Abstract
Membranoproliferative glomerulonephritis (MPGN) type II (dense deposit disease) is an inflammatory renal disease characterized by electron-dense deposits and complement C3 on the glomerular basement membrane. There is no effective therapy. We investigated the role of C5 activation in a model of MPGN that develops spontaneously in complement factor H-deficient mice (Cfh−/−). At 12 months there was a significant reduction in mortality, glomerular cellularity, neutrophil numbers, and serum creatinine levels in Cfh−/− mice deficient in C5. Excessive glomerular neutrophil numbers, frequently seen in patients with MPGN during disease flares, were also observed in Cfh−/− mice after the administration of an antiglomerular basement membrane antibody. This exaggerated injurious phenotype was absent in Cfh−/− mice deficient in C5 but not in Cfh−/− mice deficient in C6, indicating a key role for C5 activation in the induction of renal lesions. Importantly, the renal injury was completely reversed in Cfh−/− mice pretreated with an anti-murine C5 antibody. These results demonstrate an important role for C5 in both spontaneous MPGN and experimentally induced nephritis in factor H-deficient mice and provide preliminary evidence that C5 inhibition therapy might be useful in human MPGN type II.
Keywords: complement, inflammation
The complement system is a key component of innate immunity contributing to host defenses against invading pathogens through multiple mechanisms, which include opsonization, cell lysis, and inflammatory cell recruitment, an action principally mediated through the anaphylatoxin C5a. Complement activation is regulated by a complex group of membrane-bound and fluid-phase proteins (1). Factor H is an abundant serum complement regulatory protein that inhibits the alternative pathway of complement activation. It achieves this through several mechanisms, which include inhibition of the alternative pathway C3 convertase enzyme complex (C3bBb) and acting as a cofactor for the factor I-mediated proteolytic degradation of activated C3 (termed C3b) (2, 3). Its critical importance as a regulator of C3 activation in vivo is illustrated by the complement profile reported in factor H-deficient individuals, where alternative pathway activation proceeds unhindered, resulting in markedly reduced C3 levels (4).
Factor H deficiency in humans (5, 6), pigs (7), and mice (8) is associated with membranoproliferative glomerulonephritis (MPGN) type II (dense deposit disease). MPGN is characterized by glomerular capillary wall thickening with increased mesangial matrix and mesangial cells (9). Intramembranous glomerular basement membrane (GBM) deposits together with C3 (10), C5 (11), and C9 (12) staining along the GBM in the absence of Ig characterize type II MPGN (13). Patients typically have low C3 levels while C5 levels remain normal (14). MPGN type II is frequently associated with the presence of C3 nephritic factor (C3NeF), an autoantibody that stabilizes the alternative pathway C3 convertase, preventing its inactivation by factor H and resulting in excessive C3 activation (15). MPGN and C3 dysregulation has also been reported in individuals with dysfunctional C3 molecules (16, 17) and in an individual with an autoantibody against factor H (18). Patients with MPGN type II also develop macular drusen, a feature of age-related macular degeneration that has recently been associated with factor H mutations (19–21). Notably, in a recent series of 20 patients with MPGN type II, ≈70% possessed factor H haplotypes associated with age-related macular degeneration (22), suggesting that abnormal factor H function may underlie the pathogenesis of many cases of human MPGN type II.
No treatment strategies have consistently shown benefit in MPGN type II in the limited number of controlled trials published to date (reviewed in ref. 23). Because ≈50% of patients progress to end-stage renal failure within 10 years (24, 25) and this condition frequently recurs in transplanted kidneys (24, 26), there is an urgent need to develop effective therapeutic interventions.
We have previously reported that factor H-deficient mice (Cfh−/−) mice spontaneously develop MPGN that depends on C3 activation (8). In this report we first assessed the role of C5 activation on the development of spontaneous MPGN in Cfh−/− mice. Mice deficient in both C5 and factor H still developed MPGN but displayed reduced mortality and glomerular cellularity in comparison with mice deficient in factor H alone. Second, to mimic disease flares that may occur in patients with MPGN, we investigated the mechanisms by which Cfh−/− mice with chronic C3 dysregulation and MPGN responded to an additional nephrotoxic insult. In the model of heterologous nephrotoxic nephritis (NTN), Cfh−/− mice showed increased susceptibility to renal inflammation that was critically dependent on C5 activation. Inhibition of C5 activation through the administration of a monoclonal anti-C5 antibody protected the Cfh−/− mice during NTN. Our data show a pathogenic modifying role for C5 activation in the development of spontaneous MPGN and in acute renal injury in Cfh−/− mice. These findings are of direct relevance to the treatment of individuals with MPGN type II and C3 dysregulation in view of the availability of anti-human C5 antibody therapy (27) and provide preliminary data for testing the efficacy of this therapy in human MPGN type II.
Results
Spontaneous MPGN in Factor H-Deficient Mice Lacking C5.
To analyze the role of C5 activation we studied Cfh−/− mice, mice deficient in complement C5 (C5−/−), and mice deficient in both factor H and C5 (Cfh−/−.C5−/−) over 12 months. At the end of this period all mice were killed, and renal function and histology were assessed (Table 1). During the 12 months mortality was significantly greater in Cfh−/− mice compared with Cfh−/−.C5−/− mice (P = 0.0366). Grade V glomerular hypercellularity and MPGN were evident in all of the Cfh−/− mice that died. Capillary wall double contours, used as a specific light microscopic marker of MPGN, were evident in all of the Cfh−/− mice but none of the C5−/− mice. Capillary wall double contours remained detectable in all of the Cfh−/− mice lacking C5. To ascertain whether the ultrastructural features of MPGN were present, we performed electron microscopy on three animals from each group. In all of the Cfh−/− and Cfh−/−.C5−/− mice and none of the C5−/− mice examined, GBM thickening and subendothelial electron-dense deposits, typical features of MPGN, were present (data not shown). However, glomerular cellularity and crescent formation were significantly reduced in Cfh−/−.C5−/− mice compared with those deficient in factor H alone (P < 0.001). Furthermore, glomerular neutrophil numbers were significantly greater in Cfh−/− mice compared with the Cfh−/−.C5−/− mice (P < 0.001) (Table 1). Assessment of renal function showed that serum creatinine levels were significantly higher in the Cfh−/− mice compared with both the Cfh−/−.C5−/− mice (P < 0.001) and the C5−/− mice (P < 0.01) (Table 1). In contrast, the median creatinine levels did not differ between the Cfh−/−.C5−/− mice and the C5−/− mice. The degree of albuminuria was significantly greater in the Cfh−/− mice and in the Cfh−/−.C5−/− mice compared with the C5−/− mice (P < 0.001 and P < 0.01, respectively). Albuminuria did not differ between Cfh−/− and Cfh−/−.C5−/− mice, suggesting that chronic C3 deposition along the GBM alone was sufficient to disrupt the integrity of the glomerular permeability barrier. Thus, although spontaneous MPGN in Cfh−/− mice developed independent of C5 activation, C5 deficiency was associated with a reduction in mortality, glomerular cellularity, and serum creatinine levels at 12 months. Renal function may rapidly decline in patients with chronic MPGN and C3 dysregulation during nephrotoxic insults such as intercurrent illness. To mimic this experimentally we examined the response of the Cfh−/− mice to antibody-mediated C3 activation within the kidney using heterologous NTN.
Table 1.
Renal pathology in 12-month-old Cfh−/−, Cfh−/−.C5−/−, and C5−/− mice
| Mice | n | Mortality by 12 months, n (%) | n | Renal histology |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal function |
n | GBM double contours, % | Grade of glomerulonephritis |
Median no. of glomerular neutrophils per gcs (range) | ||||||||||
| Creatinine, median μmol/liter (range) | Albuminuria, median mg/24 h (range) | 0 | I | II | III | IV | V | |||||||
| C5−/− | 29 | 0 | 29 | 37 (28–53) | 0.1 (0.1–0.4) | 29 | 0 | 25 | 2 | 2 | 0 | 0 | 0 | 0.04 (0–0.24) |
| Cfh−/− | 19 | 5* (26.3) | 14 | 44‡§ (33–56) | 6.4¶ (0.1–15.5) | 19§¶ | 100 | 0 | 0 | 0 | 5 | 9 | 5 | 0.42§¶ (0.08–1.16) |
| Cfh−/−.C5−/− | 32 | 2† (6.3) | 30 | 36 (26–47) | 2.0‡ (0.1–10) | 32¶ | 100 | 0 | 0 | 8 | 18 | 6 | 0 | 0.16 (0–0.4) |
gcs, glomerular cross section.
*, P = 0.0366 vs. Cfh−/−.C5−/− mice and P = 0.0035 vs. C5−/− mice (log-rank test).
†, P = 0.0045 vs. C5−/− mice (log-rank test).
‡, P < 0.01 vs. C5−/− mice.
§, P < 0.001 vs. Cfh−/−.C5−/− mice.
¶, P < 0.001 vs. C5 −/− mice (Bonferroni’s multiple comparison test).
Heterologous NTN in Factor H-Deficient Mice.
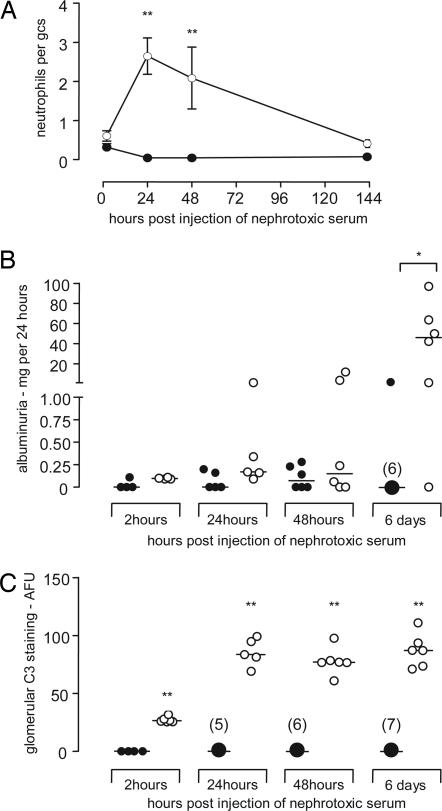
In this model binding of heterologous antibody along the GBM results in glomerular neutrophil influx and proteinuria. Importantly, increased glomerular neutrophil numbers are a feature of human MPGN type II (24, 28). Hence, we first examined glomerular neutrophil numbers and proteinuria in Cfh−/− and wild-type mice after induction of heterologous NTN using a sheep anti-mouse GBM antibody (Fig. 1). Although glomerular neutrophil numbers were similar in the two groups at 2 h (Fig. 1A), significant neutrophils were still present 24 h after injection of heterologous antibody in the Cfh−/− mice, in marked contrast to the resolution seen in wild-type mice at this time point. Increased glomerular neutrophils persisted in the Cfh−/− mice at 48 h and were still detectable 6 days after administration of the antibody (Fig. 1A). Furthermore, by day 6 significantly greater proteinuria was evident in the Cfh−/− mice compared with wild-type mice (Fig. 1B). Although large amounts of C3 are present spontaneously in the glomeruli of Cfh−/− mice, quantitation of glomerular C3 after antibody injection clearly demonstrated that further C3 deposition occurred (Fig. 1C). However, no further increase in C3 deposition was evident after 24 h, although median values at 48 h and 6 days after administration of antibody remained significantly greater (P < 0.01) than the 2-h values (Fig. 1C). In contrast, the deposition of mouse IgG and sheep IgG did not differ at any of the time points examined (data not shown). Hence, with this model, the Cfh−/− mice developed excessive glomerular neutrophil numbers associated with further deposition of glomerular C3 and proteinuria.
Fig. 1.
Glomerular neutrophil numbers per glomerular cross section (gcs) (A), albuminuria (B), and glomerular C3 deposition (C) at 2 h, 24 h, 48 h, and 6 days after injection of heterologous nephrotoxic antibody in wild-type (●) and factor H-deficient (○) mice. Intervals in A represent mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01 (Mann–Whitney test). Horizontal bars denote median values.
Heterologous NTN in Factor H-Deficient Mice Lacking C5 or C6.
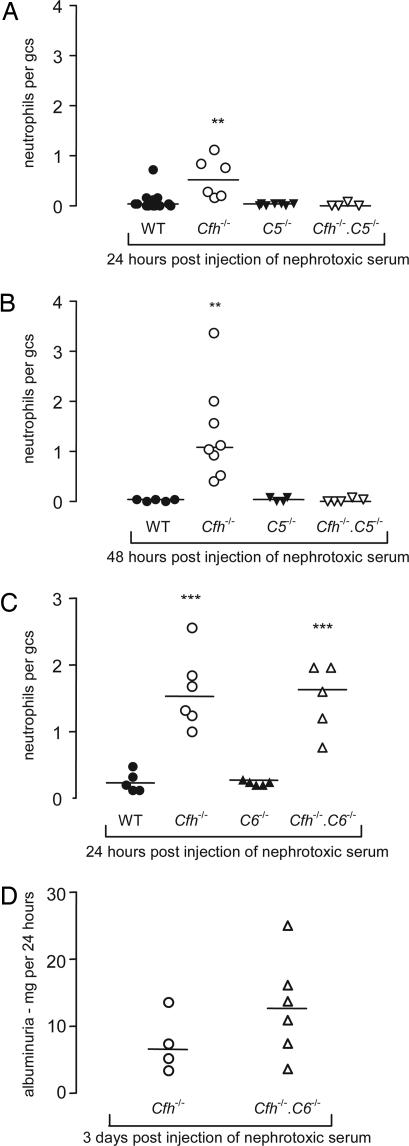
To determine the effect of C5 activation on the response of Cfh−/− mice to heterologous NTN we compared Cfh−/−, Cfh−/−.C5−/−, and C5−/− mice using this model (Fig. 2A and B). At 24 h glomerular neutrophil numbers were significantly lower in the Cfh−/−.C5−/− mice compared with the Cfh−/− mice (P < 0.01) (Fig. 2A). At 48 h, glomerular neutrophil counts remained significantly lower in the Cfh−/−.C5−/− mice compared with the Cfh−/− animals (P < 0.01) (Fig. 2B). Comparable to the 24-h time point data, the glomerular neutrophil counts at 48 h did not differ between the wild-type mice and either the C5−/− mice or the Cfh−/−.C5−/− mice. No difference in glomerular C3 deposition was evident between the Cfh−/− mice and the Cfh−/−.C5−/− mice at either time point (data not shown). We therefore concluded that the enhanced glomerular neutrophil numbers seen in the Cfh−/− mice with this model depended on C5 activation.
Fig. 2.
Heterologous NTN in Cfh−/− mice lacking C5 or C6. Glomerular neutrophil numbers per glomerular cross section (gcs) 24 h (A) and 48 h (B) after injection of heterologous nephrotoxic antibody in Cfh−/−.C5−/− (▿), C5−/− (▾), Cfh−/− (○), and wild-type (●) mice. ∗∗, P < 0.01, Cfh−/− versus wild-type and versus Cfh−/−.C5−/− mice (Bonferroni’s multiple comparison test). Horizontal bars denote median values. (C) Glomerular neutrophil numbers 24 h after injection of heterologous nephrotoxic antibody in Cfh−/−.C6−/− (▵), C6−/−(▴), Cfh−/− (○), and wild-type (●) mice. ∗∗∗, P < 0.001, Cfh−/− versus either wild-type or C6−/− mice; ∗∗∗, P < 0.001, Cfh−/−.C6−/− versus either wild-type or C6−/− mice (Bonferroni’s multiple comparison test). Horizontal bars denote median values. (D) Albuminuria 3 days after injection of heterologous nephrotoxic antibody in Cfh−/−.C6−/− (▵) and Cfh−/− (○) mice. Median albuminuria values did not differ significantly between the two groups. Horizontal bars denote median values.
Deficiency of C5 prevents both the formation of the potent anaphylatoxin C5a and the membrane attack complex (MAC). To determine which of these mechanisms was responsible for reversing the exaggerated response of the Cfh−/− mice to heterologous NTN we assessed glomerular neutrophil numbers and proteinuria in mice deficient in factor H and C6 (Cfh−/−.C6−/−). These animals cannot form the MAC but remain able to generate the C5a anaphylatoxin. At 24 h glomerular neutrophil numbers were significantly increased in both Cfh−/− and Cfh−/−.C6−/− mice compared with either wild-type or C6−/− mice, but the levels between the Cfh−/− mice and Cfh−/−.C6−/− mice did not differ (Fig. 2C). Furthermore, proteinuria, which was present in both Cfh−/− and Cfh−/−.C6−/− mice at day 3 after injection of antibody, did not differ between the two groups. Together with our data in the Cfh−/−.C5−/− mice, these observations indicate that the glomerular neutrophil numbers and proteinuria seen in Cfh−/− mice using this model depend on C5 activation, but are independent of MAC formation and hence likely to be mediated through the generation of C5a.
Heterologous NTN in Factor H-Deficient Mice Treated with Anti-Mouse C5 Antibody.
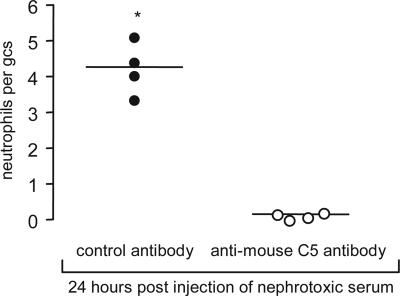
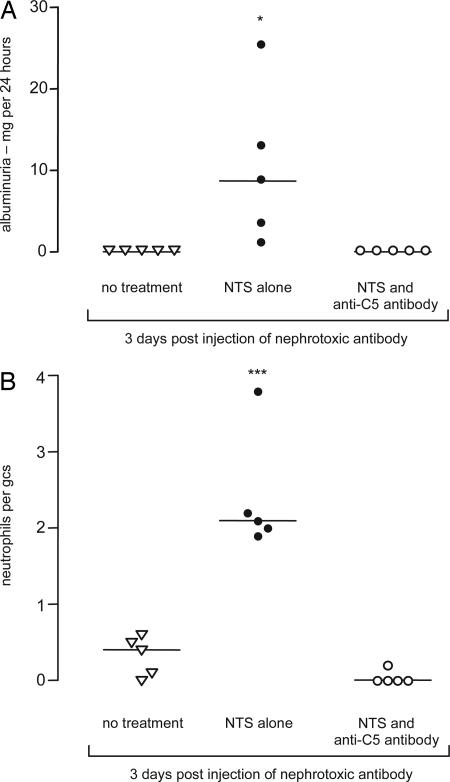
We next examined the effect of blocking C5 activation exogenously using a monoclonal anti-mouse C5 antibody. In this experiment Cfh−/− mice received either 4 mg of anti-C5 antibody or identical amounts of isotype-matched control antibody i.p. 24 h and 4 h before the i.v. injection of nephrotoxic serum. At 24 h anti-C5 antibody-treated Cfh−/− mice had minimal glomerular neutrophil numbers, in striking contrast to the response seen in the Cfh−/− mice treated with control antibody (P < 0.05) (Fig. 3). Consistent with inhibition of C5 activation was the demonstration that the total serum hemolytic activity was significantly reduced in the anti-C5 antibody-treated group (median, 21.3%; range, 6.4–24.8) compared with that seen in the control group (median, 64.3; range, 41.4–73.8) (P < 0.05). We next examined whether the administration of the anti-C5 antibody could prevent the development of proteinuria at day 3 after injection of antibody (Fig. 4). Although significant proteinuria was present at day 3 in the Cfh−/− mice that received nephrotoxic serum alone, treatment with anti-C5 antibody completely prevented the development of proteinuria (Fig. 4A). Furthermore, hematuria was detectable in all of the mice that had received nephrotoxic serum alone, whereas none of the mice that had been treated with anti-C5 antibody developed hematuria (data not shown). Neither proteinuria nor hematuria was detectable in the age-matched unmanipulated Cfh−/− mice. Consistent with our observations at the 24-h time point, glomerular neutrophils were detected only at day 3 in mice that had not received anti-C5 antibody (Fig. 4B). Thus, prevention of C5 activation through the administration of an anti-mouse C5 antibody prevented the development of both glomerular neutrophil influx and proteinuria in Cfh−/− mice during heterologous NTN.
Fig. 3.
Glomerular neutrophil numbers per glomerular cross section (gcs) 24 h after injection of heterologous nephrotoxic antibody in Cfh−/− mice treated with either anti-mouse C5 antibody (○) or isotype-matched control antibody (●). ∗, P < 0.05 versus anti-C5 antibody-treated group (Mann–Whitney test). Horizontal bars denote median values.
Fig. 4.
Albuminuria (A) and glomerular neutrophil (B) numbers per glomerular cross section (gcs) 3 days after injection of heterologous nephrotoxic serum in Cfh−/− animals treated with anti-mouse C5 antibody (○) or mice given nephrotoxic serum alone (●). Unmanipulated age-matched Cfh−/− (▿) mice had no significant proteinuria and very low numbers of glomerular neutrophils. ∗, P < 0.05; and ∗∗∗, P < 0.001 versus anti-CS antibody-treated group (Bonferroni’s multiple comparison test). Horizontal bars denote median values.
Discussion
In this study we first examined the role of C5 activation on the development of spontaneous MPGN in factor H-deficient mice. Because MPGN is evident in Cfh−/− mice by 8 months of age (8) we chose, a priori, to assess the impact of C5 deficiency on MPGN associated with factor H deficiency in 12-month-old Cfh−/− mice where MPGN will have been present for several months. Through the generation of mice deficient in both factor H and C5 we were able to show that, although MPGN still developed, the inability to activate C5 was associated with reduced mortality, glomerular cellularity, and lower serum creatinine levels in comparison to mice deficient in factor H alone. In contrast, proteinuria did not differ between the Cfh−/− and the Cfh−/−.C5−/− mice. Proteinuria in the Cfh−/− mice may result from disruption of the glomerular permeability barrier as a consequence of (i) chronic C3 deposition along the GBM with subsequent morphological changes to basement membrane proteins, (ii) chronic deposition of MAC along the GBM with similar consequences, and (iii) recruitment of acute inflammatory cells due to the generation of C5a. However, the persistence of comparable levels of proteinuria in the Cfh−/−.C5−/− mice indicated that the predominant factor determining spontaneous proteinuria in the Cfh−/− mice is GBM damage secondary to chronic C3 deposition.
Histological analysis demonstrated a significant reduction in glomerular cellularity, specifically glomerular neutrophil numbers, in the Cfh−/−.C5−/− mice. The inability of these mice to generate C5a, a potent anaphylatoxin (29), would be the obvious mechanism to explain this observation. However, C5 deficiency would also prevent MAC formation, and it is well documented that sublytic MAC formation on endothelial cells results in increased expression of adhesion molecules (e.g., intercellular adhesion molecule-1 and E-selectin) (30), production of chemokines interleukin-8 and monocyte chemoattractant protein 1 (31), and release of growth factors from endothelial cells, such as platelet-derived growth factor and basic fibroblast growth factor, which are known to be mitogenic for glomerular mesangial cells (32). Hence, both the inability to form C5a and the MAC may have contributed to the reduction in glomerular cellularity observed in the Cfh−/−.C5−/− mice.
We next examined how Cfh−/− mice would respond to a nephrotoxic insult by using heterologous NTN. After the deposition of heterologous antibody along the GBM, enhanced and sustained glomerular neutrophil infiltration was observed in the Cfh−/− mice together with further deposition of glomerular C3. Previous reports have suggested that heterologous NTN is partially complement-dependent, as is evident from the observation that C3-deficient mice developed less severe disease (33–35). Importantly, our data show that, although the Cfh−/− mice have marked fluid-phase consumption of C3, these animals retained the ability to deposit further C3 within the kidney after the binding of heterologous antibody. This C3 may derive from local C3 production because it is known that human glomerular epithelial cells and mesangial cells can synthesize C3 (36, 37).
We then dissected the factors that predisposed the Cfh−/− animals to this exaggerated phenotype by studying the response to heterologous nephritis in Cfh−/− mice lacking either C5 or C6. Our data showed that the enhanced glomerular neutrophil numbers seen in Cfh−/− mice could be abrogated in the Cfh−/− mice deficient in C5 but not in Cfh−/− mice that were deficient in C6. Similarly, proteinuria in the Cfh−/− mice measured on day 3 after injection of NTS was prevented by antibody-mediated inhibition of C5 activation, but not in mice genetically deficient in C6. These data demonstrate that both glomerular neutrophil influx and subsequent proteinuria depended on formation of C5a but not the formation of the MAC. Neutrophil accumulation during NTN has been shown to involve CR3 (CD18/11b, Mac-1). In CR3-deficient mice, although the initial influx of neutrophils into the glomerulus after injection of heterologous antibody was normal, neutrophil numbers declined rapidly thereafter, and renal injury measured as albuminuria did not occur (38). Thus, in Cfh−/−mice the presence of large amounts of C3 along the GBM may have sustained glomerular neutrophil numbers through interactions with neutrophil CR3.
We next examined the effect of inhibiting C5 activation during experimental nephritis through the administration of a monoclonal anti-murine C5 antibody. Administration of this antibody prevented both glomerular neutrophil accumulation and proteinuria in Cfh−/− mice. The anti-C5 antibody used in our studies has been shown to be effective in reducing proteinuria and renal damage in murine models of lupus nephritis (39, 40). Anti-C5 antibody therapy in humans appears to be safe and well tolerated, with efficacy demonstrated in patients with paroxysmal nocturnal hemoglobinuria (41, 42). However, its efficacy in human glomerular diseases is yet to be established.
C5 deficiency was associated with an improvement in renal histology and function in both spontaneous MPGN and heterologous NTN in Cfh−/− mice. Patients with MPGN type II may remain stable clinically for long periods; however, relapses associated with acute inflammatory changes within the kidney often result in a significant decline in renal function or threaten transplant kidney survival. Increased glomerular neutrophil numbers, often with crescent formation, are important features of MPGN type II (24, 28) and of MPGN type II developing in transplant kidneys in the absence of transplant-related pathology (43). Furthermore, during rapidly progressive disease all glomerular deposits, including paramesangial deposits, react strongly with anti-C5 antibodies (11), suggesting that C5 activation is an important component of renal injury during acute inflammatory episodes. These observations taken together suggest that anti-C5 therapy could be effective in preserving renal function in human MPGN type II. In summary, we have shown that prevention of C5 activation ameliorated both spontaneous MPGN and experimentally induced renal inflammation in Cfh−/− mice. These data provide a basis for investigating the therapeutic effects of anti-C5 therapy in human MPGN type II.
Materials and Methods
Animals.
Factor H-deficient mice were developed as reported (8). Cfh−/− mice deficient in C5 (Cfh−/−.C5−/−) were generated by intercrossing the factor H-deficient mice with commercially available DBA/2 mice (Harlan), which are naturally C5-deficient (44). To control for possible genetic background effects, the Cfh−/− and C5−/− animals used in the spontaneous cohort analysis were littermates of the Cfh−/−.C5−/− mice generated during this intercross. Spontaneous C6-deficient mice on the C3H/HeN C6-genetic background were kindly provided by B. P. Morgan (University of Wales, Cardiff). Cfh−/− mice deficient in C6 (Cfh−/−.C6−/−) were generated by intercrossing the C6-deficient animals with factor H-deficient mice that had been backcrossed onto the C3H/HeN genetic background for 10 generations. For analysis of the effects of anti-C5 antibody treatment the mice used were Cfh−/− mice backcrossed onto the C57BL/6 genetic background for 10 generations. Mice used in the experimental nephritis models were 8–12 weeks of age. All procedures were performed in accordance with institutional guidelines.
Histological Studies.
For light microscopy, kidneys were fixed in Bouin’s solution, and sections were stained with periodic acid Schiff reagent. For immunofluorescence studies kidneys were snap-frozen. Glomerular histology was graded as follows: grade 0, normal; grade I, hypercellularity in 10–25% of the glomeruli; grade II, hypercellularity in 25–50% of glomeruli; grade III, hypercellularity in 50–75% of glomeruli; grade IV, glomerular hypercellularity in >75% or crescents in <25% of glomeruli; grade V, crescents in >25% of glomeruli. Specific changes of MPGN were the presence of GBM double contours and capillary wall thickening. Histological analysis was performed in a blinded fashion, and 50 glomeruli per section were analyzed. FITC-conjugated goat anti-mouse C3 (ICN) and FITC goat anti-mouse IgG (Sigma) were used at dilutions of 1/50 and 1/200 in PBS, respectively. FITC-conjugated goat Igs were used as a control for these two antibodies. Quantitative immunofluorescence studies were performed as described (45), and results are expressed as arbitrary fluorescence units. Electron microscopy was performed as described (8).
Assessment of Renal Function.
Serum creatinine was measured by using an Olympus AU600 analyzer. Mice were placed in metabolic cages for 24 h to allow collection of urine. Urinary albumin was measured by radial immunodiffusion by using a rabbit anti-mouse albumin antibody (Biogenesis, Poole, U.K.) and purified mouse albumin (Sigma) as standards, as described (45).
Induction of Experimental Glomerulonephritis.
For induction of heterologous NTN mice received a single i.v. injection of sheep anti-mouse GBM antibody preparation (nephrotoxic serum). Preparation of nephrotoxic serum has been described (45). Although the same nephrotoxic serum was always administered to each subgroup within an individual experiment, different nephrotoxic serum preparations were used between experiments.
Hemolytic Assay.
In this assay we assessed the ability of mouse sera to replete the hemolytic activity of C5-depleted human sera (Techniclone, Dorking, U.K.). Sensitized sheep erythrocytes (Tissue Culture Sciences, Buckingham, U.K.) were prepared by incubating a 10% red cell suspension with rabbit hemolytic serum (Tissue Culture Sciences). Twenty percent mouse sera and 10% human C5-depleted sera diluted in complement fixation diluent (Oxoid, Basingstoke, U.K.) was incubated with 0.2% sensitized sheep erythrocyte preparation. After incubation red cell lysis was calculated by measuring the OD414 of the supernatant less background lysis (calculated by measuring the OD414 of the supernatant of samples prepared in an identical fashion except for the addition of 0.05 M EDTA to prevent complement activation). Hemolytic activity was expressed as a percentage of 100% lysis (OD414 of the supernatant after incubation of sensitized erythrocytes with water).
C5 Inhibition.
C5 inhibition was achieved by i.p. injection of a murine IgG1 antibody specific for the mouse C5 protein (BB5.1) (46). In these experiments control animals received injections of the isotype-matched antibody HFN7.1. The specificity and production of these antibodies have been detailed (47).
Statistical Analysis.
The Mann–Whitney test was used for comparison of two groups; for analysis of three or more groups Bonferroni’s multiple comparison test was used. Survival curves were analyzed by using the log-rank test. Data were analyzed by using prism 3.0 for Windows (GraphPad, San Diego).
Acknowledgments
We thank Dr. Jill Moss and Ian Shore for their expert help with the electron microscopy work, Margarita Lewis for technical assistance with the processing of histological specimens, J. Meeks for measurement of serum creatinine, and the staff of the Biological Services Unit at Imperial College for the care of the animals involved in this study. M.C.P. is a Wellcome Trust Research Fellow (GR071390). This work was also supported by KidNeeds.
Abbreviations
- GBM
glomerular basement membrane
- MAC
membrane attack complex
- MPGN
membranoproliferative glomerulonephritis
- NTN
nephrotoxic nephritis.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Liszewski M. K., Farries T. C., Lublin D. M., Rooney I. A., Atkinson J. P. Adv. Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 2.Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Proc. Natl. Acad. Sci. USA. 1976;73:3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pangburn M. K., Schreiber R. D., Muller-Eberhard H. J. J. Exp. Med. 1977;146:257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson R. A., Winterborn M. H. Clin. Exp. Immunol. 1981;46:110–119. [PMC free article] [PubMed] [Google Scholar]
- 5.Levy M., Halbwachs-Mecarelli L., Gubler M. C., Kohout G., Bensenouci A., Niaudet P., Hauptmann G., Lesavre P. Kidney Int. 1986;30:949–956. doi: 10.1038/ki.1986.278. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Larrea C., Dieguez M. A., Enguix A., Dominguez O., Marin B., Gomez E. Biochem. Soc. Trans. 1987;15:648–649. [Google Scholar]
- 7.Hogasen K., Jansen J. H., Mollnes T. E., Hovdenes J., Harboe M. J. Clin. Invest. 1995;95:1054–1061. doi: 10.1172/JCI117751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickering M. C., Cook H. T., Warren J., Bygrave A. E., Moss J., Walport M. J., Botto M. Nat. Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 9.Rennke H. G. Kidney Int. 1995;47:643–656. doi: 10.1038/ki.1995.82. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y., Vernier R. L., Fish A. J., Michael A. F. Lab. Invest. 1979;40:474–480. [PubMed] [Google Scholar]
- 11.West C. D., Witte D. P., McAdams A. J. Am. J. Kidney Dis. 2001;37:1120–1130. doi: 10.1053/ajkd.2001.24511. [DOI] [PubMed] [Google Scholar]
- 12.Falk R. J., Dalmasso A. P., Kim Y., Tsai C. H., Scheinman J. I., Gewurz H., Michael A. F. J. Clin. Invest. 1983;72:560–573. doi: 10.1172/JCI111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger J., Galle P. J. Urol. Nephrol. 1962;68:116–122. [PubMed] [Google Scholar]
- 14.Varade W. S., Forristal J., West C. D. Am. J. Kidney Dis. 1990;16:196–206. doi: 10.1016/s0272-6386(12)81018-3. [DOI] [PubMed] [Google Scholar]
- 15.Daha M. R., Fearon D. T., Austen K. F. J. Immunol. 1976;116:1–7. [PubMed] [Google Scholar]
- 16.Marder H. K., Coleman T. H., Forristal J., Beischel L., West C. D. Kidney Int. 1983;23:749–758. doi: 10.1038/ki.1983.89. [DOI] [PubMed] [Google Scholar]
- 17.Linshaw M. A., Stapleton F. B., Cuppage F. E., Forristal J., West C. D., Schreiber R. D., Wilson C. B. Am. J. Nephrol. 1987;7:470–477. doi: 10.1159/000167525. [DOI] [PubMed] [Google Scholar]
- 18.Meri S., Koistinen V., Miettinen A., Tornroth T., Seppala I. J. J. Exp. Med. 1992;175:939–950. doi: 10.1084/jem.175.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards A. O., Ritter R., III, Abel K. J., Manning A., Panhuysen C., Farrer L. A. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 20.Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert J. R., et al. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 21.Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., Sangiovanni J. P., Mane S. M., Mayne S. T., et al. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hageman G. S., Anderson D. H., Johnson L. V., Hancox L. S., Taiber A. J., Hardisty L. I., Hageman J. L., Stockman H. A., Borchardt J. D., Gehrs K. M., et al. Proc. Natl. Acad. Sci. USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appel G. B., Cook H. T., Hageman G., Jennette J. C., Kashgarian M., Kirschfink M., Lambris J. D., Lanning L., Lutz H. U., Meri S., et al. J. Am. Soc. Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 24.Habib R., Gubler M. C., Loirat C., Maiz H. B., Levy M. Kidney Int. 1975;7:204–215. doi: 10.1038/ki.1975.32. [DOI] [PubMed] [Google Scholar]
- 25.Cameron J. S., Turner D. R., Heaton J., Williams D. G., Ogg C. S., Chantler C., Haycock G. B., Hicks J. Am. J. Med. 1983;74:175–192. doi: 10.1016/0002-9343(83)90606-x. [DOI] [PubMed] [Google Scholar]
- 26.Galle P., Hinglais N., Crosnier J. Transplant. Proc. 1971;3:368–370. [PubMed] [Google Scholar]
- 27.Kaplan M. Curr. Opin. Invest. Drugs. 2002;3:1017–1023. [PubMed] [Google Scholar]
- 28.Vargas R., Thomson K. J., Wilson D., Cameron J. S., Turner D. R., Gill D., Chantler C., Ogg C. S. Clin. Nephrol. 1976;5:73–82. [PubMed] [Google Scholar]
- 29.Gerard N. P., Gerard C. Nature. 1991;349:614–617. doi: 10.1038/349614a0. [DOI] [PubMed] [Google Scholar]
- 30.Kilgore K. S., Shen J. P., Miller B. F., Ward P. A., Warren J. S. J. Immunol. 1995;155:1434–1441. [PubMed] [Google Scholar]
- 31.Kilgore K. S., Flory C. M., Miller B. F., Evans V. M., Warren J. S. Am. J. Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- 32.Benzaquen L. R., Nicholson-Weller A., Halperin J. A. J. Exp. Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert M. J., Takano T., Papayianni A., Rennke H. G., Minto A., Salant D. J., Carroll M. C., Brady H. R. Nephrol. Dial. Transplant. 1998;13:2799–2803. doi: 10.1093/ndt/13.11.2799. [DOI] [PubMed] [Google Scholar]
- 34.Sheerin N. S., Springall T., Carroll M. C., Hartley B., Sacks S. H. Clin. Exp. Immunol. 1997;110:403–409. doi: 10.1046/j.1365-2249.1997.4261438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheerin N. S., Springall T., Abe K., Sacks S. H. Eur. J. Immunol. 2001;31:1255–1260. doi: 10.1002/1521-4141(200104)31:4<1255::aid-immu1255>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Sacks S., Zhou W., Campbell R. D., Martin J. Clin. Exp. Immunol. 1993;93:411–417. doi: 10.1111/j.1365-2249.1993.tb08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacks S. H., Zhou W., Pani A., Campbell R. D., Martin J. Immunology. 1993;79:348–354. [PMC free article] [PubMed] [Google Scholar]
- 38.Tang T., Rosenkranz A., Assmann K. J., Goodman M. J., Gutierrez-Ramos J. C., Carroll M. C., Cotran R. S., Mayadas T. N. J. Exp. Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., Hu Q., Madri J. A., Rollins S. A., Chodera A., Matis L. A. Proc. Natl. Acad. Sci. USA. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravirajan C. T., Wang Y., Matis L. A., Papadaki L., Griffiths M. H., Latchman D. S., Isenberg D. A. Rheumatology (Oxford) 2004;43:442–447. doi: 10.1093/rheumatology/keh083. [DOI] [PubMed] [Google Scholar]
- 41.Hill A., Hillmen P., Richards S. J., Elebute D., Marsh J. C., Chan J., Mojcik C. F., Rother R. P. Blood. 2005;106:2559–2565. doi: 10.1182/blood-2005-02-0564. [DOI] [PubMed] [Google Scholar]
- 42.Hillmen P., Hall C., Marsh J. C., Elebute M., Bombara M. P., Petro B. E., Cullen M. J., Richards S. J., Rollins S. A., Mojcik C. F., Rother R. P. N. Engl. J. Med. 2004;350:552–559. doi: 10.1056/NEJMoa031688. [DOI] [PubMed] [Google Scholar]
- 43.Andresdottir M. B., Assmann K. J., Hoitsma A. J., Koene R. A., Wetzels J. F. Nephrol. Dial. Transplant. 1999;14:1723–1731. doi: 10.1093/ndt/14.7.1723. [DOI] [PubMed] [Google Scholar]
- 44.Wetsel R. A., Fleischer D. T., Haviland D. L. J. Biol. Chem. 1990;265:2435–2440. [PubMed] [Google Scholar]
- 45.Robson M. G., Cook H. T., Botto M., Taylor P. R., Busso N., Salvi R., Pusey C. D., Walport M. J., Davies K. A. J. Immunol. 2001;166:6820–6828. doi: 10.4049/jimmunol.166.11.6820. [DOI] [PubMed] [Google Scholar]
- 46.Frei Y., Lambris J. D., Stockinger B. Mol. Cell. Probes. 1987;1:141–149. doi: 10.1016/0890-8508(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 47.Peng T., Hao L., Madri J. A., Su X., Elias J. A., Stahl G. L., Squinto S., Wang Y. J. Clin. Invest. 2005;115:1590–1600. doi: 10.1172/JCI22906. [DOI] [PMC free article] [PubMed] [Google Scholar]