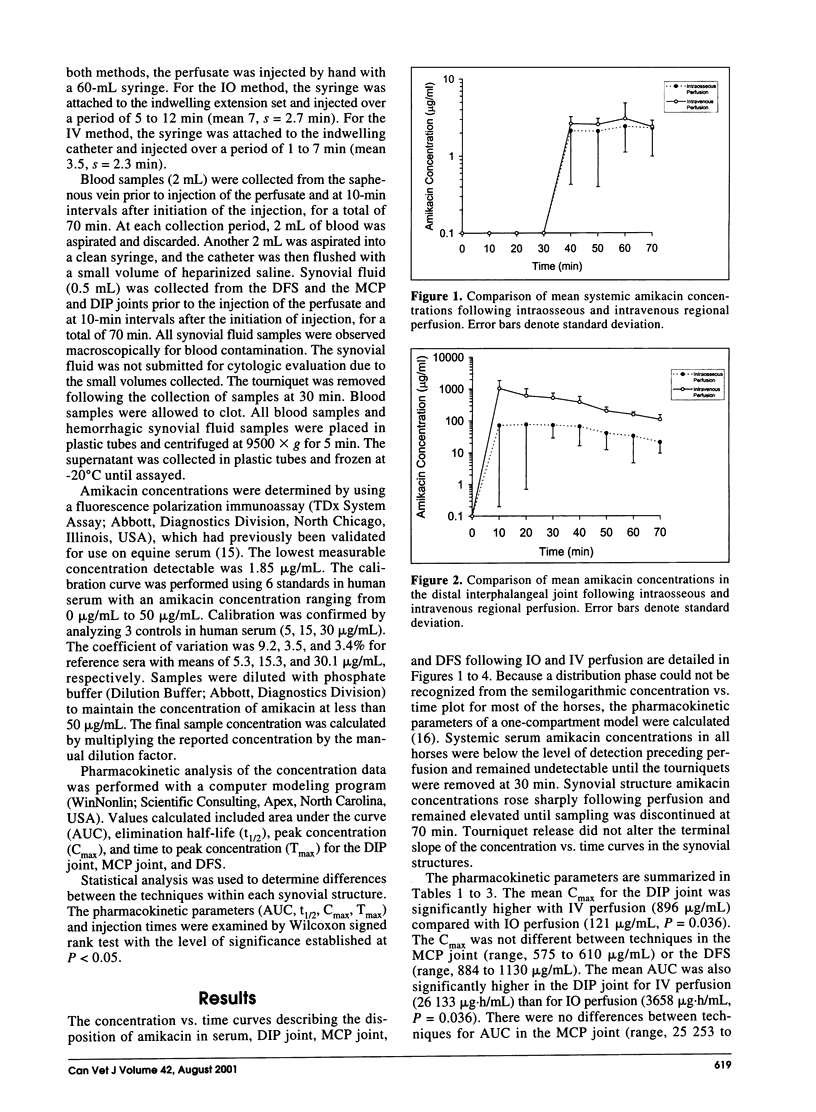
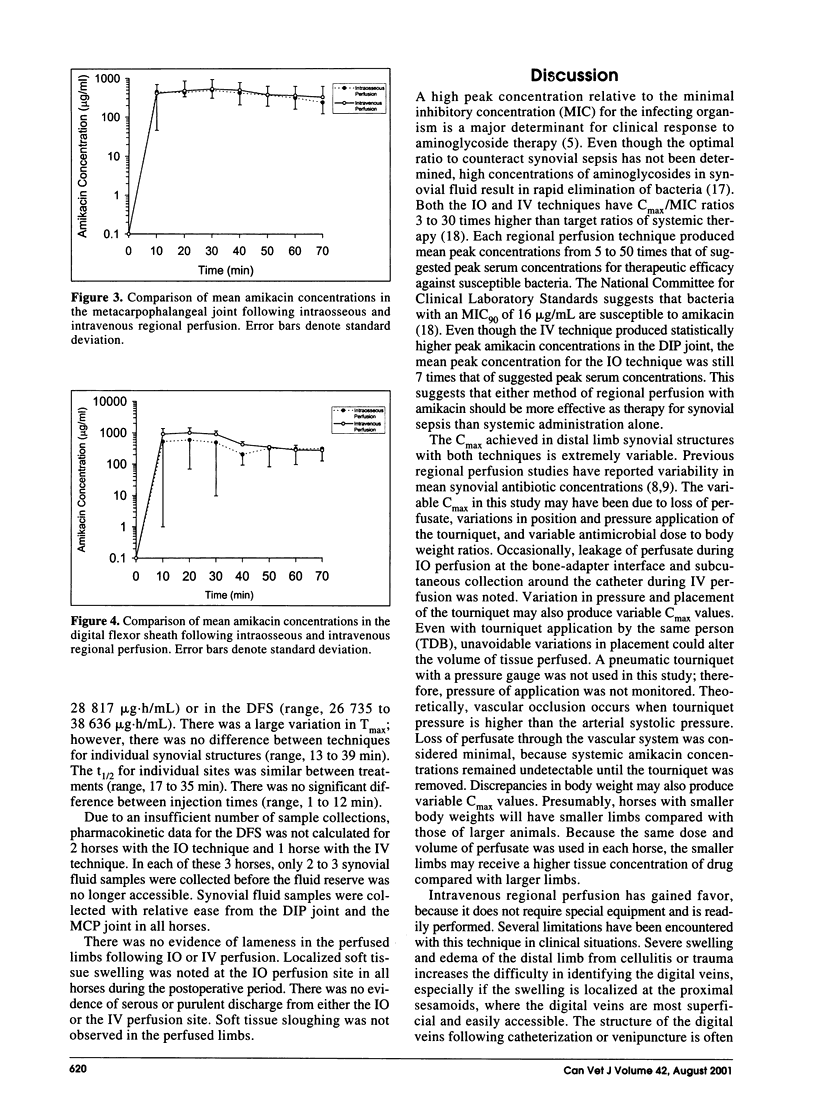
The purpose of this study was to compare the synovial fluid concentrations and pharmacokinetics of amikacin in the equine limb distal to the carpus following intraosseous and intravenous regional perfusion. The front limbs of 6 horses were randomly assigned to either intraosseous or intravenous perfusion. A tourniquet was placed distal to each carpus and the limb perfused with 500 mg of amikacin. Systemic blood samples and synovial fluid samples were collected over 70 min from the distal interphalangeal (DIP) joint, metacarpophalangeal joint, and digital flexor sheath. The tourniquet was removed following the 30 min sample collection. The mean peak amikacin concentration for the DIP joint was significantly higher with intravenous perfusion. There were no significant differences in time to peak concentration or elimination half-life between methods at each synovial structure. Each technique produced mean peak concentrations ranging from 5 to 50 times that of recommended peak serum concentrations for therapeutic efficacy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. P., Embertson R. M., Gronwall R. R., Beal C., Mayhew I. G., Curry S. H. Amikacin sulfate in mares: pharmacokinetics and body fluid and endometrial concentrations after repeated intramuscular administration. Am J Vet Res. 1984 Aug;45(8):1610–1613. [PubMed] [Google Scholar]
- Finsterbush A., Weinberg H. Venous perfusion of the limb with antibiotics for osteomyelitis and other chronic infections. J Bone Joint Surg Am. 1972 Sep;54(6):1227–1234. [PubMed] [Google Scholar]
- Gagnon H., Ferguson J. G., Papich M. G., Bailey J. V. Single-dose pharmacokinetics of cefazolin in bovine synovial fluid after intravenous regional injection. J Vet Pharmacol Ther. 1994 Feb;17(1):31–37. doi: 10.1111/j.1365-2885.1994.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Golenz M. R., Wilson W. D., Carlson G. P., Craychee T. J., Mihalyi J. E., Knox L. Effect of route of administration and age on the pharmacokinetics of amikacin administered by the intravenous and intraosseous routes to 3 and 5-day-old foals. Equine Vet J. 1994 Sep;26(5):367–373. doi: 10.1111/j.2042-3306.1994.tb04405.x. [DOI] [PubMed] [Google Scholar]
- Green S. L., Conlon P. D., Mama K., Baird J. D. Effects of hypoxia and azotaemia on the pharmacokinetics of amikacin in neonatal foals. Equine Vet J. 1992 Nov;24(6):475–479. doi: 10.1111/j.2042-3306.1992.tb02880.x. [DOI] [PubMed] [Google Scholar]
- Lloyd K. C., Stover S. M., Pascoe J. R., Adams P. Synovial fluid pH, cytologic characteristics, and gentamicin concentration after intra-articular administration of the drug in an experimental model of infectious arthritis in horses. Am J Vet Res. 1990 Sep;51(9):1363–1369. [PubMed] [Google Scholar]
- McIlwraith C. W. Treatment of infectious arthritis. Vet Clin North Am Large Anim Pract. 1983 Jul;5(2):363–379. doi: 10.1016/s0196-9846(17)30083-6. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Moore R. M., Schneider R. K., Kowalski J., Bramlage L. R., Mecklenburg L. M., Kohn C. W. Antimicrobial susceptibility of bacterial isolates from 233 horses with musculoskeletal infection during 1979-1989. Equine Vet J. 1992 Nov;24(6):450–456. doi: 10.1111/j.2042-3306.1992.tb02875.x. [DOI] [PubMed] [Google Scholar]
- Murphey E. D., Santschi E. M., Papich M. G. Regional intravenous perfusion of the distal limb of horses with amikacin sulfate. J Vet Pharmacol Ther. 1999 Feb;22(1):68–71. doi: 10.1046/j.1365-2885.1999.00180.x. [DOI] [PubMed] [Google Scholar]
- Orsini J. A., Benson C. E., Spencer P. A., Van Miller E. Resistance to gentamicin and amikacin of gram-negative organisms isolated from horses. Am J Vet Res. 1989 Jun;50(6):923–925. [PubMed] [Google Scholar]
- Orsini J. A., Soma L. R., Rourke J. E., Park M. Pharmacokinetics of amikacin in the horse following intravenous and intramuscular administration. J Vet Pharmacol Ther. 1985 Jun;8(2):194–201. doi: 10.1111/j.1365-2885.1985.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Schneider R. K., Bramlage L. R., Moore R. M., Mecklenburg L. M., Kohn C. W., Gabel A. A. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. 1992 Nov;24(6):436–442. doi: 10.1111/j.2042-3306.1992.tb02873.x. [DOI] [PubMed] [Google Scholar]
- Snyder J. R., Pascoe J. R., Hirsh D. C. Antimicrobial susceptibility of microorganisms isolated from equine orthopedic patients. Vet Surg. 1987 May-Jun;16(3):197–201. doi: 10.1111/j.1532-950x.1987.tb00938.x. [DOI] [PubMed] [Google Scholar]
- Whitehair K. J., Blevins W. E., Fessler J. F., Van Sickle D. C., White M. R., Bill R. P. Regional perfusion of the equine carpus for antibiotic delivery. Vet Surg. 1992 Jul-Aug;21(4):279–285. doi: 10.1111/j.1532-950x.1992.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Whithair K. J., Bowersock T. L., Blevins W. E., Fessler J. F., White M. R., Van Sickle D. C. Regional limb perfusion for antibiotic treatment of experimentally induced septic arthritis. Vet Surg. 1992 Sep-Oct;21(5):367–373. doi: 10.1111/j.1532-950x.1992.tb01713.x. [DOI] [PubMed] [Google Scholar]



