Abstract
The yeast vacuolar sorting protein Vps4p is an ATPase required for endosomal trafficking that couples membrane association to its ATPase cycle. To investigate the function of mammalian VPS4 in endosomal trafficking, we have transiently expressed wild-type or ATPase-defective human VPS4 (hVPS4) in cultured cells. Wild-type hVPS4 was cytosolic, whereas a substantial fraction of hVPS4 that was unable to either bind or hydrolyze ATP was localized to membranes, including those of specifically induced vacuoles. Vacuoles were exclusively endocytic in origin, and subsets of enlarged vacuoles stained with markers for each stage of the endocytic pathway. Sorting of receptors from the early endosome to the recycling compartment or to the trans-Golgi network was not significantly affected, and no mutant hVPS4 associated with these compartments. However, many hVPS4-induced vacuoles were substantially enriched in cholesterol relative to the endosomal compartments of untransfected cells, indicating that expression of mutant hVPS4 gives rise to a kinetic block in postendosomal cholesterol sorting. The phenotype described here is largely consistent with the defects in vacuolar sorting associated with class E vps mutants in yeast, and a role for mammalian VPS4 is discussed in this context.
INTRODUCTION
Cell surface receptors are internalized in clathrin-coated vesicles and targeted to the early endosome. Within this compartment receptors and ligands are sorted, either for recycling back to the cell surface or for degradation in the lysosome (Gruenberg and Maxfield, 1995). Although the molecular basis for transport to the early endosome is increasingly well understood, the organization of the transport pathway between the sorting endosome and the lysosome remains an area of considerable debate (Mellman, 1996). Moreover, few of the molecular determinants of this process have been identified.
Receptors that are destined for lysosomal degradation are enriched in internal vesicles, which bud inwardly from the limiting endosomal membrane (Hopkins et al., 1990; Futter et al., 1996). These internal membranes are also heavily enriched in lysobisphosphatidic acid (Kobayashi et al., 1998), indicating that lipid sorting may play a role in multivesicular body (MVB) formation. Indeed, recent studies have shown that in Saccharomyces cerevisiae generation of phosphatidylinositol-3,5-bisphosphate by Fab1p is essential for sorting into MVB (Gary et al., 1998; Odorizzi et al., 1998), and MVB formation in mammalian cells requires phosphoinositide 3-kinase activity (Fernandez-Borja et al., 1999). It has been argued that the MVB is a transport intermediate, budding from the sorting endosome and targeted to a stable late endosome, which also receives newly synthesized lysosomal hydrolases from a mannose-6-phosphate receptor (M6PR)-dependent post-Golgi sorting pathway (Gruenberg et al., 1989; Gruenberg and Maxfield, 1995). In an alternative model, MVBs are seen to mature into late endosomes from sorting endosomes by continued removal of recycling receptors and acquisition of Golgi-derived membrane. Such maturation would be accompanied by morphological changes, including an increase in the volume density of internal vesicles (van Deurs et al., 1993; Futter et al., 1996). Distinction between models for MVB formation/maturation is hampered by cell-specific differences in exact sorting pathways and variations in endosome morphology. Transfer between the mature MVB/late endosome and the lysosome likewise has been argued to proceed via maturation, via a vesicular intermediate, or via direct fusion (see Mellman, 1996). The direct fusion model is supported by in vivo studies that have identified extensive contact zones between late endosomes and dense-core lysosomes (Futter et al., 1996; Bright et al., 1997). Indeed, there is evidence from in vivo and in vitro studies that fusion between late endosomes and lysosomes gives rise to transient degradative compartments from which lysosomal membrane is retrieved (Bright et al., 1997; Mullock et al., 1998).
Many recent advances in our understanding of the molecular organization of the late endocytic pathway in eukaryotic cells have come from genetic studies in S. cerevisiae. In particular, a number of mutants defective in the endocytic pathway or in the sorting of newly synthesized vacuolar (yeast lysosomal) hydrolases have provided powerful reagents for both morphological and molecular characterization of this pathway (Bryant and Stevens, 1998). Among the vacuolar protein sorting (vps) mutants that have been isolated (Jones, 1977; Robinson et al., 1988; Rothman et al., 1989), 13 complementation groups belong to a class defined morphologically by the formation of a novel, multilamellar, perivacuolar compartment called the class E compartment (Raymond et al., 1992). Class E vps mutants fail to efficiently transport a subset of hydrolases, including carboxypeptidase Y (CPY), to the vacuole. Instead, these hydrolases accumulate in the class E compartment, which is acidified. The class E compartment may therefore be considered an aberrant endocytic compartment arising from a sorting defect (Piper et al., 1995; Rieder et al., 1996; Babst et al., 1997; Finken-Eigen et al., 1997). Maturation of CPY to its vacuolar form is delayed and incomplete, and some CPY is missorted to the secretory pathway. Many class E vps mutants were also isolated in a genetic screen, which selected for mutants defective in efficient retention of late-Golgi proteins, which normally in yeast requires recycling of material from an endocytic compartment (Nothwehr et al., 1996). It therefore appears that sorting and/or transport of proteins from the class E compartment to both the vacuole and Golgi complex is defective in class E vps mutants. Further evidence that the class E compartment is distal to the point at which the endocytic and vacuolar sorting compartments converge is provided by the finding that some class E vps mutants have also been isolated as endocytosis mutants that are unable to transport material from the cell surface all the way to the vacuole (Davis et al., 1993; Munn and Riezman, 1994).
The recent molecular characterization of one class E vps mutant, vps4, (Babst et al., 1997, 1998), also identified as end13, grd13, and csc1 (Munn and Riezman, 1994; Nothwehr et al., 1996; Shirahama et al., 1997), suggests how the wild-type gene product might act in sorting from a late endosomal compartment. Vps4p belongs to the ATPases associated with cellular activities (AAA) family of proteins (Babst et al., 1997). Although AAA proteins participate in a wide variety of cellular activities, an emerging theme for their function is in molecular rearrangement and/or unfolding reactions (Confalonieri and Duguet, 1995; Patel and Latterich, 1998; Leonhard et al., 1999). It is possible, therefore, that Vps4p acts to modulate interactions between other components of the vacuolar sorting pathway, including other class E proteins. Indeed, there is evidence that Vps4p influences binding of the class E proteins Vps24p and Vps32p to endosomal membranes (Babst et al., 1998). Previous studies have shown that Vps4p is a conserved protein (Périer et al., 1994; Babst et al., 1997), suggesting that its function is common to all eukaryotic cells. Hence, to further characterize the pathway of lysosomal delivery in mammalian cells, we have expressed wild-type and ATPase-defective mammalian VPS4.
MATERIALS AND METHODS
DNA Manipulations
Standard protocols for recombinant DNA manipulation were used (Sambrook et al., 1989). The Applied Biosystems (Foster City, CA) PRISM big dye terminator system was used for DNA sequencing, and mutagenesis was performed using either the QuikChange or ExSite methods (Stratagene, La Jolla, CA) according to the manufacturer's instructions. All mutagenized and subcloned constructs were confirmed by DNA sequencing. Escherichia coli strain XL1Blue (Stratagene) was used for maintenance, sequencing, and mutation of plasmids.
Plasmid pYES containing the mouse cDNA for full-length mouse suppressor of potassium transport growth defect 1 (mSKD1) from a macrophage library (Périer et al., 1994) was obtained from Dr. C.A. Vandenberg (University of California, Santa Barbara, CA). The mSKD1 gene was excised from this vector using EcoRI and BglI and subcloned blunt-ended into the EcoRV site of mammalian expression vector pcDNA3.1myc/His6 (Invitrogen, San Diego, CA). This vector was designed to express mSKD1 with an in-frame myc/His6 tag at the C-terminal end.
A human cDNA clone from a brain library (Adams et al., 1992) containing expressed sequence tag (EST) M85872 in pBluescript SK(−) was obtained from the The Institute for Genomic Research/American Type Culture Collection (Manassas, VA). The EST sequence corresponded to the 5′ end of yeast VPS4 (Babst et al., 1997). We sequenced the DNA insert of clone M85872 using walking primers and found the cDNA encoded a human homologue of yeast Vps4p, which we have called human VPS4 (hVPS4). After the hVPS4 stop codon, a 3′ flanking region of 880 bp was present, and this was deleted using ExSite mutagenesis to produce a clone designated hVPS4/d3′-pBS. The cDNA was then resequenced in the reverse direction and subsequently deposited on the database (GenBank accession number AF038960). Compared with the full-length DNA sequence of human SKD1 (Mao et al., 1998), hVPS4 lacks the first seven N-terminal amino acids.
The cDNA insert for hVPS4 was excised using XhoI and PstI and subcloned blunt-ended into the SmaI site of the mammalian expression vector pEGFP-C (Clontech, Cambridge, UK). This vector was designed to express hVPS4 with an in-frame green fluorescent protein (GFP) tag at the N terminus of the protein.
Cell Culture, Transfection, and Immunofluorescence
Normal rat kidney (NRK) cells and baby hamster kidney (BHK) cells were grown in Dulbecco's modified minimal essential medium (Life Technologies, Gaithersburg, MD) containing 10% fetal calf serum. Cells grown on glass coverslips were transfected with GFP-hVPS4 or mSKD1-myc/His6 when ∼30% confluent using the nonliposomal lipid-based FuGene method (Boehringer Mannheim, Mannheim, Germany). Controls of expression vectors without insert and FuGene alone were used. Cells were fixed 24 h after transfection using methanol or 4% (wt/vol) formaldehyde with identical results. In some cases, NRK cells were treated with 0.01% saponin, and BHK cells were treated with 0.05% saponin before fixation to release cytosolic proteins. mSKD1-myc/His6 was detected using monoclonal antibody 9E10, followed by Texas Red-conjugated (TRITC) anti-mouse (Sigma, St. Louis, MO) antibodies. Nuclei were stained using Hoechst 33258 (Sigma). For detection of cholesterol, fixed cells were incubated in PBS containing 10 μg/ml filipin (Sigma). Cells were mounted using ProLong antifade (Molecular Probes, Eugene, OR).
For localization studies, the following antibodies were used: mouse anti-transferrin receptor (Zymogen, Cambridge, United Kingdom); mouse anti-120-kDa lysosomal glycoprotein, rabbit anti-M6PR, and rabbit anti-TGN38 (gifts from J.-P. Luzio, University of Cambridge); rabbit anti-GM130 (a gift from Graham Warren, Imperial Cancer Research Fund, London, United Kingdom); and mouse anti-PDI (a gift from N. Bulleid, University of Manchester). TRITC-conjugated anti-rabbit and Cy5-conjugated anti-rabbit were from Sigma and Jackson ImmunoResearch (West Grove, PA), respectively. Cells were examined using a Leica (Nussloch, Germany) microscope, and images were captured using a charge-coupled device camera. For some experiments, a Leica confocal microscope was used.
RESULTS
Mammalian VPS4
The nucleotide sequence of the cDNA present in the pBluescript clone containing EST M85872 was determined. Sequence comparisons at the amino acid level with those on the databases indicated that the cDNA encoded a human homologue of yeast Vps4p and was designated hVPS4. Comparison with a mouse Vps4p-related protein, mSKD1 (Périer et al., 1994), and human SKD1 (designated hSKD1; Mao et al., 1998), indicated that the hVPS4 insert was missing the first seven amino acids of the protein (Figure 1). For our studies we used cDNA constructs containing this truncated hVPS4 or full-length mouse SKD1. A DNA probe corresponding to full-length hVPS4 hybridized to an mRNA species of ∼3.0 kb that was detected in all human tissues examined (our unpublished observations). Hence, hVPS4 mRNA is expressed ubiquitously, as is mSKD1 mRNA (Périer et al., 1994).
Figure 1.
Characterization of human VPS4. The amino acid sequence of human VPS4 was compared with those of human and mouse SKD1 and yeast Vps4p. Identical amino acids are outlined in black. Those amino acids whose identity is conserved only between three proteins are outlined in dark gray. Light gray indicates residues where identity is limited to two proteins. The location of the N-terminal coiled coil–forming region that was deleted for some experiments is indicated by a double overline, and that of the AAA domain is indicated by a single line. Point mutations used in this study are also shown. The sequence data for hVPS4 are available from GenBank under accession number AF038960.
Membrane Localization of Mammalian VPS4 Is Coupled to an ATPase Cycle
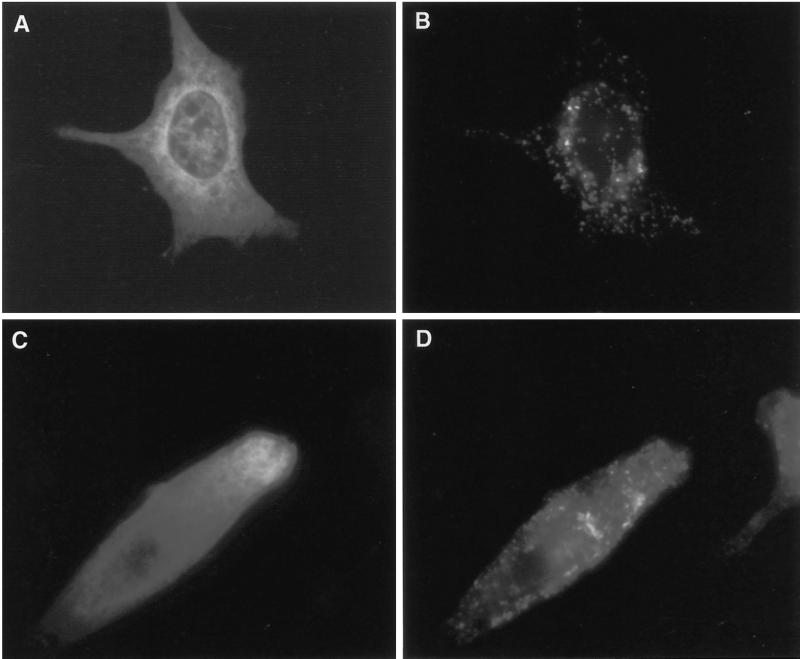
To determine the cellular localization of mammalian VPS4, we used mammalian expression vectors containing hVPS4 with a GFP tag at the N terminus or mSKD1 with a myc/His6 tag at the C terminus. NRK cells were used for most experiments, although some localization studies required the use of BHK cells. Essentially identical results were obtained using both these and other cell lines. As expected, wild-type GFP-hVPS4 (Figure 2A) and wild-type mSKD1-myc/His6 (our unpublished observations) were exclusively cytosolic.
Figure 2.
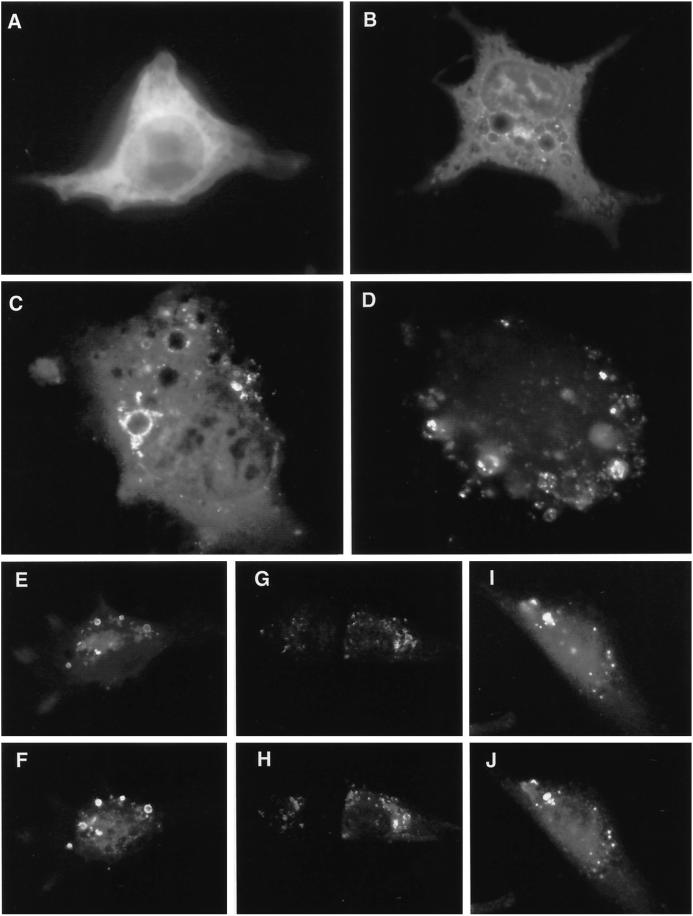
Expression of GFP-tagged hVPS4 in cultured NRK cells. (A) Wild-type GFP-hVPS4 was transiently expressed in NRK cells and visualized by conventional fluorescence microscopy. (B) As in A, but with cells expressing GFP-hVPS4(KQ), an ATP binding-defective mutant. (C) As in A, but with cells expressing GFP-hVPS4(EQ), an ATP hydrolysis-defective mutant. (D) Cells expressing GFP-hVPS4(EQ) were treated with 0.01% (wt/vol) saponin immediately before fixation. NRK cells were cotransfected with GFP-hVPS4(EQ) and mSKD1-myc/His6(KQ) (E and F), hVPS4(EQ) and mSKD1-myc/His6(wt) (G and H), or hVPS4(KQ) and mSKD1-myc/His6(wt) (I and J). Cells were visualized by direct fluorescence microscopy (A–D, E, G, and I) or by indirect fluorescence microscopy using anti-myc antibody (F, H, and J).
Other members of the AAA family, notably NSF (Wilson et al., 1992; Whiteheart et al., 1994), use an ATPase cycle for binding and release of protein substrates. Indeed, yeast Vps4p stably associates with a class E compartment when it is prevented from hydrolyzing ATP (Babst et al., 1998). To study the effects of loss of ATPase activity on mammalian VPS4 localization, well-characterized mutations were made to abrogate ATP binding (KQ), or ATP hydrolysis (EQ), as outlined in Figure 1. Transfection of cells with GFP-hVPS4(KQ) (Figure 2B) or GFP-hVPS4(EQ) (Figure 2C) resulted in the formation of large vacuoles that could readily be identified against the background fluorescence from cytosolic GFP-hVPS4. Although vacuoles were occasionally observed in cells transfected with GFP alone or with wild-type GFP-hVPS4, the occurrence and extent of vacuolation were dramatically increased by transfection with ATPase-defective VPS4.
In addition to their broad cytosolic location, both GFP-hVPS4(KQ) (Figure 2B) and GFP-hVPS4(EQ) (Figure 2C) were localized to brightly fluorescent foci. Although isolated foci were sometimes observed, most were clearly associated with vacuoles, and in some instances the entire vacuolar rim was evenly stained (Figure 2C). Treatment of cells with saponin before fixation reduced the cytosolic staining without substantially affecting staining at foci (Figure 2D), consistent with binding of the ATPase-defective mutants to specific membrane receptor(s). Cotransfection experiments revealed that EQ mutants of GFP-hVPS4 and mSKD1-myc/His6 localized to the same vacuolar structures, as did cotransfected KQ mutants (our unpublished results). Therefore, addition of tags to either the N or C termini did not influence protein localization, nor did the small N-terminal deletion of hVPS4 affect its localization or phenotype compared with full-length mSKD1. In addition to large vacuoles stained with GFP-hVPS4, a number of enlarged vacuoles lacked staining altogether.
The finding that both ATP binding- and ATP hydrolysis-defective mutants of hVPS4 were localized to membranes was initially surprising, because ATP binding-defective yeast Vps4p is exclusively cytosolic when expressed against a null background (Babst et al., 1998). When cells were cotransfected with GFP-hVPS4(EQ) and mSKD1-myc/His6(KQ), the markers colocalized almost exactly (Figure 2, E and F), demonstrating that the two mutants localized to the same membranes. The same result was achieved when cells were cotransfected with GFP-hVPS4(KQ) and mSKD1-myc/His6(EQ) (our unpublished observations). Because the ATPase activity of Vps4p is highly cooperative (Babst et al., 1998), one explanation for the membrane localization of ATP binding-defective mammalian VPS4 is that it coassembles with endogenous wild-type protein to form an inactive complex that is unable to hydrolyze ATP. Consistent with this, cotransfecting either GFP-hVPS4(EQ) (Figure 2, G and H) or GFP-hVPS4(KQ) (Figure 2, I and J) with wild-type mSKD1-myc/His6 led to significant association of wild-type protein with membranes.
ATPase-defective Mammalian VPS4 Binds Endosomal Compartments and Alters Endosome Morphology
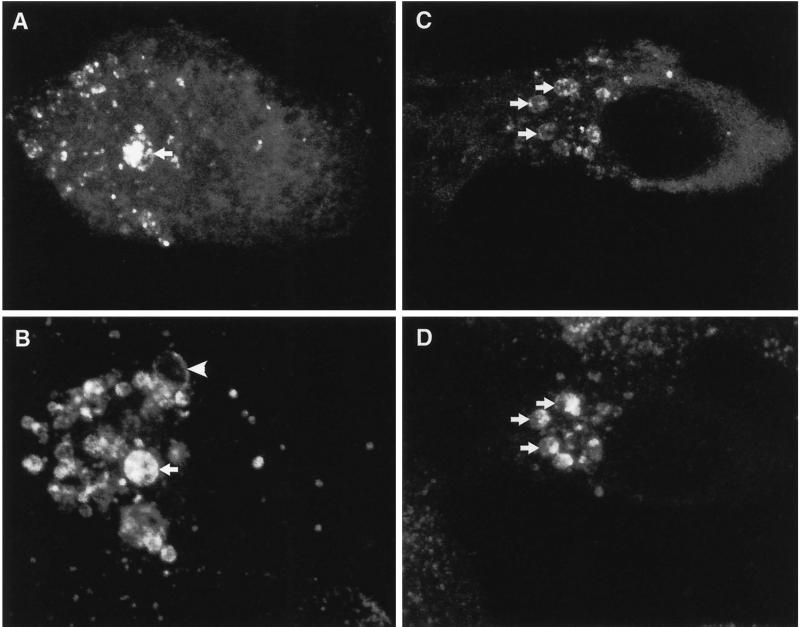
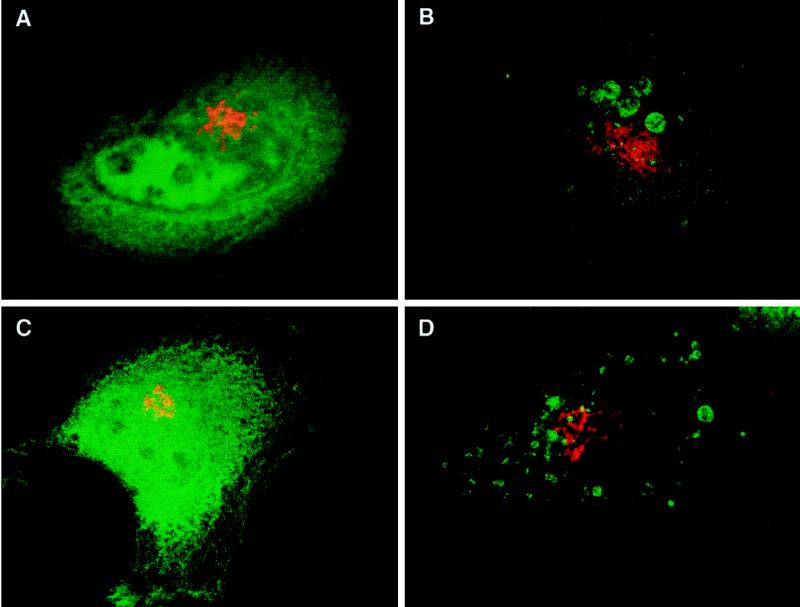
ATPase-defective mutants of mammalian VPS4 were localized to vacuolar membranes, many of which were enlarged. To identify these, transfected cells were examined by indirect immunofluorescence microscopy. In NRK cells the M6PR, which cycles between the trans-Golgi Network (TGN) and an endocytic compartment (see Mellman, 1996), is distributed mainly to the endosome (Bright et al., 1997). The 120-kDa lysosomal glycoprotein (lgp120) localizes to dense-core lysosomes and to late endosomes (Bright et al., 1997). Both markers showed partial colocalization with foci of GFP-hVPS4(EQ) staining in saponin-treated transfected cells (Figure 3, A–D). As expected, a similar extent of colocalization was found with GFP-hVPS4(KQ) (also see Figure 8). Moreover, the distributions of both endosomal markers were profoundly altered in transfected cells compared with neighboring untransfected cells. Lgp120-positive structures, which normally are punctate and throughout the cytoplasm (for example, see Figure 4B), were significantly enlarged and vacuolated (Figure 3B). Although a subset of these contained GFP-hVPS4(EQ), several swollen lgp120-positive vacuoles were devoid of GFP label. M6PR labeling in control cells was also punctate, although often more confined in its cellular distribution than lgp120 labeling (Figure 4, compare B and C). In GFP-hVPS4(EQ)-transfected cells this marker was also redistributed to vacuolar structures of varying size (Figure 3D). As was seen with lgp120, GFP-hVPS4(EQ) did not colocalize with all of the swollen M6PR-positive compartments.
Figure 3.
Human VPS4(EQ) binds to aberrant late endosomal compartments. NRK cells transfected with GFP-hVPS4(EQ) and permeabilized before fixation were visualized by direct confocal fluorescence microscopy (A) or indirectly using antibody against lgp120 and TRITC-conjugated secondary antibody (B). Arrows indicate lgp120-positive compartments that contain GFP-hVPS4(EQ). The arrowhead indicates an lgp120-positive compartment which lacks GFP-hVPS4(EQ). Alternatively, cells transfected with GFP-hVPS4(EQ) were visualized by direct confocal fluorescence microscopy (C) or indirectly using antibody against M6PR (D). Arrows indicate examples of M6PR-positive compartments that contain GFP-hVPS4(EQ).
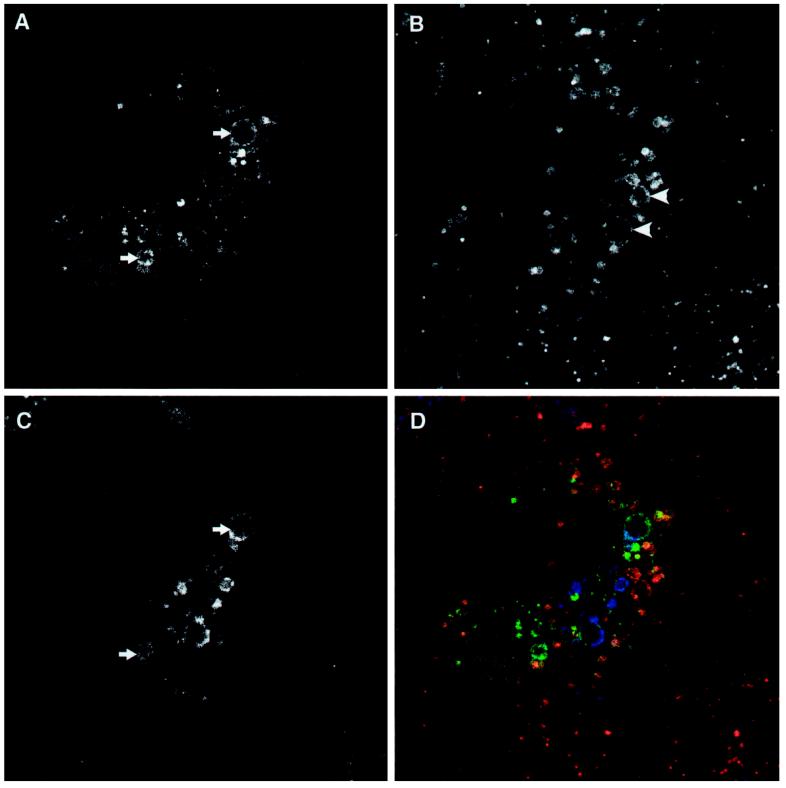
Figure 8.
Expression of ATPase-defective hVPS4 affects cholesterol sorting. NRK cells were transfected with GFP-hVPS4(KQ) (A–H) or GFP-hVPS4(wt) (I–L) and examined by conventional fluorescence microscopy for GFP (green; A, E, and I), M6PR (red; B), lgp120 (red; F and J), and cholesterol (blue, filipin staining; C, G, and K). Merged images (D, H, and L) are shown. Examples of vacuoles that costain for GFP, marker, and filipin are indicated by arrows. Note that exposure conditions for filipin staining were identical in all samples. No increase in filipin staining was observed in the cell transfected with GFP-hVPS4(wt) relative to the neighboring untransfected cell.
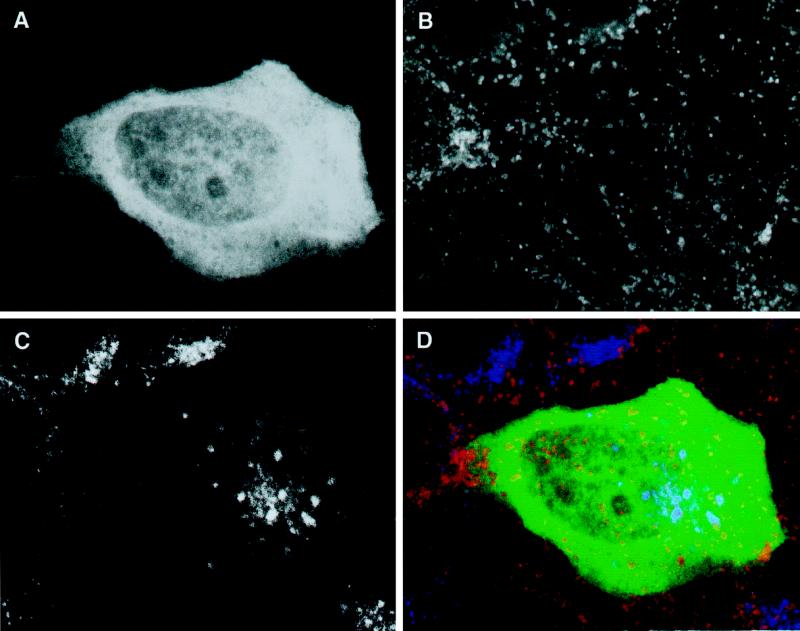
Figure 4.
Distribution of lgp120 and M6PR in cells transfected with GFP-hVPS4(wt). NRK cells transfected with GFP-hVPS4(wt) were visualized directly (A; green) or using anti-lgp120 (B; red) or anti-M6PR (C; blue). The merged image (D) shows the limited codistribution of lgp120 and M6PR. Note the similar distributions of these markers in transfected and neighboring untransfected cells.
Importantly, the distribution of lgp120 and M6PR was unaffected in mock-transfected cells or cells transfected with GFP vector alone (our unpublished results). Moreover, in cells transfected with wild-type GFP-hVPS4 (Figure 4), both lgp120 and M6PR retained very similar distributions compared with neighboring, untransfected, cells. Precisely the same results were found using cells expressing the mSKD1-myc/His6 constructs (our unpublished observations). Membrane localization of hVPS4 and vacuolation of membranes was not simply a consequence of high levels of protein expression, because this phenotype was observed in essentially all cells in which GFP-hVPS4 could be detected. Indeed, membrane localization appeared more striking in cells expressing low levels of GFP-hVPS4; higher levels of expression of GFP-hVPS4 simply led to increased cytosolic staining, consistent with the presence of a limited pool of membrane-associated VPS4 receptors (our unpublished observations).
We also examined whether mammalian VPS4 bound to early, transferrin receptor (TfR)-positive, sorting endosomes. In NRK cells, TfR predominantly localized to a perinuclear recycling compartment (see Figure 7). In contrast, in BHK cells TfR staining is almost exclusively of early sorting endosomes. Several GFP-positive vacuoles in transfected BHK cells were enriched in TfR (Figure 5, A and B, arrows), indicating that early endosomes, as well as late endosomes and lysosomes, possess receptors for mammalian VPS4. Like late endocytic compartments, early endosomes appeared abnormally vacuolated in transfected cells. The degree of vacuolation of these endosomes was variable, however, and in some cells the distribution of TfR was not substantially altered. As with lgp120- and M6PR-positive structures, aberrant TfR-containing vacuoles were not confined to membranes containing GFP-hVPS4. In cells transfected with wild-type GFP-hVPS4, transferrin receptor distribution was not significantly affected compared with that in neighboring, untransfected cells (Figure 5, D–F).
Figure 7.
Human VPS4 expression does not affect sorting of TGN38 or transferrin receptor. NRK cells expressing GFP-hVPS4(wt) (A and C) or GFP-hVPS4(EQ) (B and D) were treated with saponin before fixation and examined by confocal fluorescence microscopy for GFP (all samples), TfR (C and D), and TGN38 (A and B).
Figure 5.
Expression of hVPS4(EQ) gives rise to aberrant early endosomes. BHK cells were transfected with GFP-hVPS4(EQ) (A–C) or GFP-hVPS4(wt) (D–F) and visualized directly (A and D; green) or after incubation with anti-TfR (B and E; red). Merged images are shown (C and F). Arrows (A and B) provide examples of where GFP-hVPS4(EQ) and TfR colocalize. Note the change in TfR distribution in cells transfected with GFP-hVPS4(EQ) compared with GFP-hVPS4(wt) or untransfected cells. Cells in A–C were permeabilized with saponin before fixation.
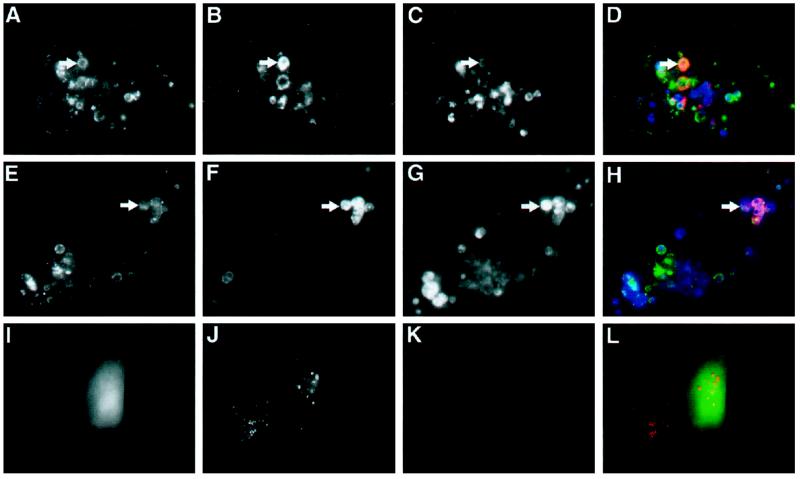
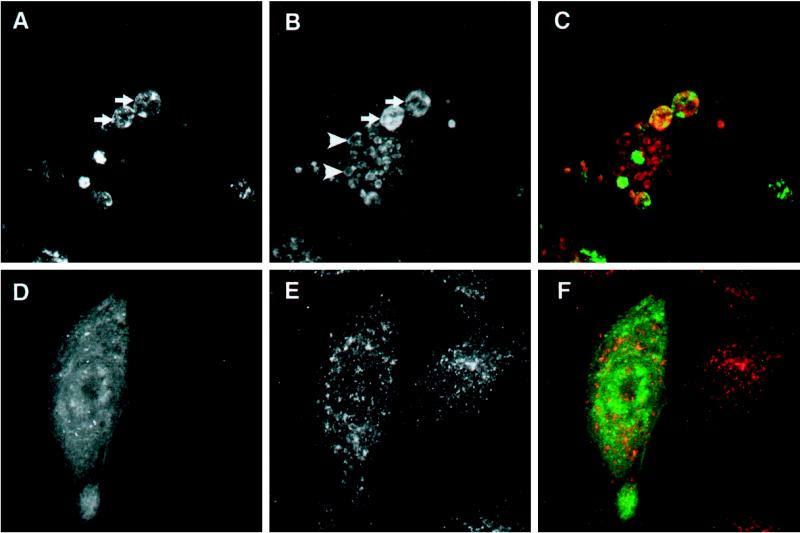
It appeared likely that vacuolation of endosomal compartments in cells expressing ATPase-defective mutants of hVPS4/mSKD1 results from altering the balance of membrane fusion/budding reactions. One consequence of this might be increased mixing of membrane markers. In particular, it has been demonstrated by several groups that direct fusion between late endosomes and lysosomes occurs (van Deurs et al., 1995; Futter et al., 1996), which may give rise to a transient hybrid compartment rich in both M6PR and lgp120 (Bright et al., 1997; Mullock et al., 1998). Under certain conditions, such as after sucrose uptake, these hybrid compartments are stabilized (Bright et al., 1997). We therefore sought, by triple-labeling experiments and confocal fluorescence microscopy, to investigate whether extensive mixing between M6PR and lgp120-containing compartments occurred in transfected cells. As illustrated in the confocal slice shown in Figure 6, both M6PR- and lgp120-containing compartments were profoundly affected in GFP-hVPS4(EQ)-transfected cells. However, no substantial mixing of these markers was observed. Estimating whether any mixing occurred between early and late endosomal markers in transfected NRK cells proved problematic, because the majority of TfR was localized to the perinuclear recycling compartment (Figure 7). However, the appearance in several cells of vacuoles that stained for neither M6PR nor lgp120 (our unpublished observations) indicated the presence of additional endocytic compartments that remained discrete.
Figure 6.
Human VPS4 expression does not cause mixing of lgp120 and M6PR compartments. NRK cells expressing GFP-hVPS4(EQ) were treated with saponin before fixation and examined by direct fluorescence microscopy for GFP (A; green) or by indirect fluorescence microscopy for lgp120 (B; red) and M6PR (C; blue). (D) Merged image. All images are from a single confocal slice. GFP-positive structures that label with M6PR are indicated by arrows. Examples of lgp120-positive structures that lack GFP are indicated by arrowheads.
Mammalian or hVPS4-induced vacuoles labeled with endocytic tracers such as TRITC-conjugated ovalbumin and with lysotracker, a reagent specific for acidified endocytic compartments (our unpublished observations). This confirmed the endocytic nature of enlarged vacuoles and indicated that, as in yeast (Babst et al., 1997), the mutant VPS4-induced compartments can still receive fluid-phase marker and remain acidic.
To assess the impact of mutant hVPS4 or mSKD1 expression on sorting of receptors from an endosomal compartment, we examined the distributions of TfR and TGN38 in transfected NRK cells. TfR distribution in NRK cells is dominated by the perinuclear recycling compartment. As shown in Figure 7, A and B, the distribution of receptor in this compartment is minimally affected in cells expressing GFP-hVPS4(EQ) compared with that in cells expressing the wild-type protein. Likewise, the localization of TGN38, which recycles from the cell surface to the TGN via early endosomes, was unaltered in cells expressing GFP-hVPS4(EQ) (Figure 7, C and D). The distribution of both markers in cells expressing wild-type protein was essentially identical in mock-transfected cells or cells expressing GFP alone (our unpublished observations). The distributions of the Golgi marker GM130 and the endoplasmic reticulum marker PDI were similarly unaffected (our unpublished observations).
Recent evidence indicates that cholesterol, endocytosed as part of low-density lipoprotein particles, travels to late endosomal compartment(s), from where it is sorted by an unidentified mechanism (Mukherjee and Maxfield, 1999). This sorting step can be impaired by administering either hydrophobic amines (Liscum and Faust, 1989) or antibodies to the endosome-specific lipid lysobisphosphatidic acid (Kobayashi et al., 1999) or by mutating the Niemann-Pick C disease (NPC1) gene (Neufeld et al., 1999). Importantly, many of the mutant GFP-hVPS4-induced vacuoles stained with the cholesterol-specific fluorescent marker filipin (Figure 8, A–H). This confirmed their identity as late endosomal compartments that could receive endocytosed markers, but from which transport of cholesterol was substantially reduced relative to that from endosomes of neighboring untransfected cells or cells transfected with wild-type GFP-hVPS4 (see Figure 8, I–L). As expected, several of the cholesterol-rich vacuoles were also positive for either M6PR (Figure 8, A–D) or lgp120 (Figure, 8 E–H), although more extensive colocalization was observed between cholesterol and lgp120.
The putative coiled coil–forming region toward the N terminus of yeast Vps4p has been implicated in membrane association of the protein (Babst et al., 1998). Likewise, we observed that GFP-hVPS4(EQ) with a deletion spanning this region was unable to associate with membranes (Figure 9). As expected, GFP-hVPS4(EQ,ΔCC) expression did not alter the morphology of early sorting endosomes (Figure 9, A and B) or lgp120-positive late endosomes/lysosomes (Figure 9, C and D). Likewise, M6PR distribution was not altered (our unpublished results).
Figure 9.
The coiled coil domain of hVPS4 is required for endosome localization and formation of aberrant compartments. NRK cells (A and B) or BHK cells (C and D) were transfected with GFP-hVPS4(EQ/ΔCC) and examined for GFP (A and C), lgp120 (B), or TfR (D).
DISCUSSION
Mammalian VPS4 Localizes to Endosomes
This report describes the endosome association of the mammalian homologue of a yeast protein required for vacuolar sorting. Vps4p and mammalian VPS4/SKD1 belong to the family of AAA ATPases, which couple their ATPase cycle to the binding and release of substrate proteins (Confalonieri and Duguet, 1995; Patel and Latterich, 1998). For yeast Vps4p, this coupling leads to ATPase-dependent localization of the protein. Vps4p that can bind but not hydrolyze ATP is substantially membrane associated when expressed against a null background, whereas nucleotide-free or wild-type protein (which rapidly hydrolyses ATP) is cytosolic (Babst et al., 1998). We now demonstrate that ATP hydrolysis-defective hVPS4/mSKD1 is similarly impaired in its release from membranes. However, we also find that expression of nucleotide-free mammalian VPS4 gives rise to an identical phenotype. These results are consistent with the ATPase activity of Vps4p being cooperative and reliant on formation of oligomers (Babst et al., 1998). Thus, nucleotide-free mammalian VPS4, when expressed in the presence of wild-type protein, may form heterodimers that are incorporated into complexes that are incapable of hydrolyzing ATP and thus being disassembled (alternatively, dimers consisting of nucleotide-free hVPS4 alone could act as dominant-negative inhibitors by oligomerizing with wild-type dimers).
Unlike yeast Vps4p, which binds exclusively to the class E perivacuolar compartment (Babst et al., 1998), ATPase-defective mammalian VPS4 bound to multiple endosomal compartments. Although M6PR-positive endosomes were frequently stained with GFP-hVPS4, a significant proportion of lgp120-positive late endosomes/lysosomes were also stained. Transferrin receptor-positive sorting endosomes also bound GFP-hVPS4, although this was observed less frequently than binding to later compartments. It is unlikely that we have observed inappropriate binding of exogenously expressed protein, given that essentially no binding of hVPS4 was seen to other membranes, including the endoplasmic reticulum, Golgi, and plasma membrane. Also consistent with binding of hVPS4 to early endosomal compartments is the finding that Hrs, a mammalian homologue of another class E vacuolar sorting protein, Vps27p, associates predominantly with TfR-positive endosomes (Komada et al., 1997).
Expression of Mutant Mammalian VPS4 Dramatically Affects Endosome Morphology
Expression of ATPase-defective hVPS4 had a striking effect on the morphology of multiple endosomal compartments. The vacuolation observed was specific, rather than a consequence of the transfection strategy, because no such vacuolation was observed in mock-transfected cells, cells transfected with GFP alone, or in cells transfected with wild-type GFP-hVPS4 [GFP-hVPS4(wt)]. Furthermore, deletion of the coiled coil–forming domain of hVPS4 completely abrogated membrane binding and the formation of enlarged vacuoles. Similarly, no change in the morphology of other cellular membranes was observed upon transfection with ATPase-defective hVPS4. In addition, Hoechst staining revealed no abnormalities in nuclear morphology (our unpublished results). Certain alleles of VPS4 in yeast give rise to a gain-of-function constitutive autophagy phenotype (Shirahama et al., 1997). However, although we occasionally observed vacuoles that appeared to contain cytosolic material and thus could be autophagic, these were not a general feature of the induced vacuoles. We conclude that the effect on endosome morphology is a specific consequence of the action of mutant hVPS4. The finding that several endosomal compartments are affected is consistent with the observation that these compartments possess receptors for hVPS4. We have ruled out the possibility that binding of mammalian VPS4 to both endosomes and lysosomes is a secondary consequence of aberrant mixing and fusion of compartments, because markers remain separated.
Yeast Vps4p has been proposed to release complexes of soluble class E proteins, including Vps24p and Vps32p, from the endosomal membrane (Babst et al., 1998). Although the substrates for mammalian VPS4 have yet to be identified, the phenotypes described in this report are consistent with this function. Indeed, the demonstration that some endosomal compartments will vacuolate despite the absence from their membranes of hVPS4 implies that the action of hVPS4 on endosomes is not direct. More likely, hVPS4 is required to recycle cytosolic components essential for normal endosome function, and the presence of mutant hVPS4 will essentially deplete these components. In this respect it is significant that putative mammalian homologues of a number of class E proteins, including Vps24p and Vps32p, have been identified (Odorizzi et al., 1998).
The Function of Mammalian VPS4
The ability of endosomes in VPS4-transfected cells to accumulate endocytic tracers and to acidify is consistent with the phenotype of yeast cells lacking functional Vps4p, because those cells retain a significant ability to transport hydrolases to the vacuole and are able to maintain acidified late endosomal compartments, despite the exaggeration of these organelles. The ability of mutant hVPS4-transfected cells to sort both TGN38 and TfR from the early endosome appears at first perhaps more surprising, given that Vps4p-deficient yeast cells show significant defects in retention of late Golgi markers (Nothwehr et al., 1996). However, it is likely that yeast Golgi markers recycle via a later endocytic compartment than do mammalian TfR and TGN38. Our finding that many aberrant endosomal compartments accumulate cholesterol is further consistent with a mammalian VPS4-induced defect in postendosomal sorting, because it demonstrates that cholesterol transport distal to these compartments is retarded relative to that which is proximal. At present, the precise role of mammalian VPS4 is not understood, although the findings presented here are consistent with previous suggestions that yeast Vps4p participates, directly or indirectly, in the formation of MVB (Odorizzi et al., 1998). Moreover, the phenotype observed in this study is strikingly similar to that observed in wortmannin-treated cells (Fernandez-Borja et al., 1999), in which vacuolation of a number of endosomal compartments, including those containing TfR, M6PR, and the lysosomal protein CD63, was seen. It has been suggested that this phenotype is a direct consequence of the impairment of MVB formation (Fernandez-Borja et al., 1999). Further work will be required to assess in detail the effect of mammalian VPS4 expression on formation of MVB, receptor sorting, and other aspects of endosome function.
ACKNOWLEDGMENTS
We are grateful to our colleagues for their generous provision of reagents used in this study. Bill Wickstead participated in the construction of some of the DNA constructs. We thank Ruth Slater for DNA sequencing, supported by Wellcome Trust grant 044327/Z/95/Z. The confocal microscope was also funded by a Wellcome Trust grant. This work is supported by Medical Research Council grants G9722026 and G117/153 and Biotechnology and Biological Sciences Research Council grant CO5969.
Abbreviations used:
- AAA
ATPases associated with cellular activities
- BHK
baby hamster kidney
- CPY
carboxypeptidase Y
- EST
expressed sequence tag
- GFP
green fluorescent protein
- h
human
- lgp120
120-kDa lysosomal glycoprotein
- m
mouse
- M6PR
mannose-6-phosphate receptor
- MVB
multivesicular body
- NRK
normal rat kidney
- SKD
suppressor of potassium transport growth defect
- TfR
transferrin receptor
- TGN
trans-Golgi network
- VPS
vacuolar protein sorting
- wt
wild-type
REFERENCES
- Adams MD, Dubnick M, Kerlavage AR, Moreno R, Kelley JM, Utterback TR, Nagle JW, Fields C, Venter JC. Sequence identification of 2375 human brain genes. Nature. 1992;355:632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 1997;16:1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, Wendland B, Estapa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Reaves BJ, Mullock BM, Luzio JP. Dense core lysosomes can fuse with late endosomes and are reformed from the resultant hybrid organelles. J Cell Sci. 1997;110:2027–2040. doi: 10.1242/jcs.110.17.2027. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confalonieri F, Duguet M. A 200-amino acid ATPase module in search of a basic function. Bioessays. 1995;17:639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Davis NG, Horecka JL, Sprague GF. Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Borja M, Wubbolts R, Calafat J, Janssen H, Divecha N, Dusseljee S, Neefjes J. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr Biol. 1999;9:55–58. doi: 10.1016/s0960-9822(99)80048-7. [DOI] [PubMed] [Google Scholar]
- Finken-Eigen M, Röhricht RA, Köhrer K. The VPS4 gene is involved in protein transport out of a yeast prevacuolar endosome-like compartment. Curr Genet. 1997;31:469–480. doi: 10.1007/s002940050232. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Miller K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature. 1990;346:335–340. doi: 10.1038/346335a0. [DOI] [PubMed] [Google Scholar]
- Jones EW. Proteinase mutants of Saccharomyces cerevisiae. Genetics. 1977;85:23–33. doi: 10.1093/genetics/85.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat M-H, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nature Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- Komada M, Masaki R, Yamamoto A, Kitamura N. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J Biol Chem. 1997;272:20538–20544. doi: 10.1074/jbc.272.33.20538. [DOI] [PubMed] [Google Scholar]
- Leonhard K, Stiegler A, Neupert W, Langer T. Chaperone-like activity of the AAA domain of the yeast Yme1 AAA protease. Nature. 1999;398:348–351. doi: 10.1038/18704. [DOI] [PubMed] [Google Scholar]
- Liscum L, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-beta-[2-(diethylamino)ethoxy]androst-5-en-17-one. J Biol Chem. 1989;264:11796–11806. [PubMed] [Google Scholar]
- Mao M, et al. Identification of genes expressed in human CD34+ hematopoietic stem/progenitor cells by expressed sequence tags and efficient full-length cDNA cloning. Proc Natl Acad Sci USA. 1998;95:8175–8180. doi: 10.1073/pnas.95.14.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Maxfield FR. Cholesterol: stuck in traffic. Nature Cell Biol. 1999;1:E37–E38. doi: 10.1038/10030. [DOI] [PubMed] [Google Scholar]
- Mullock BM, Bright NA, Fearon CW, Gray SR, Luzio JP. Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J Cell Biol. 1998;140:591–601. doi: 10.1083/jcb.140.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn AL, Riezman H. Endocytosis is required for the growth of vacuolar H+-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994;127:373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem. 1999;274:9627–9635. doi: 10.1074/jbc.274.14.9627. [DOI] [PubMed] [Google Scholar]
- Nothwehr SF, Bryant NJ, Stevens TH. The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol Cell Biol. 1996;16:2700–2707. doi: 10.1128/mcb.16.6.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Babst M, Emr SD. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell. 1998;95:847–858. doi: 10.1016/s0092-8674(00)81707-9. [DOI] [PubMed] [Google Scholar]
- Patel S, Latterich M. The AAA team: related ATPases with diverse functions. Trends Cell Biol. 1998;8:65–71. [PubMed] [Google Scholar]
- Périer F, Coulter KL, Liang H, Radeke CM, Gaber RF, Vandenberg CA. Identification of a novel mammalian member of the NSF/CDC48p/Pas1p/TBP-1 family through heterologous expression in yeast. FEBS Lett. 1994;351:286–290. doi: 10.1016/0014-5793(94)00879-5. [DOI] [PubMed] [Google Scholar]
- Piper RC, Cooper AA, Yang H, Stevens TH. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J Cell Biol. 1995;131:603–617. doi: 10.1083/jcb.131.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Banta LM, Köhrer K, McCaffery JM, Emr SD. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol Biol Cell. 1996;7:985–999. doi: 10.1091/mbc.7.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Howald I, Stevens TH. Characterization of genes required for sorting and vacuolar function in the yeast Saccharomyces cerevisiae. EMBO J. 1989;8:2057–2065. doi: 10.1002/j.1460-2075.1989.tb03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shirahama K, Noda T, Ohsumi Y. Mutational analysis of Csc1/Vps4p: involvement of endosome in regulation of autophagy in yeast. Cell Struct Funct. 1997;22:501–509. doi: 10.1247/csf.22.501. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K. Delivery to lysosomes in the human carcinoma cell line HEp-2 involves an actin filament-facilitated fusion between mature endosome and preexisting lysosomes. Eur J Cell Biol. 1995;66:309–323. [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Kayser L, Sandvig K, Hansen SH. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur J Cell Biol. 1993;61:208–224. [PubMed] [Google Scholar]
- Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. N-Ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE. A multisubunit particle implicated in membrane fusion. J Cell Biol. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]