Abstract
The Nck family of Src homology (SH) 2/SH3 domain adaptors functions to link tyrosine phosphorylation induced by extracellular signals with downstream regulators of actin dynamics. We investigated the role of mammalian Nck adaptors in signaling from the activated platelet-derived growth factor (PDGF) receptor (PDGFβR) to the actin cytoskeleton. We report here that Nck adaptors are required for cytoskeletal reorganization and chemotaxis stimulated by PDGF-B. Analysis of tyrosine-phosphorylated proteins demonstrated that Crk-associated substrate (p130Cas), not the activated PDGFβR itself, is the major Nck SH2 domain-binding protein in PDGF-B-stimulated cells. Both Nck- and p130Cas-deficient cells fail to display cytoskeletal rearrangements, including the formation of membrane ruffles and the disassembly of actin bundles, typically shown by their WT counterparts in response to PDGF-B. Furthermore, Nck and p130Cas colocalize in phosphotyrosine-enriched membrane ruffles induced by PDGF-B in NIH 3T3 cells. These results suggest that Nck adaptors play an essential role in linking the activated PDGFβR with actin dynamics through a pathway that involves p130Cas.
Keywords: Crk-associated substrate, actin cytoskeleton, SH2 domain, SH3 domain, tyrosine phosphorylation
Cell motility is critically important in developmental processes and in the pathogenesis of a variety of diseases. Remodeling of the actin cytoskeleton, i.e., the dynamic assembly and disassembly of filamentous actin, governs essential aspects of cell motility such as the formation of membrane protrusions and cell adhesion to other cells or to the substrate (1). Extracellular signals can induce remodeling of the actin cytoskeleton through changes in tyrosine phosphorylation. For example, ligand-induced dimerization of platelet-derived growth factor (PDGF) receptors (PDGFβR) stimulates their intrinsic kinase activity, leading to the autophosphorylation in trans of multiple intracellular tyrosine residues on the receptor (2, 3). This event results in the creation of phosphotyrosine docking sites for Src homology (SH) 2 domain-containing signaling molecules (4). Cellular effects mediated by signaling pathways activated by PDGFβR involve proliferation, survival, actin reorganization, and migration (3). Despite extensive efforts aimed at characterizing effectors that associate with PDGFβR (2), the dynamic assembly of signaling complexes induced by the activation of this receptor and their relation to specific cellular responses are still poorly understood.
Nck, a two-gene family in mammals, is an important link in transducing signals from tyrosine phosphorylation to the cytoskeleton (5, 6). Nck proteins (termed Nck, Nckα or Nck1, and Nckβ or Nck2) consist of three N-terminal SH3 domains followed by a single C-terminal SH2 domain. The SH2 domain of Nck can bind a number of activated receptor tyrosine kinases and tyrosine-phosphorylated docking proteins; on the other hand, the Nck SH3 domains engage proline-rich binding sites on a host of effectors implicated in cytoskeletal regulation, including members of the WASp/Scar family (reviewed in refs. 5 and 6). Nck adaptors have been shown to play an important role during embryogenesis potentially linked to cell motility and cytoskeletal organization (7).
Although circumstantial evidence suggests that the Nck adaptors can interact with the activated PDGFβR (8–10), their role in signaling to the actin cytoskeleton downstream of this receptor remains largely unknown. Here we report that Nck adaptors are strictly required for cytoskeletal reorganization and chemotaxis stimulated by PDGF-B. Furthermore, we provide mechanistic insights suggesting that Nck adaptors transduce signals downstream of the activated PDGFβR by an indirect mechanism involving the scaffolding protein p130Cas.
Results
Nck Adaptors Are Required for Cytoskeletal Changes Induced by PDGF-B.
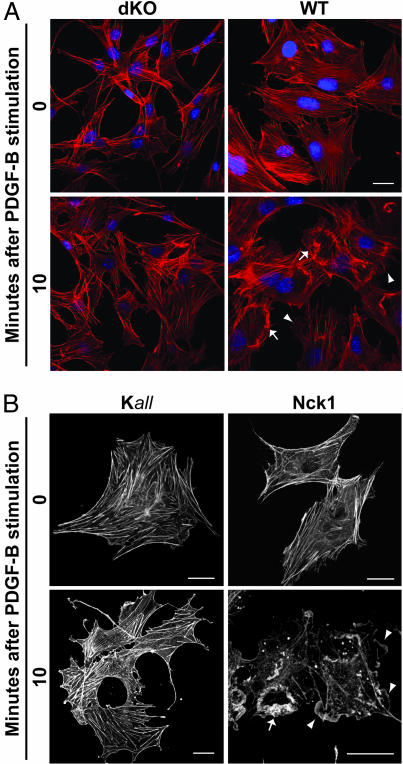
To determine the significance of Nck adaptors in cytoskeletal changes induced by PDGF-B, serum-starved, Nck-deficient (both Nck genes inactivated; dKO), and WT mouse embryonic fibroblasts (MEFs) were left untreated or were stimulated with PDGF-B. Profound remodeling of the cytoskeleton, including the disassembly of actin bundles and the formation of various types of membrane protrusions, occurred in WT but not dKO cells after PDGF-B stimulation (Fig. 1A and Fig. 7A, which is published as supporting information on the PNAS web site). Occurrence of “peripheral” or “dorsal” ruffles (11) was compared between the genotypes. Whereas dKO cells remained mostly in a “quiescent” state, the WT cells, in contrast, showed a dramatic increase in peripheral and dorsal ruffling after PDGF-B stimulation (Fig. 7B). These observations suggested a critical role for Nck adaptors in transducing signals from the activated PDGFβR to the actin cytoskeleton.
Fig. 1.
Requirement of Nck in actin rearrangements induced by PDGF-B. Shown are confocal images of serum-starved MEFs left untreated (time 0) or stimulated with PDGF-B for 10 min. (A) Actin rearrangements in Nck-deficient (dKO) and WT cells fixed and stained with Texas red phalloidin and Hoechst 33342 to visualize the actin cytoskeleton and nuclei, respectively. (B) Cytoskeletal rearrangements in Nck-deficient cells coexpressing actin-GFP and WT (Nck1) or a loss-of-function mutant Nck (Kall). Arrows and arrowheads indicate dorsal and peripheral ruffles, respectively. (Scale bars: 25 μm.)
Expression of Nck Rescues the Response to PDGF-B in Nck-Deficient Cells.
Given the striking contrast in the response to PDGF-B stimulation of WT vs. dKO cells, we next tested whether the ectopic expression of Nck1 or Nck2 in dKO cells could rescue the responsiveness to PDGF-B. Cells were cotransfected with actin-GFP to visualize cytoskeletal changes and a vector expressing Nck1, Nck2, a loss-of-function mutant Nck1 (with inactivating mutation of all three SH3 domains; Kall), or empty vector (EBB). No major cytoskeletal changes were observed after PDGF-B stimulation in serum-starved dKO cells cotransfected with a loss-of-function mutant Nck1 (Fig. 1B, Kall) or with the empty vector (EBB in Fig. 8A, which is published as supporting information on the PNAS web site). In contrast, actin bundles disassembled and peripheral and dorsal ruffles formed in cells transfected with either Nck1 (Fig. 1B) or Nck2 (Fig. 8 A and B) after PDGF-B stimulation. To further evaluate the role of Nck in cytoskeletal dynamics induced by PDGF-B, we performed live-cell imaging of Nck-deficient cells cotransfected with actin-GFP and either empty vector or Nck1. As shown in Movie 1, which is published as supporting information on the PNAS web site, dKO cotransfected with the empty vector (EBB) showed a very stable cytoskeleton. Conversely, cells cotransfected with Nck1 exhibited formation of very dynamic peripheral (Movie 2, which is published as supporting information on the PNAS web site) and dorsal (Movie 3, which is published as supporting information on the PNAS web site) membrane ruffles after PDGF-B stimulation. Thus, these results demonstrate that either Nck1 or Nck2 is required for PDGF-B-stimulated actin rearrangements. Importantly, phosphorylation of the PDGFβR and activation of p42/44Erk, cell proliferation-related signaling molecules, was not affected in the absence of Nck adaptors (see below). Actin dynamics is the cellular function most profoundly altered in the absence of Nck adaptors.
Lack of Nck Adaptors Leads to Impaired Chemotactic and Haptotactic Responses.
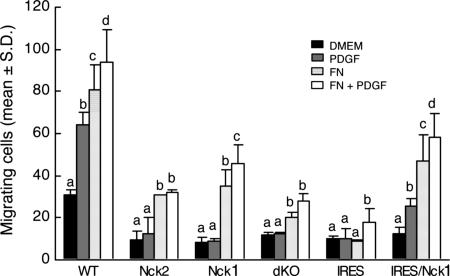
We took advantage of the availability of MEFs of various genotypes and compared their ability to migrate using a conventional transwell migration assay. Nck-deficient (dKO) cells had a readily apparent migration disadvantage compared with their WT counterparts (Fig. 9, which is published as supporting information on the PNAS web site). As shown in Fig. 2, more WT MEFs migrated in the presence of PDGF-B, fibronectin, or both than in starvation medium alone (P < 0.001). In contrast, the lack of either Nck1 or Nck2 abrogated the chemotactic response to PDGF (P > 0.05). The haptotactic response to fibronectin was severely compromised in MEFs with inactivation of both Nck genes (dKO and IRES). However, more (P < 0.001) MEFs with only one of the two Nck genes migrated in the presence of fibronectin than in the presence of medium alone. Interestingly, Nck-deficient MEFs rescued with a retrovirus expressing Nck (expression levels ≈3-fold higher than in WT cells) showed a slight, but significant (P < 0.01), increase in migration in response to PDGF and/or fibronectin compared with cells exposed to medium alone. These results strongly suggest that both Nck genes are required for normal directed cell motility and that Nck1 and Nck2 are at least partially redundant.
Fig. 2.
Nck deficiency impairs PDGF-B- and fibronectin-stimulated cell migration. Data show the number of migrating cells per microscopic field. Bars represent means ± SD from eight pictures taken at random from each treatment combination in each of two independent experiments. Genotypes compared are WT, Nck1−/− Nck2−/+ (Nck2), Nck1−/+ Nck2−/− (Nck1), Nck−/− Nck2−/− (dKO), and Nck-deficient cells rescued with an empty retroviral vector (IRES) or a retroviral vector expressing Nck (IRES/Nck1). Within each genotype, means with a different letter over the bar are different (P < 0.01).
Profile of Tyrosine Phosphorylation in Response to Activation of PDGFβR.
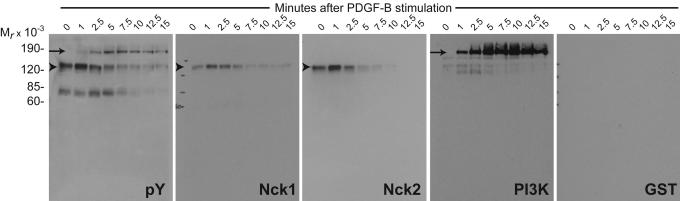
To begin to understand the mechanism by which Nck adaptors link signaling from the activated PDGFβR to the cytoskeleton, we examined tyrosine-phosphorylated proteins in cell lysates obtained from serum-starved NIH 3T3 cells left untreated or stimulated with PDGF-B. Western blot analysis using an antibody against phosphotyrosine (Fig. 3, pY) showed that three major proteins of ≈185, 130, and 65 kDa underwent changes in their phosphotyrosine levels in association with PDGF-B stimulation. We also determined the pattern of SH2-binding proteins by far-Western blot analysis using GST fusions of the isolated SH2 domain from Nck1, Nck2, or phosphatidylinositol 3-kinase (PI3K) or GST alone as a control (Fig. 3). A band of ≈185 kDa, the PDGFβR, was clearly observed in pY and PI3K blots soon after PDGF-B stimulation but was undetectable in lysates of cells left untreated. Noticeably, this band was absent in filters probed with SH2 domains from Nck1 and Nck2, indicating that the activated PDGFβR is not efficiently bound directly by Nck adaptors. In contrast, a single major band of ≈130 kDa was consistently detected in membranes probed with Nck1 and Nck2 SH2 domains; this band was not seen in the PI3K SH2 blot. The ≈130-kDa band showed low but detectable levels of tyrosine phosphorylation in lysates from serum-starved, unstimulated cells and a modest (≈3- to 5-fold) increase in lysates obtained soon after PDGF-B stimulation. Interestingly, the ≈130-kDa band exhibited a dynamic, biphasic pattern of phosphorylation, with a rapid increase soon after PDGF-B stimulation (2–5 min) followed by an equally rapid and sustained decrease thereafter to a level lower than that in starved cells (Figs. 3 and 4 and Figs. 10 and 11, which are published as supporting information on the PNAS web site). Because the ≈130-kDa Nck-interacting protein is likely to mediate Nck recruitment to sites of actin rearrangements, we sought to determine its identity in subsequent experiments.
Fig. 3.
Pattern of tyrosine phosphorylation in response to activation of PDGFβR. Lysates from NIH 3T3 cells left untreated (time 0) or stimulated with PDGF-B for various intervals were transferred to membranes and probed with anti-pTyr antibody (pY) or subjected to far-Western blot analysis using GST fusions of the isolated SH2 domain from Nck1 (Nck1), Nck2 (Nck2), or PI3K p85 subunit and GST alone as a control. Apparent molecular weights (Mr × 10−3) of standards are indicated. Arrows indicate a band corresponding to the activated PDGFβR, and arrowheads indicate a band of ≈130 kDa in size that binds Nck SH2 domains. Similar kinetics of tyrosine phosphorylation were observed in three independent experiments.
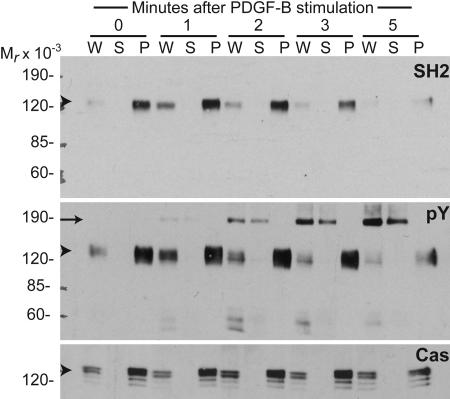
Fig. 4.
p130Cas is the major tyrosine-phosphorylated protein that binds Nck in cells stimulated with PDGF-B. Lysates obtained from NIH 3T3 cells left untreated (time 0) or stimulated with PDGF-B for various intervals were immunoprecipitated with polyclonal antibodies against p130Cas. Whole-cell lysates (W) and supernatant (S) and pellet (P) fractions were subjected to SDS/PAGE, and proteins were transferred to nitrocellulose filters. Pellet fractions represent five times more lysate than whole-cell lysate or supernatant fractions. Membranes were probed with GST fusions of the isolated SH2 domain from Nck1 (SH2) in a far-Western blot or with monoclonal anti-phosphotyrosine (pY) or anti-p130Cas (Cas) antibodies in Western blot analysis. Apparent molecular weights (Mr × 10−3) are indicated. Arrowheads indicate a band of ≈130 kDa in size corresponding to p130Cas, and the arrow indicates the PDGFβR.
p130Cas Is the Major Tyrosine-Phosphorylated Protein That Binds Nck in PDGF-B-Stimulated Cells.
We investigated the identity of the ≈130-kDa Nck SH2-binding protein by immunoprecipitation. Focal adhesion kinase (Fak) and p130Cas were likely candidates based on their molecular weights and previous work suggesting possible association with Nck (12, 13). Lysates from serum-starved NIH 3T3 cells were subjected to immunoprecipitation with polyclonal anti-p130Cas (Fig. 4) or anti-Fak antibodies (Fig. 10). Immunoprecipitation with anti-Cas almost completely cleared the ≈130-kDa Nck SH2-binding protein from the lysates. In contrast, only a minor fraction of the ≈130-kDa protein was cleared from the lysates by immunoprecipitation with anti-Fak, and this fraction did not bind Nck SH2 domains (Fig. 10). Western blot analysis of the same IP:Cas (Fig. 4) and IP:Fak (Fig. 10) filters (IP, immunoprecipitation) stripped and reprobed with specific antibodies against p130Cas (Cas) and Fak (Fak), respectively, demonstrated the specificity of the immunoprecipitation.
The identity of the ≈130-kDa Nck SH2 domain-binding protein as p130Cas was confirmed by comparing the pattern of tyrosine-phosphorylated proteins in Cas-deficient and WT MEFs or NIH 3T3 cells. As shown in Fig. 11, tyrosine phosphorylation of the ≈130-kDa Nck SH2 domain-binding protein was absent in samples from Cas-deficient cells, whereas it increased after PDGF-B stimulation in cells containing p130Cas. Taken together, these results show unambiguously that p130Cas is the major tyrosine-phosphorylated protein that can bind Nck SH2 domains in cells stimulated with PDGF-B.
p130Cas Is Required for Cytoskeletal Changes Induced by PDGF-B.
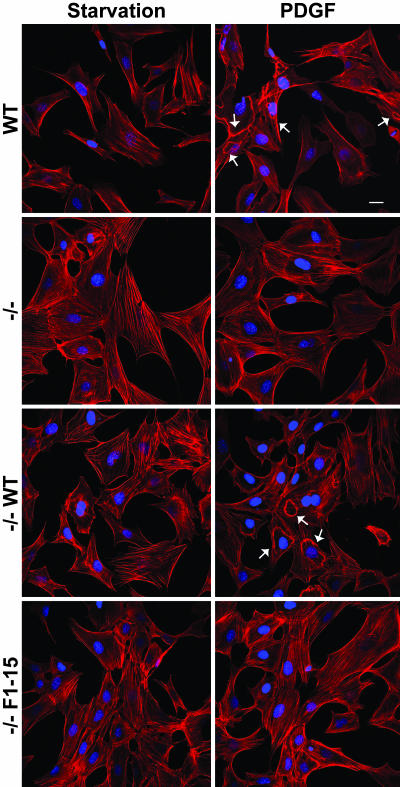
The striking finding that p130Cas is the major Nck SH2 domain-binding protein in fibroblasts stimulated with PDGF-B led us to test the hypothesis that cells lacking p130Cas, or expressing p130Cas lacking the Nck SH2 binding sites, would fail to undergo cytoskeletal rearrangements in response to PDGF-B. Accordingly, we compared the PDGF-responsiveness of WT vs. p130Cas-deficient MEFs or p130Cas-deficient MEFs rescued with a retroviral vector expressing either WT p130Cas or a phosphorylation-defective mutant (Y→F substitutions of the 15 YXXP tyrosine residues of the substrate domain, which include the Nck SH2 binding sites; ref. 14). After PDGF-B stimulation, major cytoskeletal changes, including the formation of membrane protrusions, were readily apparent in WT and p130Cas-deficient MEFs reexpressing WT p130Cas (Fig. 5). In contrast, the formation of membrane protrusions induced by PDGF-B was severely compromised in p130Cas-deficient and p130Cas-deficient cells reexpressing the phosphorylation-defective p130Cas mutant, although a few cells displayed small foci of actin on the dorsal surface resembling disorganized dorsal ruffles. Quantitative analysis showed that ≈50% of the WT and p130Cas-deficient MEFs reexpressing WT p130Cas formed readily distinguishable dorsal ruffles in response to PDGF-B stimulation whereas only ≈10–15% of the p130Cas-deficient and p130Cas-deficient cells reexpressing the phosphorylation-defective p130Cas mutant showed actin foci on the dorsal surface (Fig. 12, which is published as supporting information on the PNAS web site).
Fig. 5.
p130Cas is required for membrane ruffling induced by PDGF-B stimulation. Shown are confocal images of WT, p130Cas-deficient (−/−), and p130Cas-deficient MEFs reexpressing either WT (−/− WT) or a phosphorylation-deficient mutant (−/− F1–15) p130Cas. Cells were left untreated (Starvation) or were stimulated with 30 ng/ml PDGF-B. Arrows indicate dorsal ruffles. (Scale bar: 25 μm.)
Nck and p130Cas Colocalize in Phosphotyrosine-Enriched Membrane Ruffles Induced by PDGF-B.
We first investigated the effects of PDGF-B stimulation on the subcellular distribution of GFP fusions of either full-length Nck1 or its isolated SH2 domain in Nck-deficient MEFs or NIH 3T3 cells. In Nck-deficient cells, full-length Nck1-GFP was recruited to structures resembling peripheral and dorsal ruffles (Fig. 13A Upper, which is published as supporting information on the PNAS web site). Consistent with the failure of the Nck SH3 domain mutant (Fig. 1B, Kall) to rescue the response to PDGF-B, no structures resembling peripheral or dorsal ruffles were detected in dKO cells transfected with Nck1 SH2-GFP after PDGF-B stimulation (Fig. 13A Lower). In contrast, the GFP-Nck1 SH2 domain fusion localized strongly to membrane ruffles induced by PDGF-B in NIH 3T3 cells where endogenous Nck is present (Fig. 13B). This observation suggested that Nck recruitment depends on SH2-domain-mediated interactions with tyrosine-phosphorylated proteins enriched at sites of actin polymerization.
Next we analyzed by immunofluorescence the subcellular distribution of tyrosine-phosphorylated proteins and Nck in relation to F-actin structures in NIH 3T3 cells. We found that both endogenous p130Cas and Nck colocalized to actin structures induced by PDGF-B stimulation in NIH 3T3 fibroblasts (Fig. 6). In Nck-deficient and NIH 3T3 cells, respectively, ectopically expressed (Fig. 13C) and endogenous (Fig. 14B, which is published as supporting information on the PNAS web site) Nck displayed a diffuse cytoplasmic distribution under serum starvation and localized strongly to PDGF-B-induced membrane ruffles. In serum-starved NIH 3T3 cells, tyrosine-phosphorylated proteins accumulated in foci at the cell periphery, presumably sites of cell-substrate adhesion that coincided with tips of actin bundles. In sharp contrast, tyrosine-phosphorylated proteins accumulated at the edges of membrane ruffles in PDGF-B-stimulated cells, and, consistent with the disassembly of actin bundles, the foci of phosphotyrosine proteins at the cell periphery were no longer observed (Fig. 14A). Taken together, these results suggest that PDGF-stimulated accumulation of tyrosine-phosphorylated proteins recruits Nck to the membrane through SH2 domain-mediated interactions, where it colocalizes with p130Cas at sites of active actin polymerization.
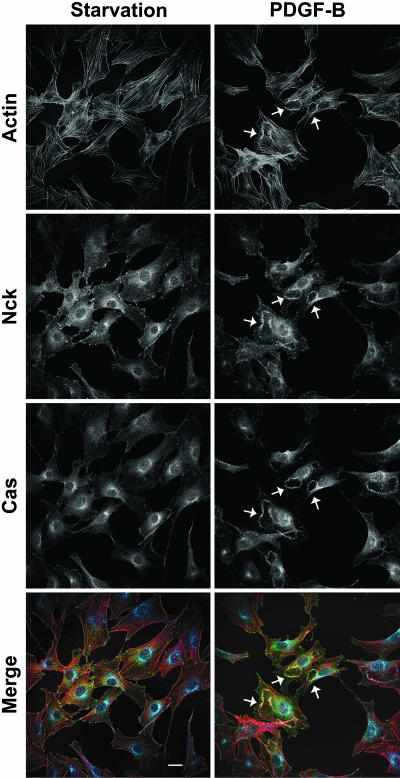
Fig. 6.
Recruitment of endogenous Nck and p130Cas to membrane ruffles induced by PDGF-B. Shown are confocal images of serum-starved NIH 3T3 fibroblasts left untreated (Starvation) or stimulated with PDGF for 10 min (PDGF-B). Fixed cells were stained with Texas red phalloidin and anti-Nck and anti-p130Cas antibodies. In the merged images, filamentous actin, Nck, and p130Cas are colored red, green, and blue, respectively. Arrows indicate dorsal ruffles. (Scale bar: 25 μm.)
Actin Dynamics Is the Cellular Function Most Profoundly Altered in the Absence of Nck Adaptors.
To analyze whether other aspects of PDGFβR signaling were affected in the absence of Nck adaptors, we compared the overall patterns of tyrosine phosphorylation, p42/44Erk activation, and p130Cas phosphorylation of Nck-deficient vs. Nck-deficient cells rescued with Nck1 (Fig. 15, which is published as supporting information on the PNAS web site). The overall pattern of tyrosine phosphorylation did not differ significantly between dKO and IRES/Nck1 cells. Importantly, the expression and functionality of PDGFβR are not affected in cells lacking Nck adaptors, as evidenced by similar patterns of PDGFβR autophosphorylation in dKO and IRES/Nck1 cells (pY blot). Furthermore, PDGF-B-dependent activation of the cell proliferation-related signaling molecules p42/44Erk did not differ between dKO and IRES/Nck1 cells.
Tyrosine phosphorylation of proteins in the ≈120- to 130-kDa range showed similar basal levels under starvation conditions and an ≈3- to 5-fold increase after PDGF-B stimulation (Fig. 15). In IRES/Nck1 cells, tyrosine phosphorylation of these proteins followed a biphasic pattern that resembled that observed in WT NIH 3T3 cells. Interestingly, in cells lacking Nck adaptors (dKO cells), phosphorylation of proteins in the ≈120- to 130-kDa range appeared to increase linearly after PDGF-B stimulation (i.e., down-regulation was not apparent in this time scale). Last, the phosphorylation of p130Cas in response to PDGF-B increased to the same extent, as detected by far-Western blotting with SH2 domains from Nck, in dKO and IRES/Nck1 cells (Fig. 15, SH2 blot). Taken together, these observations suggest that actin dynamics, but not other aspects of PDGF signaling, is the cellular function most profoundly altered in the absence of Nck adaptors.
Discussion
Using a combination of genetics, cell biology, and biochemistry we have uncovered an essential role of Nck adaptors in linking tyrosine phosphorylation induced by activation of the PDGFβR, remodeling of the actin cytoskeleton, and directed cell motility. Furthermore, our studies provide mechanistic insights suggesting that Nck adaptors transduce signals to the cytoskeleton downstream of the PDGFβR through a signaling pathway that involves p130Cas.
Inactivation of both Nck1 and Nck2 results in profound defects in development of mesoderm-derived tissues and the formation of the lamellipodial actin meshwork leading to embryonic lethality (7). Here we report that MEFs lacking functional Nck proteins fail to show the typical cytoskeletal changes observed in their WT counterparts in response to PDGF-B stimulation and have seriously compromised directional motility. Importantly, the responsiveness to PDGF-B in Nck-deficient cells was rescued by ectopic expression of WT Nck1 or Nck2 but not loss-of-function mutants. These results are entirely consistent with an essential role of Nck adaptors in promoting the dynamic assembly of actin in response to tyrosine kinase signals and are in agreement with previous studies demonstrating that the Nck/Dock adapter protein is required in photoreceptor axon guidance and target recognition in the Drosophila visual system (reviewed in ref. 15).
One of the potential mechanisms by which Nck adaptors could couple to the PDGFβR is by direct binding to autophosphorylated docking sites induced on the receptor upon ligand-mediated activation. Indeed, it has been shown that Nck1 (8) and Nck 2 (10) can bind in vitro, through SH2 domain-mediated interactions, to phosphotyrosine residues (Tyr-751 and Tyr-1009, respectively) in the ligand-activated PDGFβR. Our data, however, support a model in which Nck proteins could couple to PDGFβR through an indirect mechanism. We used an SH2 domain-based far-Western blot analysis (16) to determine Nck SH2 domain-binding partners in lysates obtained from serum-starved and PDGF-B-stimulated cells. SH2 domains from both Nck1 and Nck2 consistently detected a single band of ≈130 kDa that was unambiguously identified as p130Cas. In contrast, an ≈190-kDa species, presumably the activated PDGFβR, was readily detected by anti-pY and PI3K SH2 domains but not Nck SH2 domains. In previous studies (8) the presence of tyrosine-phosphorylated proteins in addition to the PDGFβR in GST-Nck pull-down fractions was not investigated, and thus the possibility of an indirect interaction was not ruled out.
Crk-associated substrate (p130Cas) is implicated in cytoskeletal regulation and cell migration, forms a complex with cell-matrix adhesion-associated proteins, and is phosphorylated upon integrin receptor engagement (17). Integrin receptor clustering by fibronectin promoted p130Cas phosphorylation via Fak/Src and the formation of a complex between phosphorylated p130Cas and Nck (12). Consistent with the PDGF-B-stimulated phosphorylation of p130Cas previously reported (18), our results point to a complex between p130Cas and Nck as a critical node in the signaling network linking the activated PDGFβR with the actin cytoskeleton. Several converging observations lend support to this notion: (i) the rapid increase (3- to 5-fold) in phosphorylation of p130Cas after stimulation with PDGF-B; (ii) the change in subcellular distribution of endogenous p130Cas and Nck from cytoplasmic/focal adhesion-associated in quiescent cells to the phosphotyrosine-enriched membrane ruffles induced by PDGF-B; (iii) the preferential binding of Nck SH2 domain to p130Cas in far-Western blot analysis; (iv) the strikingly similar cytoskeletal phenotypes, i.e., the absence of actin rearrangements after PDGF-B stimulation, in Nck- and p130Cas-deficient cells; and (v) the inability of a phosphorylation-defective mutant of p130Cas (which cannot bind Nck SH2 domains) to rescue the response to PDGF-B in p130Cas-deficient cells. Interestingly, unpublished work from our laboratory shows that specific down-regulation by small interfering RNA interference of Crk adaptors (Crk and Crk L), strong binding partners of p130Cas, does not alter membrane ruffling induced by PDGF-B stimulation. This finding is consistent with our observation of increased phosphorylation of Crk II and L, which renders them in an inactive conformation, after PDGF-B stimulation. Thus, the above observations suggest that a molecular complex involving p130Cas and Nck, but not Crk adaptors, is a critical link in signaling from the activated PDGFβR to the actin cytoskeleton. Future studies will be necessary to elucidate the molecular mechanism linking the activated PDGFβR with the formation of a complex between p130Cas and Nck, including the mechanisms of p130Cas phosphorylation and relocalization.
p130Cas exhibited a biphasic pattern of phosphorylation, with a rapid increase soon after PDGF-B stimulation (2–5 min) followed by an equally rapid and sustained decrease thereafter. This observation suggests the existence of a limiting or “off” mechanism, presumably mediated by a protein tyrosine phosphatase, that could be important in fine-tuning actin rearrangements induced by PDGF-B. Further studies will be necessary to uncover the molecular mechanisms underlying the dynamics of p130Cas phosphorylation and its physiological consequences in the context of PDGFβR activation. Using a very sensitive probe, such as the SH2 domains of the regulatory subunit of PI3K, we could detect significant autophosphorylation of the PDGFβR within 1 min after PDGF-B stimulation (Fig. 3). We recently found a similarly rapid increase in the GTP loading of Rac (G.M.R. and B.J.M, unpublished data), and major cytoskeletal rearrangements were seen soon after (3.5 min) PDGF-B stimulation (Figs. 7A and 8A). During T cell receptor activation, a rapid increase in tyrosine phosphorylation recruits Nck into a signaling complex that subsequently migrates peripherally and accumulates at a ring-shaped actin-rich structure (19). It is likely that highly dynamic processes similar to those occurring during T cell receptor engagement also occur during activation of the PDGFβR. Our data are consistent with a model in which p130Cas serves to recruit Nck to the membrane to initiate actin polymerization and that these complexes are remodeled over time as additional proteins are recruited and/or phosphorylated.
Nck can signal to the actin cytoskeleton by interaction of its SH3 domains with a variety of downstream effectors. PAK-1 (p21-activated kinase 1) is recruited by the middle SH3 domain of Nck (20, 21), and Nck-mediated targeting of PAK to the plasma membrane is sufficient for its activation by members of the Rho family of GTPases (22). Under these conditions PAK may also serve as an adaptor to recruit guanine nucleotide exchange factors for Rho GTPases (23) Importantly, activation of PDGFβR leads to Nck-mediated recruitment of PAK-1 to dorsal ruffles and to the edges of lamellipodia (24). Alternatively, Nck adaptors could stimulate actin polymerization through interactions with members of the WASp/WAVE family and their binding partners. We have shown recently that an increased local concentration of membrane-targeted Nck SH3 domains is sufficient to trigger localized actin polymerization through a pathway that requires N-WASp (25). N-WASp localizes to several actin structures, including podosomes, invadopodia, and circular dorsal ruffles (26). Interestingly, invadopodium formation in metastatic carcinoma cells downstream of epidermal growth factor receptor requires Nck-mediated recruitment of the N-WASp-Arp2/3 complex (27). Also, Nck adaptors have been implicated in activation of WAVE proteins (28). Further studies will be necessary to clarify the role of Nck adaptors in activation and targeting of the various downstream effectors upon PDGF stimulation.
In summary, our results demonstrate that Nck adaptors are strictly required for cytoskeletal reorganization and chemotaxis stimulated by PDGF-B. Furthermore, these studies provide mechanistic insights suggesting that Nck adaptors transduce signals to the cytoskeleton downstream of the PDGFβR through a signaling pathway that involves p130Cas. The Nck family of SH2/SH3 domain adaptors may constitute an important target of intervention in diseases concurring with dysregulated signaling from the PDGFβR, altered actin dynamics, and aberrant cell migration.
Materials and Methods
Cell Culture, Transfections, PDGF-B Stimulation, and Migration Assay.
NIH 3T3 cells and MEF lacking Nck (7) or p130Cas (29) were cultured in DMEM supplemented with antibiotics and 10% calf serum or 10% FBS, respectively. Transient transfections were carried out by using the Lipofectamine reagent according to instructions provided by the manufacturer. Cells were serum-starved (DMEM plus 0.1% FBS) for 24 h before stimulation with 30 ng/ml PDGF-BB (Upstate Biotechnology). Migration assay of serum-starved MEFs was performed by using a transwell migration assay (Boyden chamber) as detailed in Supporting Text, which is published as supporting information on the PNAS web site.
Immunofluorescence and Confocal Microscopy.
Immunofluorescence was performed as previously described (25), and further details are provided in Supporting Text. The affinity-purified polyclonal anti-Nck antibody was raised in rabbits immunized with a GST fusion of full-length human Nck1. The anti-phosphotyrosine antibody was from Cell Signaling Technology (P-Tyr-100, catalog no. 9411), and the polyclonal anti-Fak antibody was from Santa Cruz Biotechnology (C-20, sc-558). The monoclonal anti-Cas (6G11) antibody was described previously (30). In some experiments, cells were stained with Texas red phalloidin (Molecular Probes) and Hoechst 33342 dye to visualize the actin cytoskeleton and nuclei, respectively.
Fluorescent images were collected on a Zeiss LSM 510 confocal microscope with a ×63 NA 1.25 Plan-NEOFLUAR oil-immersion objective. Live images were obtained on a Nikon TE2000 inverted microscope with a 60 × 1.4 NA oil-immersion objective. Additional details are provided in Supporting Text.
Pull-Down Assay, Immunoprecipitation, Western Immunoblotting, and Far-Western Blot Analysis.
Serum-starved or PDGF-B-treated cells were harvested, and lysates were subjected to immunoprecipitation with polyclonal anti-Fak or anti-Cas (Cas-B) antibodies (30). Procedural details are described in Supporting Text. Pull-down assays were performed with glutathione-Sepharose beads precomplexed with GST-Nck SH2 domains. Western immunoblotting was carried out by using a monoclonal anti-phosphotyrosine antibody (dilution 1:5,000), a monoclonal anti-Cas antibody (dilution 1:1,000), or a polyclonal anti-Fak antibody (dilution 1:1,000). We used GST fusions of Nck1, Nck2, and PI3K SH2 domains in a far-Western blot analysis as previously described (16). A detailed description of these procedures is provided in Supporting Text.
Supplementary Material
Acknowledgments
We thank Amy Bouton (University of Virginia School of Medicine, Charlottesville) for reagents including anti-Cas antibodies and for critically reading the manuscript. We are grateful to Drs. A. Cowan and W. Mohler for expert advice in imaging techniques and Dr. Kazuya Machida for helping with the far-Western blot analysis. This work was supported by National Institutes of Health Grant CA82258 (to B.J.M.) and a postdoctoral fellowship from the American Heart Association (to G.M.R.).
Abbreviations
- PDGF
platelet-derived growth factor
- PDGFβR
PDGF receptor
- SH
Src homology
- MEF
mouse embryonic fibroblast
- PI3K
phosphatidylinositol 3-kinase
- Fak
focal adhesion kinase.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Pollard T. D., Borisy G. G. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 2.Tallquist M., Kazlauskas A. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Heldin C. H., Westermark B. Physiol. Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 4.Pawson T., Nash P. Genes Dev. 2000;14:1027–1047. [PubMed] [Google Scholar]
- 5.Li W., Fan J., Woodley D. T. Oncogene. 2001;20:6403–6417. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- 6.Buday L., Wunderlich L., Tamas P. Cell. Signalling. 2002;14:723–731. doi: 10.1016/s0898-6568(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 7.Bladt F., Aippersbach E., Gelkop S., Strasser G. A., Nash P., Tafuri A., Gertler F. B., Pawson T. Mol. Cell. Biol. 2003;23:4586–4597. doi: 10.1128/MCB.23.13.4586-4597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura R., Li W., Kashishian A., Mondino A., Zhou M., Cooper J., Schlessinger J. Mol. Cell. Biol. 1993;13:6889–6896. doi: 10.1128/mcb.13.11.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen M., She H., Davis E. M., Spicer C. M., Kim L., Ren R., Le Beau M. M., Li W. J. Biol. Chem. 1998;273:25171–25178. doi: 10.1074/jbc.273.39.25171. [DOI] [PubMed] [Google Scholar]
- 10.Chen M., She H., Kim A., Woodley D. T., Li W. Mol. Cell. Biol. 2000;20:7867–7880. doi: 10.1128/mcb.20.21.7867-7880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. Dev. Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 12.Schlaepfer D. D., Broome M. A., Hunter T. Mol. Cell. Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruest P. J., Shin N. Y., Polte T. R., Zhang X., Hanks S. K. Mol. Cell. Biol. 2001;21:7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin N. Y., Dise R. S., Schneider-Mergener J., Ritchie M. D., Kilkenny D. M., Hanks S. K. J. Biol. Chem. 2004;279:38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- 15.Rao Y. Int. J. Biol. Sci. 2005;1:80–86. doi: 10.7150/ijbs.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nollau P., Mayer B. J. Proc. Natl. Acad. Sci. USA. 2001;98:13531–13536. doi: 10.1073/pnas.241215998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouton A. H., Riggins R. B., Bruce-Staskal P. J. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 18.Casamassima A., Rozengurt E. J. Biol. Chem. 1997;272:9363–9370. doi: 10.1074/jbc.272.14.9363. [DOI] [PubMed] [Google Scholar]
- 19.Barda-Saad M., Braiman A., Titerence R., Bunnell S. C., Barr V. A., Samelson L. E. Nat. Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 20.Bokoch G. M., Wang Y., Bohl B. P., Sells M. A., Quilliam L. A., Knaus U. G. J. Biol. Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- 21.Galisteo M. L., Chernoff J., Su Y. C., Skolnik E. Y., Schlessinger J. J. Biol. Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- 22.Lu W., Mayer B. J. Oncogene. 1999;18:797–806. doi: 10.1038/sj.onc.1202361. [DOI] [PubMed] [Google Scholar]
- 23.Li Z., Hannigan M., Mo Z., Liu B., Lu W., Wu Y., Smrcka A. V., Wu G., Li L., Liu M., et al. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- 24.Dharmawardhane S., Sanders L. C., Martin S. S., Daniels R. H., Bokoch G. M. J. Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivera G. M., Briceno C. A., Takeshima F., Snapper S. B., Mayer B. J. Curr. Biol. 2004;14:11–22. doi: 10.1016/j.cub.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Buccione R., Orth J. D., McNiven M. A. Nat. Rev. Mol. Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H., Lorenz M., Kempiak S., Sarmiento C., Coniglio S., Symons M., Segall J., Eddy R., Miki H., Takenawa T., Condeelis J. J. Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eden S., Rohatgi R., Podtelejnikov A. V., Mann M., Kirschner M. W. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- 29.Honda H., Oda H., Nakamoto T., Honda Z., Sakai R., Suzuki T., Saito T., Nakamura K., Nakao K., Ishikawa T., et al. Nat. Genet. 1998;19:361–365. doi: 10.1038/1246. [DOI] [PubMed] [Google Scholar]
- 30.Bouton A. H., Burnham M. R. Hybridoma. 1997;16:403–411. doi: 10.1089/hyb.1997.16.403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.