Summary
Objective
Human metapneumovirus (hMPV) is a recently described paramyxovirus that has been associated with acute upper and lower respiratory infection (LRI) in infants and children worldwide. We previously observed that one-third of the children with hMPV-associated LRI had been diagnosed with a concomitant acute otitis media (AOM). In the current study, we sought to investigate an association between hMPV and children presenting with AOM as a primary diagnosis.
Methods
We used realtime RT-PCR for hMPV to retrospectively test 144 paired nasal wash (NW) and middle ear fluid (MEF) specimens that had been prospectively collected from children with AOM during a 3-year period from 1990–1992. RNA was extracted from archived, frozen samples and realtime RT-PCR for hMPV was performed.
Results
We detected hMPV in 8/144 (6%) NW and 1/144 MEF. Several of the children still tested positive for hMPV in NW 3 days later, showing persistent virus shedding. All were detected from November–May and six had bacterial co-pathogens. Two of the eight (25%) hMPV-infected children had no bacterial pathogen isolated, suggesting that hMPV may be associated with AOM as a sole pathogen.
Conclusions
These data show that hMPV is associated with a proportion of AOM and thus has additional morbidity and healthcare impact related to these illnesses.
Keywords: Paramyxovirus, Metapneumovirus, Otitis media
1. Introduction
Human metapneumovirus (hMPV) is a recently described paramyxovirus associated with acute respiratory tract infection in children worldwide [1–5]. In our previous study of lower respiratory infection (LRI) in a 25-year cohort of children, we noted that one-third of the children with hMPV-associated LRI had been diagnosed with a concomitant acute otitis media (AOM). Respiratory viruses such as rhinovirus, respiratory syncytial virus (RSV), parainfluenzavirus (PIV) and influenza virus have been detected in nasopharyngeal samples, as well as MEF samples from children with AOM; their role in the etiology and pathogenesis of AOM has become evident [6–8]. We previously detected other respiratory viruses in a significant proportion of middle ear fluid (MEF) samples from children with AOM [8]. In this study, we sought to investigate the role of hMPV in children presenting with AOM as a primary diagnosis by retrospectively testing samples that were previously collected in a prospective cohort study.
2. Methods
2.1. Patients
Children with AOM were prospectively enrolled in clinical trials of antibiotic therapy during a 3-year period from 1990–1992 [9,10]. All children were healthy outpatients seen at our primary care clinics, and none had received antibiotics during the preceding week. The diagnosis of AOM was based on symptoms of fever, irritability or earache, signs of inflammation (red or yellow color or bulging) of the tympanic membrane, and the presence of MEF as documented by tympanocentesis. Informed consent was obtained from the parents or guardians of all children, and all studies were approved by the Institutional Review Board of the University of Texas Medical Branch.
2.2. Specimens
At enrollment MEF was obtained for bacterial and viral studies by needle tympanocentesis. A nasal wash (NW) specimen was obtained following tympanocentesis for viral studies by flushing the nostril with 3–5 ml of phosphate-buffered saline solution in a 30-ml rubber bulb. A second needle tympanocentesis and a second set of viral studies were performed at days 3–5. Venous blood was collected at study entry and follow up as described [9].
2.3. Specimen processing and virologic procedures
The MEF specimen was processed immediately for aerobic bacterial cultures. The collected MEF was then diluted in 1 ml of PBS and aliquoted for viral culture and RSV antigen detection by enzyme immunoassay (EIA). The original dilutions of the MEF were from 4- to 300-fold (median 11). The MEF specimens tested in the present study were samples remaining from previous studies that had been processed, further diluted, frozen and thawed many times. For viral culture of MEF and NW specimens, the specimen was inoculated in two tubes each of primary monkey kidney cells and human fibroblasts. In addition, Hep-2 cells or buffalo green monkey kidney cells were used during RSV or enterovirus seasons, respectively. Inoculation into cell cultures was performed within 2–3 h of specimen collection.
Rapid viral antigen detection was performed on both MEF and NW specimens as previously described [9]. Viral serologic tests for the same respiratory viruses were also performed by the same indirect fluorescent antibody technique; acute and convalescent sera from the same patient were tested at the same time.
2.4. Definitions
The viral respiratory infection was considered documented if a positive result was obtained by viral culture, antigen detection tests or RT-PCR of either NW or MEF specimens. Also, a 4-fold or higher rise in viral titers between acute and convalescent sera was considered as proof of the viral infection. When calculating the rates of detection of different viruses in the MEF, the dual viral infections observed in nine children were regarded as separate viral infections.
2.5. HMPV testing
Specimens were thawed at 37 °C and RNA was extracted using the QIAMP Viral RNA kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Metapneumovirus testing was performed by a realtime RT-PCR assay on a Smart Cycler (Cepheid, Sunnyvale, CA) using primers and probe for the N gene [11] with the Quantitect RT-PCR kit (Qiagen, Valencia, CA). The probe was altered slightly, with BHQ-3 (Invitrogen, Carlsbad, CA) substituted for TAMRA as the 3′ fluorescent quencher. These primers and probe have been shown to detect all four genetic lineages of MPV and the limit of detection in our assay was 50 copies of viral genome per reaction. RNA was tested at several dilutions to determine the most sensitive conditions (data not shown) and 10 μl of undiluted RNA was used in the reaction. Each run included positive hMPV RNA and negative water controls, and positive samples were retested in a second assay for confirmation. Samples that had been collected at enrollment that tested positive for hMPV had the same patients’ follow-up NW and MEF (collected on days 3–5 of the study) tested by the same methods. Serum specimens were not available for hMPV serological testing.
3. Results
Four-hundred and thirty-two children with AOM had previously been enrolled from January 1990 through December 1992. The study cohort was described in detail previously [8]. The median age of the children was 14 months (range 2 months to 7 years) and the male:female ratio was 1.1:1. The race and ethnicity of the children was 38% white, 35% black, and 26% Hispanic. Sixty-eight percent of all AOM episodes occurred from November through April (Fig. 1).
Fig. 1.
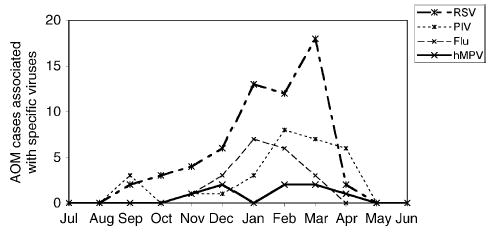
Monthly isolation of viruses from either NW or MEF of children during the study period. Data are cumulative from 1990to 1992. AOM, acute otitis media; RSV, respiratory syncytial virus; PIV, parainfluenza viruses 1, 2 and 3; flu, influenza viruses A and B; hMPV, human metapneumovirus.
We previously identified 191 viruses in 174/432 (40%) cases in NW or MEF by culture, IFA or serology. The viruses identified were RSV (60/432; 14%), PIV3 (20/432; 5%), PIV1 (6/432), PIV2 (3/432), influenza A (9/432; 2%), influenza B (11/432; 2%), enteroviruses (EV) (25/432; 6%), rhinovirus (RV) (3/432), adenovirus (23/432; 5%), CMV (32/432; 7%) and herpes virus (2/432). There were a number of co-infections detected, including seven adenovirus (two CMV, three EV, two RSV), six CMV (three RSV, one PIV2, one RSV/PIV3, one PIV3/RV), two EV (one PIV3, one CMV) and one influenza A (one RSV).
Thus, of 432 cases, 174 previously had a virus identified and 258 previously had no virus identified. Of these 258, paired NW and MEF specimen from the same patient obtained at the day 1 visit were available in 144 cases. Bacteria had been isolated previously from MEF samples of 102 of these 144 cases (71%). Of these, 81 grew one bacterial species, 20 grew two species and one grew three species. S. pneumoniae, H. influenzae, and M. catarrhalis, alone or in combination accounted for 88 cases and the remainder consisted of Group A beta hemolytic streptococci, S. aureus or gram-negative bacteria.
Of the 144 paired NW/MEF specimens collected at the time of diagnosis, realtime RT-PCR detected hMPV in eight (6%) NW and one MEF (Table 1). A 13-month-old black male with AOM grew S. pneumoniae, M. catarrhalis, and S. aureus in the MEF; hMPV was positive in both MEF and NW at the time of the diagnosis. He was treated with antibiotic but did not return for a follow-up visit. Of the seven other hMPV-positive patients, six had available follow-up NW or MEF samples obtained 3 days later. Three of these NW samples also tested positive for hMPV.
Table 1.
Demographic and bacteriological data of patients with AOM and hMPV infection
| Case # | Age (months) | Gender | Race | Date of AOM | HMPV in NW | HMPV in MEF | Bacteria in MEF |
|---|---|---|---|---|---|---|---|
| 1 | 22 | M | C | 11/29/90 | + | − | Nontypable H. influenzae |
| 2 | 6 | F | H | 12/4/90 | +a | − | S. pneumoniae/M. catarrhalis |
| 3 | 13 | M | B | 3/25/91 | + | + | S. pneumoniae/M. catarrhalis/S. aureus |
| 4 | 34 | F | C | 2/17/92 | + | − | No bacteria |
| 5 | 10 | M | H | 2/21/92 | + | − | S. pneumoniae/nontypable H. influenzae |
| 6 | 6 | M | B | 3/27/92 | +a | − | S. pneumoniae/M. catarrhalis |
| 7 | 20 | F | B | 4/1/92 | +a | − | No bacteria |
| 8 | 31 | F | C | 12/4/92 | + | − | Nontypable H. influenzae |
F: female; M: male; H: Hispanic; B: black; C: Caucasian.
These patients’ follow-up nasal wash samples (3 days later) also tested positive for hMPV.
The characteristics of the eight hMPV-infected patients are shown in Table 1. Bacterial co-pathogens were previously cultured from 6/8 MEF of the hMPV-infected patients, including S. pneumoniae, M. catarrhalis, nontypable H. influenzae and S. aureus. This was similar to the distribution of bacterial species that we observed in MEF of the entire cohort. The hMPV infections were all detected from November though April (Fig. 1). The seasonality of hMPV overlapped with that of RSV, influenza and PIV.
4. Discussion
We detected hMPV in eight of 144 (6%) NW samples from virus-negative AOM cases; in three of these cases, hMPV continued to be present in NW collected 3 days later. We also found hMPV in MEF in one case. Our results suggest that hMPV, like other respiratory viruses, plays a role in the pathogenesis of AOM and the virus can enter the middle ear. Extrapolation of these results would suggest an expected 14 hMPV infections from the entire group of 258 cases of previously negative AOM, leading to an overall hMPV prevalence of 3% in this cohort of children with AOM. This is lower than the prevalence in the same AOM cohort for RSV (14%), but similar to the prevalence of influenza virus (4%) or PIV3 (5%). These rates are similar to those observed for hMPV in other studies of acute respiratory infections, in which hMPV prevalence is generally lower than RSV and similar to influenza and PIV3 [1–5]. Seasonality of hMPV infection in this study (November to April) was similar to what has been reported previously [1–5].
While results of viral detection in AOM cases depend largely on the diagnostic methodologies and seasonal variation, the 6% prevalence of hMPV in this cohort of children with AOM compares well with hMPV prevalence in studies of other acute respiratory infections, in which hMPV prevalence is generally lower than that of RSV and similar to the prevalence of influenza and PIV [1–5]. In this cohort, the prevalence for RSV, PIV, and influenza viruses was 14%, 7% and 5%, respectively. Rhinovirus is also frequently associated with AOM and in some studies is the most common virus detected [12]. In this study, we detected rhinovirus by culture, which is much less sensitive than RT-PCR methods.
There are some limitations of our study. First, the NW and MEF specimens we tested had been thawed and frozen several times for previous studies, which may have decreased sensitivity, although we used a sensitive RT-PCR method that is capable of detecting all four genetic lineages of hMPV at 50 copies or less [11]. In a previous study, we retested freeze–thawed samples for hMPV and other viruses and did not find a significant loss of sensitivity [5]. Second, we did not test the samples from previously virus-positive cases, and thus could have missed hMPV in cases with dual viral infection. Therefore, the prevalence of hMPV-associated AOM we detected may be an underestimate. However, in our previous study of hMPV and LRI in children, we detected co-infections with hMPV and other respiratory viruses in only 4–6% of the subjects [5].
The presence of bacterial co-pathogens in MEF from the hMPV-infected patients emphasizes the importance of upper respiratory bacteria in the pathogenesis of AOM. We isolated bacteria from 71% of the previously virus-negative MEF in this study, similar to the bacterial prevalence in MEF of AOM found in other AOM studies. However, 2/8 (25%) of the hMPV-infected children had no bacterial pathogen isolated, suggesting that hMPV may be associated with AOM as a sole pathogen. These findings were similar to what we previously reported on the role of other respiratory viruses and suggest a similar role of this virus in the etiology and pathogenesis of virus-induced AOM [7,13]. We previously found that 5% of MEF had viruses isolated as a sole pathogen [13].
AOM is the most common bacterial infection and the most frequent indication for oral antibiotic therapy in children, and thus has major health and economic impact [14,15]. The direct and indirect costs associated with AOM have been estimated to exceed 3.5 billion dollars annually in the United States [16]. Complications of AOM can be severe and require hospitalization or surgical intervention [17]. Our study adds to the two previous reports on hMPV and AOM [18,19]. The detection of hMPV in 1/144 MEF does not definitively establish a direct role for hMPV in middle ear pathogenesis. Nonetheless, the presence of hMPV in 8/144 (6%) of the nasal washes supports the role of hMPV in antecedent URI that predisposes to the development of AOM. Large prospective studies are needed to fully establish the contribution of hMPV to AOM in otherwise healthy children. HMPV is a common virus associated with acute respiratory tract disease in children, though there are limited data describing the economic impact of hMPV [20]. Our data show that hMPV is also associated with a proportion of AOM, and thus has additional morbidity and healthcare related to these illnesses.
Acknowledgments
Supported by National Institute of Health R03 AI54790 (JVW).
Footnotes
Conflict of interest statement: We have no conflicts of interests to declare in relation to the work presented in this manuscript.
References
- 1.Van Den Hoogen BG, DeJong JC, Groen J, Kuiken T, DeGroot R, Fouchier RAM, Osterhaus DME. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peiris JS, Tang WH, Chan KH, Khong PL, Guan Y, Lau YL, Chu SS. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin G, De Serres G, Cote S, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van den Hoogen BG, van Doornum GJ, Fockens JC, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 5.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruuskanen O, Arola M, Heikkinen T, Ziegler T. Viruses in acute otitis media: increasing evidence for clinical significance. Pediatr Infect Dis J. 1991;10:425–427. doi: 10.1097/00006454-199106000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Chonmaitree T, Heikkinen T. Role of viruses in middle-ear disease. Ann NY Acad Sci. 1997;830:143–157. doi: 10.1111/j.1749-6632.1997.tb51886.x. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen T, Thint M, Chonmaitree T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N Engl J Med. 1999;340:260–264. doi: 10.1056/NEJM199901283400402. [DOI] [PubMed] [Google Scholar]
- 9.Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120:856–862. doi: 10.1016/s0022-3476(05)81950-x. [DOI] [PubMed] [Google Scholar]
- 10.Chonmaitree T, Patel JA, Sim T, et al. Role of leukotriene B4 and interleukin-8 in acute bacterial and viral otitis media. Ann Otol Rhinol Laryngol. 1996;105:968–974. doi: 10.1177/000348949610501207. [DOI] [PubMed] [Google Scholar]
- 11.Maertzdorf J, Wang CK, Brown JB, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–986. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nokso-Koivisto J, Raty R, Blomqvist S, Kleemola M, Syrjanen R, Pitkaranta A, Kilpi T, Hovi T. Presence of specific viruses in the middle ear fluids and respiratory secretions of young children with acute otitis media. J Med Virol. 2004;72:241–248. doi: 10.1002/jmv.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chonmaitree T. Viral and bacterial interaction in acute otitis media. Pediatr Infect Dis J. 2000;19:S24–S30. doi: 10.1097/00006454-200005001-00005. [DOI] [PubMed] [Google Scholar]
- 14.McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office-based physicians in the United States. JAMA. 1995;273:214–219. [PubMed] [Google Scholar]
- 15.Halasa NB, Griffin MR, Zhu Y, Edwards KM. Differences in antibiotic prescribing patterns for children younger than five years in the three major outpatient settings. J Pediatr. 2004;144:200–205. doi: 10.1016/j.jpeds.2003.10.053. [DOI] [PubMed] [Google Scholar]
- 16.Stool SE, Field MJ. The impact of otitis media. Pediatr Infect Dis J. 1989;8:S11–S114. [PubMed] [Google Scholar]
- 17.Bluestone CD. Clinical course, complications and sequelae of acute otitis media. Pediatr Infect Dis J. 2000;19:S37–S46. doi: 10.1097/00006454-200005001-00007. [DOI] [PubMed] [Google Scholar]
- 18.Schildgen O, Geikowski T, Glatzel T, Schuster J, Simon A. Frequency of human metapneumovirus in the upper respiratory tract of children with symptoms of an acute otitis media. Eur J Pediatr. 2005;164:400–401. doi: 10.1007/s00431-005-1655-6. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki A, Watanabe O, Okamoto M, Endo H, Yano H, Suetake M, Nishimura H. Detection of human metapneumovirus from children with acute otitis media. Pediatr Infect Dis J. 2005;24:655–657. doi: 10.1097/01.inf.0000168755.01196.49. [DOI] [PubMed] [Google Scholar]
- 20.Bosis S, Esposito S, Niesters HG, Crovari P, Osterhaus AD, Principi N. Impact of human metapneumovirus in childhood: comparison with respiratory syncytial virus and influenza viruses. J Med Virol. 2005;75:101–104. doi: 10.1002/jmv.20243. [DOI] [PubMed] [Google Scholar]


