Abstract
Transfection of Mv1Lu mink lung type II alveolar cells with β1–6-N-acetylglucosaminyl transferase V is associated with the expression of large lysosomal vacuoles, which are immunofluorescently labeled for the lysosomal glycoprotein lysosomal-associated membrane protein-2 and the β1–6-branched N-glycan-specific lectin phaseolis vulgaris leucoagglutinin. By electron microscopy, the vacuoles present the morphology of multilamellar bodies (MLBs). Treatment of the cells with the lysosomal protease inhibitor leupeptin results in the progressive transformation of the MLBs into electron-dense autophagic vacuoles and eventual disappearance of MLBs after 4 d of treatment. Heterologous structures containing both membrane lamellae and peripheral electron-dense regions appear 15 h after leupeptin addition and are indicative of ongoing lysosome–MLB fusion. Leupeptin washout is associated with the formation after 24 and 48 h of single or multiple foci of lamellae within the autophagic vacuoles, which give rise to MLBs after 72 h. Treatment with 3-methyladenine, an inhibitor of autophagic sequestration, results in the significantly reduced expression of multilamellar bodies and the accumulation of inclusion bodies resembling nascent or immature autophagic vacuoles. Scrape-loaded cytoplasmic FITC-dextran is incorporated into lysosomal-associated membrane protein-2–positive MLBs, and this process is inhibited by 3-methyladenine, demonstrating that active autophagy is involved in MLB formation. Our results indicate that selective resistance to lysosomal degradation within the autophagic vacuole results in the formation of a microenvironment propicious for the formation of membrane lamella.
INTRODUCTION
Multilamellar bodies (MLBs) are membrane-bound cellular organelles, which vary in size from 100-2400 nm, are composed of concentric membrane layers, and frequently exhibit an electron-dense core. MLBs are found in numerous cell types where they function in lipid storage and secretion (Schmitz and Müller, 1991). In lung type II alveolar cells, MLBs function as secretory granules whose exocytosis results in the deposition of the tubular myelin forms of surfactant on the surface of the alveolae (Hatasa and Nakamura, 1965; Ryan et al., 1975; Williams, 1977). The surfactant film over the alveolar epithelium regulates the surface tension at the air–cell interface and protects the alveola from collapse during respiration (Haagman and van Golde, 1991).
Although the secretory function of MLBs in type II alveolar cells is well established, the precise mechanism of MLB biogenesis remains unclear. Autoradiographic studies of murine type II alveolar cells of mouse lungs showed that although phospholipids labeled with [3H]choline are delivered directly from the Golgi to the MLB, proteins metabolically labeled with [3H]leucine are visualized within multivesicular bodies before delivery to MLBs (Chevalier and Collet, 1972). Surfactant proteins A, B, and C are delivered via multivesicular bodies to MLBs, and multivesicular bodies are proposed as the site of processing of surfactant precursor to mature forms; both multivesicular bodies and MLBs express the lysosomal marker CD63 and are therefore part of the lysosomal pathway (Voorhut et al., 1992, 1993). The lysosomal nature of the MLB has been demonstrated by the localization of various lysosomal enzymes to this organelle (Balis and Conen, 1964; Hatasa and Nakamura, 1965; Goldfischer et al., 1968; Hoffman, 1972; DiAugustine, 1974; Heath et al., 1976; Hook and Gilmore, 1982; de Vries et al., 1985).
It has been previously suggested based on morphological criteria that MLBs form via cellular autophagy (Balis and Conen, 1964; Sorokin, 1967; Flaks and Flaks, 1972; Stratton, 1978). Autophagy is a normal degradative process that exists in all eukaryotic cells and is stimulated in response to a variety of environmental stresses, which necessitate the use of autophagic mechanisms to enable cellular survival (Seglen and Bohley, 1992; Dunn, 1994). Degradative autophagic vacuoles (AVd) form after acquisition of lysosomal properties by nascent, immature autophagic vacuoles (AVi), which present multiple limiting membranes and are considered to form by the sequestration of cytoplasm by smooth endoplasmic reticulum membranes (Dunn, 1990; Furuno et al., 1990; Ueno et al., 1991). The lysosomal nature of both the autophagic vacuole and the MLB supports a relationship between the two organelles; however, definitive evidence of a role for autophagy in MLB biogenesis has yet to be demonstrated.
Transfection of the immortalized Mv1Lu cell line, derived from mink lung type II alveolar cells, with β-1–6-N-acetylglucosaminyl transferase V (GlcNAc-TV), the enzyme responsible for the β-1–6 branching of N-glycans, which favors the addition of elongated polylactosamine chains, results in the loss of contact inhibition, decreased substrate adhesion, increased susceptibility to apoptosis and increased tumorigenicity in nude mice (Demetriou et al., 1995). GlcNAc-TV-transfected Mv1Lu cells also exhibit increased phaseolis vulgaris leucoagglutinin (L-PHA) reactivity of lysosomal-associated membrane protein-2 (LAMP-2), demonstrating that increased GlcNAc-TV activity alters the β1–6 branching of this heavily glycosylated lysosomal glycoprotein (Demetriou et al., 1995). We show here that in contrast to untransfected Mv1Lu cells, which exhibit none or at best few MLBs, GlcNAc-TV-transfected Mv1Lu cells stably express numerous cytoplasmic MLBs. MLB formation in the GlcNAc-TV transfectants is reversibly regulated by leupeptin, an inhibitor of lysosomal proteases, demonstrating that lysosomal degradation is necessary for the formation of the membrane lamella of the MLB. It is also inhibited by 3-methyladenine (3-MA), a specific inhibitor of early stages in autophagic vacuole formation (Seglen and Gordon, 1982), and we demonstrate the necessary role for autophagy and autophagic vacuole biogenesis in MLB formation.
MATERIALS AND METHODS
Cell Culture
Mv1Lu mink lung epithelial cells, mock-transfected Mv1Lu cells (C1), and the GlcNAc-TV-transfected Mv1Lu cell lines (R2, M9, and M1) (Demetriou et al., 1995) were grown in Dulbecco's modified Eagle's medium supplemented with glutamine, nonessential amino acids (Life Technologies, Oakville, Ontario, Canada), and 10% FBS (Immunocorp, Laval, Quebec, Canada) in an air-5% CO2 atmosphere at constant humidity at 37°C. The medium of the transfected cell lines (C1, R2, M9, and M1) was supplemented with 600 μg/ml G418 (Life Technologies) to maintain the transfected phenotype. For all experiments, cells were plated at a density of 40,000 cells/cm2, and the medium was replaced every 2 d. Leupeptin (Roche Diagnostics, Laval, Quebec, Canada) was added to cell cultures at a concentration of 2 μg/ml, and 3-MA (Sigma, St. Louis, MO) was added at a concentration of 10 mM.
Immunofluorescence
Cells cultured on glass coverslips were fixed by the addition of precooled (−80°C) methanol/acetone (80:20% vol/vol) directly to the coverslips and then placed at −20°C for 15 min. After fixation, the cells were rinsed extensively with PBS, pH 7.4, supplemented with 0.1 mM Ca2+ and 1 mM Mg2+ (PBS/CM), and then incubated for 15 min with PBS/CM containing 0.5% BSA at room temperature to reduce nonspecific binding. LAMP-2 distribution was determined using the AC17 anti-LAMP-2 antibody (Nabi et al., 1991; Nabi and Rodriguez-Boulan, 1993) followed by FITC- or Texas Red-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Detection of the distribution of L-PHA reactivity was performed using rhodamine-conjugated L-PHA (E-Y Laboratories, San Mateo, CA). After labeling, the coverslips were mounted in Airvol (Air Products and Chemicals, Allentown, PA). Labeled cells were viewed in a Zeiss (Thornwood, NY) Axioskop fluorescent microscope equipped with a 63× Plan Apochromat objective and fluorochrome-selective filters. Images were photographed using Eastman Kodak (Rochester, NY) T-Max 400 film. Confocal microscopy was performed with the 60× Nikon (Tokyo, Japan) Plan Apochromat objective of a dual-channel Bio-Rad (Hercules, CA) MRC 600 laser scanning confocal microscope equipped with a krypton/argon laser and printed using a Polaroid (Cambridge, MA) TX1500 video printer.
Electron Microscopy
Cells grown on Petri dishes were rinsed with 0.1 M sodium cacodylate, pH 7.3, and fixed with 2% glutaraldehyde for 60 min at 4°C. The fixed cells were rinsed in cacodylate buffer, scraped from the Petri dish, and collected by centrifugation. The cell pellet was postfixed for 60 min with 2% osmium tetroxide at 4°C, dehydrated, and embedded in LR-White resin (MecaLab, Montreal, Quebec, Canada). Ultra-thin sections (80 nm) were contrasted with uranyl acetate and lead citrate and visualized with a Phillips (Eindhoven, The Netherlands) 300 or Zeiss CEM902 electron microscope. Quantification of the expression of MLBs and of inclusion bodies in the 3-MA experiments was determined by circumscribing the cytoplasm (excluding the nucleus), the MLBs, and the inclusion bodies from 10 images at 4400× magnification and determining the area of the circumscribed regions. MLBs were defined as membrane-bound cytoplasmic organelles that present at least three distinct circumferential concentric membrane lamellae. MLBs were composed either completely of concentric lamella or of concentric lamella surrounding a single dense core. Inclusion bodies, or AVi, were defined by the presence of multiple internal structures surrounded by a limiting membrane composed of single or multiple membranes and could be morphologically distinguished from MLBs.
Scrape Loading of FITC-Dextran
FITC-dextran was scrape loaded into M9 cells essentially as previously described (McNeil et al., 1984). Cells were plated overnight to semiconfluence and then rinsed three times in cold PBS/CM and incubated on ice for 15 min to chill the cultures. Cold PBS/CM (0.5 ml) containing 2.5 mg/ml lysine-fixable 10,000 molecular weight FITC-dextran (Molecular Probes, Eugene, OR) was added to the culture dish, and the cells were immediately scraped from the dish in the concentrated FITC-dextran solution. The cell suspension was rapidly diluted in 40 ml of cold PBS/CM and centrifuged in the cold to pellet the cells. The scrape loading was performed at 4°C, and the cells were rapidly diluted in cold PBS/CM to reduce the possibility of FITC-dextran uptake by fluid phase endocytosis. The cell pellet was resuspended in culture medium, and the cells were plated for 2, 24, 48, or 72 h in regular medium or in medium containing 10 mM 3-MA before fixation with 3% paraformaldehyde and immunofluorescence labeling with anti-LAMP-2 and Texas Red-conjugated secondary antibodies. Quantification of autophagic activity was performed by counting the number of FITC-dextran–loaded cells exhibiting FITC labeling of LAMP-2-positive lysosomal structures.
RESULTS
Increased Expression of MLBs in GlcNAc-TV-transfected Mv1Lu Cells
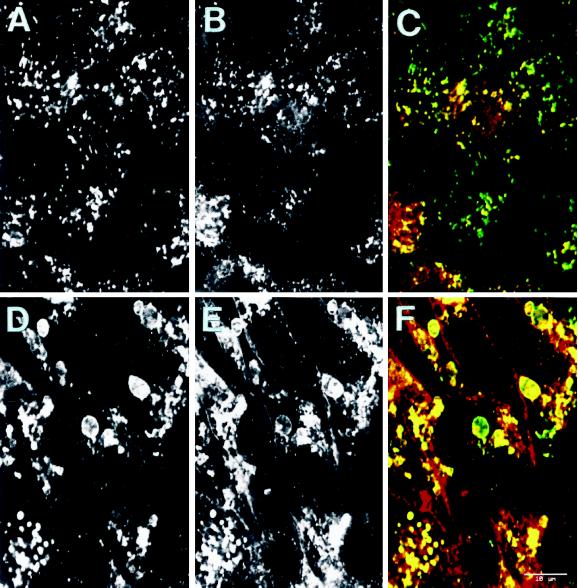
GlcNAc-TV transfection of Mv1Lu cells resulted in the obtention of clones M9 and M1, which exhibited significantly higher expression levels of GlcNAc-TV activity and increased L-PHA reactivity of LAMP-2 (Demetriou et al., 1995). To assess the distribution of LAMP-2 and lysosomes in these cells, 6-d confluent cultures of untransfected Mv1Lu cells and M9 and M1 GlcNAc-TV transfectants were immunofluorescently labeled with antibodies to LAMP-2 (Figure 1). Clusters of LAMP-2-labeled lysosomes, as indicated by the arrows, correspond to the perinuclear concentration of lysosomal organelles in an individual cell. In contrast to the punctate distribution of the lysosomal marker in Mv1Lu cells, anti-LAMP-2 antibodies label large vacuolar structures in both the M9 and M1 cell lines (Figure 1, B and C). To determine whether the increased β1–6-branched N-glycans of the GlcNAc-TV transfectants are indeed localized to the large LAMP-2-positive lysosomal vacuoles present in the M9 and M1 transfectants, Mv1Lu cells and M9 cells were double immunofluorescently labeled for LAMP-2 and L-PHA, a lectin specific for the β1–6 branching of polylactosamine chains. By confocal microscopy, LAMP-2 and L-PHA colocalize in both the punctate LAMP-2 lysosomal labeling of Mv1Lu cells as well as the swollen LAMP-2-labeled vacuoles of M9 cells (Figure 2).
Figure 1.
Expression of large lysosomal vacuoles in GlcNAc-TV-transfected Mv1Lu cells. Untransfected Mv1Lu cells (A) or GlcNAc-TV-transfected clones M9 (B) and M1 (C) plated for 6 d were immunofluorescently labeled with LAMP-2 to reveal the distribution of lysosomes in these cells. Clusters of lysosomes representing the lysosomes of individual cells are indicated by the arrows. Distinct swollen LAMP-2-positive lysosomal vacuoles are present in M9 and M1 cells but not in untransfected Mv1Lu cells. Bar, 10 μm.
Figure 2.
The swollen lysosomal vacuoles of GlcNAc-TV transfectants are labeled with L-PHA. Untransfected Mv1Lu (A–C) or GlcNAcTV-transfected M9 (D–F) cells plated for 6 d were double immunofluorescently labeled with anti-LAMP-2 followed by FITC-conjugated anti-mouse secondary antibody (A and D) or with rhodamine-conjugated L-PHA (B and E). Merged images are presented in C and F (LAMP-2 in green, L-PHA in red). LAMP-2- and L-PHA-reactive β1–6-branched oligosaccharides are localized to lysosomes of Mv1Lu cells as well as to the large lysosomal vacuoles of GlcNAc-TV M9 transfectants. Bar, 10 μm.
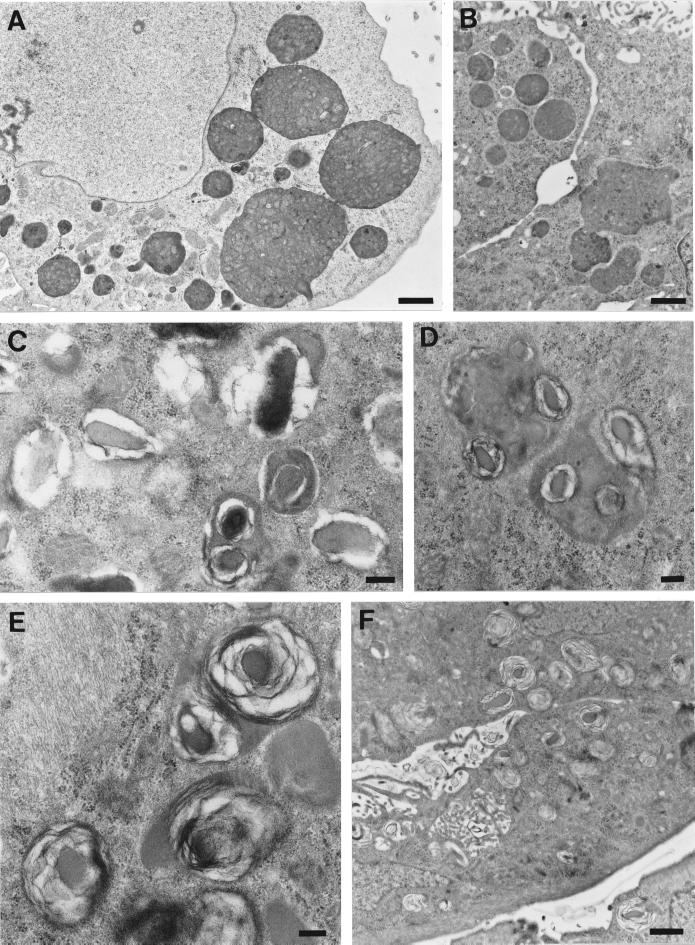
Electron microscopy of the different cell types revealed the presence of large MLBs in the M9 and M1 cell lines but not in the untransfected Mv1Lu cells (Figure 3). MLBs of M1 cells frequently exhibit a dense core surrounded by lamellae, whereas those of M9 cells exhibited a more uniform lamellar morphology and are larger. Expression of MLBs in untransfected Mv1Lu cells, mock-transfected C1 cells, and the GlcNAc-TV transfectants R2, M9, and M1 exhibiting increasing levels of GlcNAc-TV activity (Demetriou et al., 1995; Table 1) was quantified by determining the extent of cytoplasmic area that was filled by MLBs in the different cells. Both the number of MLBs and the proportion of cytoplasmic area that they cover are significantly greater in high GlcNAc-TV-expressing M9 and M1 cells compared with untransfected Mv1Lu, mock-transfected C1, or low-expressing R2 cells (Table 1). Curiously, the area covered by MLBs is greater in M9 cells than in M1 cells, which express higher GlcNAc-TV levels; the number of MLBs in M1 cells is larger than in M9 cells, indicating that the difference between the two cell types is due to an increased size of MLBs in the M9 cells. MLBs >2 μm2 in area are not observed in untransfected Mv1Lu, mock-transfected C1, or low-GlcNAc-TV–expressing R2 cells. Untransfected Mv1Lu cells can express MLBs, albeit very few, suggesting that these cells may have partially retained a differentiated type II phenotype. Mock-transfected C1 and low-GlcNAc-TV–expressing R2 cells (Demetriou et al., 1995) exhibit more MLBs than untransfected Mv1Lu cells. However, the difference between the MLB/cytoplasm ratio of C1 and R2 cells and Mv1Lu cells is not statistically significant (0.05 < p < 0.1) and suggests that MLB expression in C1 and R2 cells may be due to subtle changes in the phenotype of the cells after transfection.
Figure 3.
GlcNAc-TV transfectants express MLBs. Untransfected Mv1Lu cells (A) or GlcNAc-TV-transfected clone M9 (B and D) and M1 (C) cells plated for 6 d were processed for electron microscopy. Distinct MLBs are present in the cytoplasm of both M9 (B) and M1 (C) cells but are absent from untransfected Mv1Lu cells (A). The lamellar morphology of large MLBs is particularly evident in M9 cells (D). Bars, 1 μM (A–C); 0.5 μM (D).
Table 1.
Quantification of MLB expression in GlcNAc-TV-transfected Mv1Lu cells
| Cell type | GlcNAc-TV (pmol/mg/h)a | Total no. of MLBs | Range of MLB size (μm2) | Total cytoplasmic area (μm2) | Total MLB area (μm2) | Area MLB/cytoplasm (%) |
|---|---|---|---|---|---|---|
| Mv1Lu | 63 ± 6 | 1 | 0.53–0.53 | 2022 | 0.53 | 0.03 ± 0.03 |
| C1 | 59 ± 5 | 29 | 0.27–2.00 | 1875 | 22.5 | 1.1 ± 0.6 |
| R2 | 151 ± 15 | 15 | 0.33–1.60 | 1882 | 12.3 | 0.6 ± 0.3 |
| M9 | 567 ± 265 | 78 | 0.23–16.3 | 1690 | 223 | 13.0 ± 1.1 |
| M1 | 1082 ± 308 | 98 | 0.22–14.0 | 1821 | 109 | 6.1 ± 1.6 |
From 10 electron microscopy images (4400×) of each cell line, the area covered by MLBs and by cytoplasm (excluding the nucleus) in the complete image was determined. Using a two-tailed Student's t test, the ratio of MLB to cytoplasmic area in M9 cells is significantly different from both Mv1Lu and C1 cells (p < 10−5). For M1 cells, the ratio of MLB to cytoplasmic area is also significantly different from both Mv1Lu (p < 0.005) and C1 (p < 0.01) cells. The significance of the difference in MLB/cytoplasm area between either C1 or R2 cells and Mv1Lu cells is of the order of 0.05 < p < 0.1.
The values for GlcNAc-TV expression were taken from Dimetriou et al. (1995).
Lysosomal Degradation Is Necessary for MLB Biogenesis
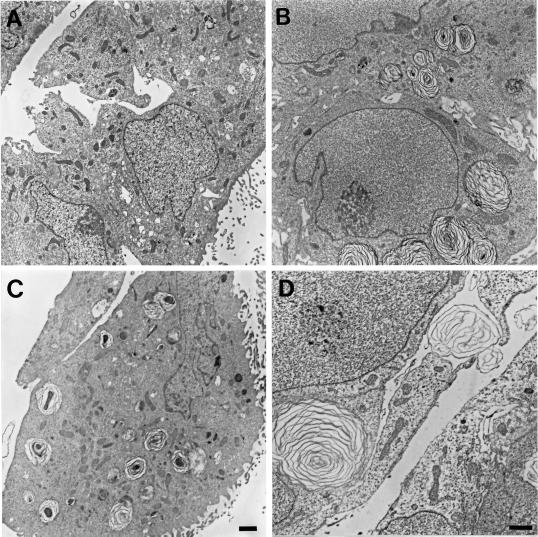
The only cytoplasmic structures visualized in M9 or M1 cells by electron microscopy large enough to correspond to the large LAMP-2- and L-PHA-positive vacuoles identified in these cells by immunofluorescence labeling are MLBs. Furthermore, the expression of LAMP-2 in the MLBs of GlcNAc-TV-transfected Mv1Lu cells is consistent with the previously described lysosomal nature of this organelle (Balis and Conen, 1964; Hatasa and Nakamura, 1965; Goldfischer et al., 1968; Hoffman, 1972; DiAugustine, 1974; Heath et al., 1976; Hook and Gilmore, 1982; de Vries et al., 1985; Voorhut et al., 1992). To assess the role of lysosomal degradation on MLB expression, M1 cells were treated with the lysosomal protease inhibitor leupeptin (Figure 4). After 15 h of leupeptin treatment, MLBs exhibit electron-dense material around the periphery of the vacuole caused by the apparent fusion of MLBs with other lysosomal organelles and transfer of nonlamellar electron-dense material. In addition to these heterogeneous structures, smaller dense vacuoles accumulate. With increasing time in leupeptin-containing media, MLBs are no longer evident, and after 4 d of incubation with leupeptin, only large dense vacuoles are present in M1 cells (Figures 4E and 5A). Leupeptin treatment has been previously shown to induce the accumulation of AVd (Furuno et al., 1982; Ueno et al., 1991; Yokota et al., 1995), and the leupeptin-induced vacuoles in M1 cells are morphologically equivalent to AVd (Figures 4E and 5A).
Figure 4.
Gradual transformation of MLBs into AVd by leupeptin treatment. GlcNAc-TV-transfected M1 cells were plated for 2 d in regular medium and then incubated for 15 (A and B), 48 (C), 72 (D), or 96 (E) h in the presence of 2 μg/ml leupeptin and processed for electron microscopy. At early times after addition of leupeptin, dense material can be observed at the periphery of MLBs (A and B), and at later times dense vacuoles proliferate such that after 96 h autophagic vacuoles predominate, and MLBs are no longer present. Bars, 0.5 μm.
Figure 5.
Formation of MLBs via autophagic vacuole degradation after leupeptin washout. GlcNAc-TV-transfected M1 cells were plated for 2 d and then incubated for 4 d in the presence of 2 μg/ml leupeptin (A) and then washed and incubated in leupeptin-free medium for 15 (B), 24 (C), 48 (D and E), or 72 (F) h before being processed for electron microscopy. After leupeptin treatment, MLBs disappear, and large autophagic vacuoles are present (A). After 24 and 48 h, single or multiple foci of lamella form within the autophagic vacuole (C–E), and after 72 h the autophagic vacuoles transform into lamellar structures resembling those of untreated cells (F). Bars, 1 μm (A, B, and F); 0.2 μm (C–E).
To determine whether removal of leupeptin and activation of lysosomal degradation could reverse this process and lead to the formation of MLBs, M1 cells treated with leupeptin for 4 d were then incubated in the absence of leupeptin and analyzed by electron microscopy. Fifteen hours after leupeptin washout, dense AVd are still present in M1 cells; however, they appear to have lost some internal structure (Figure 5B). After 24 h the morphological transformation of the vacuoles commences, and numerous dense core bodies are present with some exhibiting internal lamellae (Figure 5C). Forty-eight hours after leupeptin removal, intermediates in the transformation of AVd into MLBs can be visualized (Figure 5, D and E). The formation of single or multiple dense core lamellar structures within individual autophagic vacuoles can be clearly visualized. After 72 h in the absence of leupeptin, the cells exhibit MLBs similar to untreated cells (Figure 5F). The reversible regulation of MLB expression by leupeptin in GlcNAc-TV-transfected M1 cells demonstrates that lysosome fusion with MLBs and subsequent degradation of MLB contents by lysosomal hydrolases regulate lamella formation.
Role of Autophagy in MLB Biogenesis
Leupeptin is a general inhibitor of lysosomal protease activity, and although it blocks degradation of AVd (Furuno et al., 1982; Kovacs et al., 1982; Ueno et al., 1991; Yokota et al., 1995), it is not a specific inhibitor of autophagy. To determine whether autophagy is specifically involved in MLB formation, we used 3-MA, which has previously been demonstrated to block autophagy at the initial sequestration step (Seglen and Gordon, 1982). By immunofluorescence, 3-MA treatment results in the disappearance of large LAMP-2-positive vacuoles in M9 cells (Figure 6). The LAMP-2-positive lysosomes in both Mv1Lu and M9 cells treated with 3-MA (Figure 6, B and D) are slightly swollen compared with those of untreated Mv1Lu cells (Figure 6A); however, the large LAMP-2-positive vacuoles corresponding to MLBs in M9 cells (Figure 6C) are no longer visible in 3-MA-treated M9 cells (Figure 6D).
Figure 6.
Inhibition of autophagy by 3-MA results in the disappearance of swollen LAMP-2-positive MLBs. Untransfected Mv1Lu (A and B) and GlcNAc-TV-transfected M9 (C and D) cells were incubated in regular medium (A and C) or medium supplemented with 10 mM 3-MA for 3 d (B and D) and then immunofluorescently labeled for LAMP-2. Although 3-MA treatment induces the formation of slightly swollen lysosomal structures in both Mv1Lu and M9 cells (B and D), it also results in the disappearance of the large LAMP-2-positive vacuoles, which correspond to MLBs in M9 cells (D). Bar, 10 μm.
The ability of 3-MA to induce the disappearance of MLBs was confirmed by electron microscopy (Figure 7). In cells treated with 3-MA, distinctive inclusion bodies accumulate, which do not present the circumferential membrane layers of MLBs (Figure 7, arrows). The limiting membrane of the inclusion bodies is formed of double or multiple membranes and surround multiple internal structures including multilamellar structures. These inclusion bodies can be morphologically distinguished from MLBs, whose concentric membrane layers surround only a single dense core. These inclusion bodies are morphologically equivalent to AVi, and their expression after 3-MA treatment is consistent with the role of 3-MA as an inhibitor of autophagy. Quantitative analysis of the effect of 3-MA treatment on MLB expression in M9 and M1 cells demonstrates that cells cultured in 10 mM 3-MA for 3 d exhibit significantly decreased expression of MLBs (Table 2). In some experiments, we observed the complete disappearance of MLBs. Relative to MLB expression in control cells, expression of inclusion bodies in 3-MA-treated cells is significantly reduced in all experiments.The ability of 3-MA to inhibit MLB formation identifies a role for autophagic vacuole maturation in MLB formation.
Figure 7.
Expression of inclusion bodies in 3-MA-treated GlcNAc-TV transfectants. Treatment of GlcNAc-TV-transfected M1 cells with 10 mM 3-MA for 3 d resulted in the disappearance of MLBs and the appearance of morphologically distinct inclusion bodies (see arrows) exhibiting multiple external membranes and resembling AVi (A–D). Bar, 0.5 μm (A); 0.2 μm (B–D).
Table 2.
3-MA decreases multilamellar body expression
| Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | |
|---|---|---|---|---|
| M1 control | ||||
| MLBs (%) | 5.6 ± 1.1 | 8.6 ± 1.7 | 9.1 ± 1.6 | ND |
| M1 + 3-MA | ||||
| MLBs (%) | 0.05 ± 0.04a | 2.1 ± 0.1b | 0.08 ± 0.06a | ND |
| Inclusion bodies (%) | 3.1 ± 1.1 | 2.8 ± 0.6 | 5.5 ± 1.3 | ND |
| M9 control | ||||
| MLBs (%) | 13.7 ± 2.1 | 24.4 ± 4.3 | 12.3 ± 2.8 | 11.8 ± 3.5 |
| M9 + 3-MA | ||||
| MLBs (%) | 5.2 ± 1.6b | 10.5 ± 1.5c | 0.3 ± 0.3a | 2.3 ± 1.4c |
| Inclusion bodies (%) | 1.2 ± 0.1 | 1.8 ± 0.6 | 1.8 ± 0.5 | 0.5 ± 0.1 |
GlcNAc-TV-transfected M1 and M9 Mv1Lu clones were plated for 6 d in regular medium (control). For the 3-MA-treated cells (+ 3-MA), the culture medium was supplemented with 10 mM 3-MA for the final 3 d. The area of MLBs and of cytoplasm was quantified as for Table 1. The area covered by inclusion bodies, morphologically distinguished from MLBs (see MATERIALS AND METHODS), in the 3-MA-treated cells was also measured. Inclusion bodies were not detected in control cells. ND, not determined.
a–c The significance of the difference in MLB/cytoplasm area between control and 3-MA treated cells is as follows:
p < 0.005;
p < 0.01;
p < 0.05.
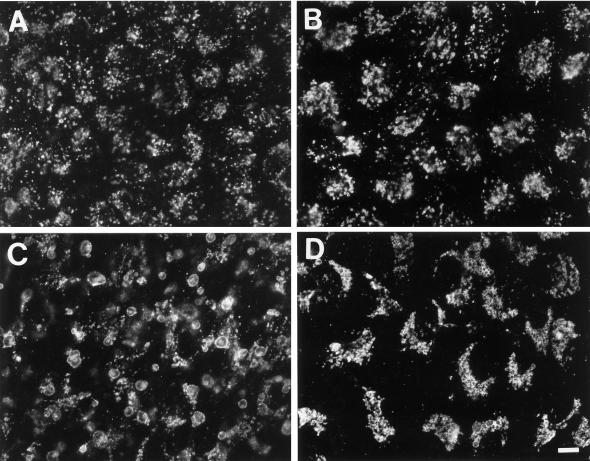
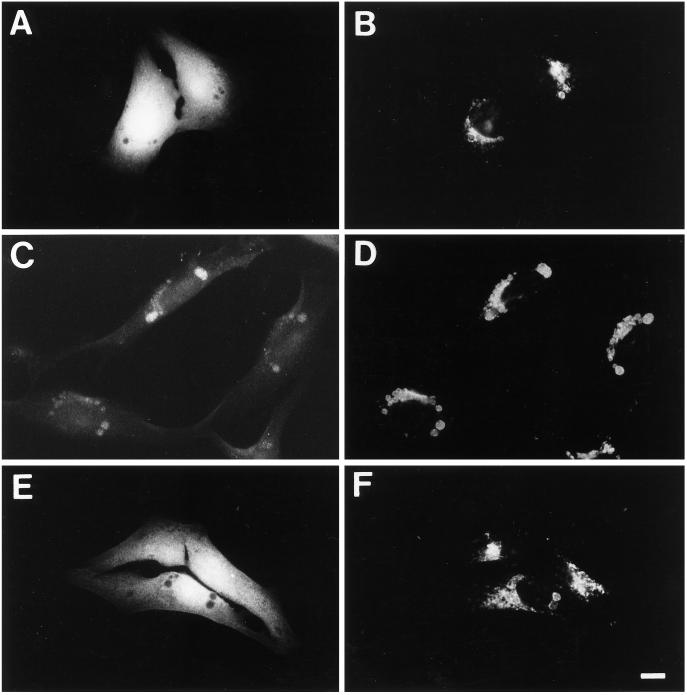
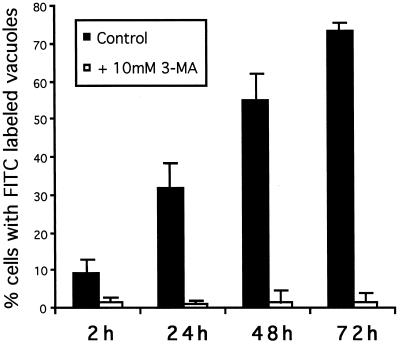
To demonstrate that 3-MA indeed blocks autophagy in GlcNAc-TV-transfected Mv1Lu cells and to confirm the role of autophagy in MLB biogenesis, M9 cells were scrape loaded with FITC-dextran. The procedure was performed at 4°C to minimize endocytic capture of FITC-dextran such that the fluorescent marker was incorporated only into the cytoplasm of the cell. The scrape-loaded cells were washed in the cold to eliminate any free FITC-dextran and then fixed at various times after plating in regular or 3-MA-supplemented medium. At early times after plating in regular medium (2 h), the majority of cells exhibit a cytoplasmic distribution of FITC-dextran, which is excluded from vacuolar structures (Figure 8, A and B). After 48 h, the majority of cells plated in regular medium exhibit an accumulation of FITC-dextran in LAMP-2-positive vacuoles including the swollen structures equivalent to MLBs (Figure 8, C and D). However, if the cells are replated in 3-MA-containing medium after scraping, the FITC-dextran remains cytosolic, and no LAMP-2-positive vacuoles are labeled (Figure 8, E and F). The number of cells presenting FITC-dextran labeling in LAMP-2 positive vacuoles was counted from six experiments (Figure 9). With time an increasing number of cells exhibit autophagic incorporation of FITC-dextran into LAMP-2-positive vacuoles. In the presence of 3-MA, essentially no cells exhibit a vacuolar labeling irrespective of the time of incubation in culture medium. The limited incorporation of scrape-loaded FITC-dextran into lysosomal vacuoles in the presence of 3-MA demonstrates that endocytic uptake of FITC-dextran by the cells was minimal and that 3-MA does indeed block autophagy in M9 cells. FITC-dextran transfer from the cytosol to large vacuoles of M9 cells, which correspond to MLBs, therefore demonstrates that active autophagy is involved in the formation of these organelles.
Figure 8.
Incorporation of cytosolic FITC-dextran into MLBs is inhibited by 3-MA. M9 cells were scraped from plastic dishes in the presence of 2.5 mg/ml FITC-dextran, washed extensively, replated on coverslips in regular medium (A–D) or medium containing 10 mM 3-MA (E and F), and then fixed after 2 (A and B) or 48 (C–F) h and labeled for LAMP-2 using Texas Red-conjugated secondary antibodies. The distribution of FITC-dextran (A, C, and E) and LAMP-2 (B, D, and F) in the same cells is presented. After only 2 h of plating, FITC-dextran is cytosolic and excluded form large LAMP-2-positive vacuoles (A and B); however, with time FITC-dextran is incorporated via autophagy into LAMP-2-positive perinuclear vacuoles equivalent to MLBs (C and D). Autophagic incorporation of FITC-dextran into MLBs is inhibited by 3-MA (E and F). Bar, 10 μm.
Figure 9.
Autophagy of scrape-loaded FITC-dextran in GlcNAc-TV-transfected cells. FITC-dextran was scrape loaded into M9 cells as in Figure 8 and incubated in regular medium (filled bars) or medium supplemented with 10 mM 3-MA (open bars) for 2, 24, 48, or 72 h (as indicated) before fixation and labeling for LAMP-2 as in Figure 8. Fifty FITC-dextran-loaded cells per slide were assessed for the presence of FITC labeling in LAMP-2-positive vacuoles. The percent of cells that exhibit FITC labeling of lysosomal vacuoles is presented and represents the average ± SD of six experiments.
DISCUSSION
Expression of MLBs in GlcNAc-TV-transfected Mv1Lu Cells
β1–6 branching of complex N-linked oligosaccharides is initiated by GlcNAc-TV and produces the preferred substrate for β-1-3-N-acetylglucosaminyl transferase (i), the rate-limiting enzyme implicated in polylactosamine elongation (Holmes et al., 1987; Yousefi et al., 1991). Increased expression of polylactosamine and the associated Lewis and blood group antigens are carcinoma markers (Fukuda, 1985; Hakamori, 1989). Modified expression of polylactosamine is also associated with cellular differentiation of various cell types (Spillmann and Finne, 1987; Youakim et al., 1989; Amos and Lotan, 1990; Lee et al., 1990; Tuo et al., 1992; Nabi and Rodriguez-Boulan, 1993). The decreasing polylactosamine glycosylation of the lysosomal LAMP glycoproteins in cultured epithelial cells with time in culture is modulated independently of glycosyltransferase activities (Brockhausen et al., 1991; Nabi and Dennis, 1998), and polylactosamine glycosylation has been shown to be regulated by the Golgi residence time of the protein (Wang et al., 1991; Nabi and Rodriguez-Boulan, 1993; Nabi and Dennis, 1998). Increased GlcNAc-TV expression is associated with increased polylactosamine glycosylation in oncogenically transformed and undifferentiated cell lines (Yamashita et al., 1985; Heffernan et al., 1989; Yousefi et al., 1991), indicating that GlcNAc-TV expression levels can regulate the expression of polylactosamine oligosaccharides.
Transfection of the contact-inhibited lung epithelial cell line with GlcNAc-TV resulted in increased expression of L-PHA-reactive β1–6-branched N-glycans and the expression of a partially transformed phenotype, including loss of the contact-inhibited phenotype, tumorigenicity in nude mice, and an increased propensity to apoptosis (Demetriou et al., 1995). GlcNAc-TV expression in Mv1Lu cells is therefore associated with the expression of early events in cellular transformation. Mv1Lu cells are derived from lung type II alveolar cells, responsible for the elaboration of alveolar surfactant in the lung. Surfactant secretion by type II lung alveolar cells is mediated by MLBs whose presence in type II cells is a phenotypic characteristic of these cells (Haagman and van Golde, 1991). However, the differentiated type II alveolar phenotype is highly unstable in culture, and the expression of MLBs by primary type II cell cultures is maintained for only days after establishment of the cultures (Diglio and Kikkawa, 1977; Dobbs et al., 1985). The ability of GlcNAc-TV transfection to induce the formation of MLBs in a cultured type II alveolar-derived cell line identifies a role for protein glycosylation in organelle biogenesis and in the expression of a differentiated phenotype by this lung type II-derived alveolar cell line in culture.
The immunofluorescent L-PHA labeling of the large LAMP-2-positive vacuoles localizes β1–6-branched L-PHA substrates to MLBs. Increased L-PHA reactivity of LAMP-2 was demonstrated in M1 and M9 cells compared with untransfected Mv1Lu cells (Demetriou et al., 1995). In both M9 and M1 cells, LAMP-2 migrates more slowly in SDS-PAGE than in Mv1Lu cells, even though M1 cells exhibit increased L-PHA reactivity of LAMP-2 relative to M9 cells, corresponding to their twofold increased expression of GlcNAcTV (Demetriou et al., 1995). The basis for the increased MLB expression of M9 cells compared with M1 cells is not clear, and the specific aspect of polylactosamine glycosylation that induces MLB formation is not known. Direct demonstration of a role for polylactosamine glycosylation in MLB biogenesis proved difficult, because inhibitors of the glycosylation biosynthetic pathway also inhibit the corresponding lysosomal glycosidases, thereby preventing autophagic vacuole degradation (Tulsiani and Touster, 1992). Putative β1–6 branching and polylactosamine glycosylation of MLB glycoproteins might enhance their resistance to degradation by lysosomal proteases or modify interactions between MLB components, thereby favoring lamella formation.
Role of Lysosomal Degradation in MLB Biogenesis
The large vacuoles immunofluorescently labeled with antibodies to LAMP-2 are present predominantly in the M9 and M1 GlcNAc-TV transfectants as are morphologically identifiable MLBs by electron microscopy. The fact that no other structure comparable in size with the MLBs is present in the transfected cells identifies the large fluorescently labeled LAMP-2- and L-PHA-positive vacuoles as MLBs. Deficiency in lysosomal galactosidases and sialidases is associated with the accumulation of lamellar bodies, demonstrating that impaired lysosomal degradation of glycoproteins or glycolipids can be associated with the formation of lamellar bodies (Amano et al., 1983; Alroy et al., 1985; Allegranza et al., 1989; Ohshima et al., 1997).
A definitive role for lysosomal degradation in MLB formation was demonstrated by leupeptin treatment of GlcNAcTV transfectants (Figures 4 and 5). Over 3–4 d, MLBs are gradually replaced by AVd, implicating leupeptin inhibition of lysosomal proteases in the prevention of de novo formation of MLBs from AVd. In the absence of new synthesis of MLBs, the disappearance of MLBs could occur via normal turnover mechanisms, which may include dilution caused by cell division or secretion. Lamellar membrane structures can be visualized in the extracellular space of GlcNAc-TV-transfected Mv1Lu cells (Figure 3D). However, the presence of peripheral dense regions in MLBs 15 h after addition of leupeptin is also indicative of the fusion of MLBs with endosomes and/or lysosomes whose contents are not transformed into membrane lamellae in the absence of lysosomal degradation. The appearance of heterologous transforming vacuoles after leupeptin treatment suggests that MLBs are continually fusing with lysosomes and that transformation of newly incorporated material into membrane lamellae requires lysosomal degradation. The endocytic pathway has been shown to deliver material to nascent autophagic vacuoles, and the autophagic pathway is therefore accessible at early stages (Gordon and Seglen, 1988; Tooze et al., 1990; Liou et al., 1997). Fusion of lysosomes with degradative autophagic vacuoles has also been documented (Ericsson, 1969; Lawrence and Brown, 1992; Yokota et al., 1995). Our data support the idea that heterologous fusion events between lysosomes and MLBs are continually occurring; whether these fusion events represent complete incorporation of the lysosome into the MLB or rather a kiss and run mechanism is not clear (Storrie and Desjardins, 1996).
The transformation of leupeptin-induced AVd into MLBs after leupeptin washout demonstrates that lysosomal degradation is a critical element in the formation of membrane lamellae. The formation of lamellae within distinct subregions of the autophagic vacuole (Figure 5, D and E) further indicates that localized degradation is responsible for the formation of a microenvironment propicious for lamellae formation. In GlcNAc-TV-transfected Mv1Lu cells, β1–6 branching of N-glycans of LAMPs and possibly other as yet unidentified MLB glycoproteins therefore generates a lipid–protein mix, which is conducive to the formation of membrane lamellae within the degradative lysosomal environment of the AVd. Continuing lysosome fusion could generate large lysosomal organelles whose contents cannot be degraded by lysosomal hydrolases, resulting in the formation of a residual body of lysosomal degradation or an MLB. However, the ability to inhibit MLB formation with 3-MA, a specific inhibitor of autophagy, demonstrates that in the cell system studied here, lysosome fusion with autophagic vacuoles is necessarily involved in MLB biogenesis.
Biogenesis of MLBs via Autophagy
A specific role for autophagic sequestration in MLB biogenesis was demonstrated by the ability of 3-MA to prevent the formation of MLBs in GlcNAc-TV-transfected cells (Figure 6 and Table 2). 3-MA treatment is associated with increased lysosomal pH and decreased lysosomal density and with inhibition of late endosome to lysosome transport (Caro et al., 1988; Punnonen et al., 1994), which may explain the slight enlargement of LAMP-2-positive structures in both GlcNAc-TV-transfected and untransfected Mv1Lu cells (Figure 6, B and D). Nevertheless, 3-MA treatment of GlcNAc-TV transfectants results in the disappearance of large LAMP-2-labeled vacuoles corresponding to MLBs as well as the significant reduction in morphologically identifiable MLBs by electron microscopy. Inhibition of autophagy with 3-MA is therefore generally associated with the disappearance of MLBs. 3-MA treatment results in the accumulation of inclusion bodies that morphologically resemble AVi, demonstrating that 3-MA is blocking autophagy in the GlcNAc-TV transfectants at an early stage of autophagic vacuole biogenesis. In the hepatocyte, 3-MA blocks the initial sequestration event in autophagic vacuole biogenesis and is associated with an approximate twofold reduction in autophagic sequestration (Kopitz et al., 1990; Seglen and Bohley, 1992), which is consistent with the reduction (between 40 and 83%) in cytoplasmic area covered by both MLBs and inclusion bodies in 3-MA-treated M1 and M9 cells observed here (Table 2).
The demonstration that cytoplasmic FITC-dextran can be incorporated into the LAMP-2-positive MLBs provides a direct illustration that autophagic sequestration is involved in MLB biogenesis (Figure 8). The fact that this sequestration process is inhibited by 3-MA clearly shows that 3-MA is inhibiting autophagy in these cells and that inhibition of autophagy is directly responsible for the decreased expression of MLBs in 3-MA-treated cells. A similar approach has been recently been used to demonstrate that the parasitophorous vacuoles of Leishmania mexicana acquire cytosolic material via autophagy, and, in a manner similar to our results, this process is inhibited by 3-MA (Schaible et al., 1999). The role of autophagic vacuole biogenesis in MLB formation implicates autophagy not only in the cellular response to stress but also in a normal cellular function, MLB formation, and surfactant secretion by the lung type II alveolar cell. The necessary role of autophagy in MLB biogenesis in Mv1Lu cells suggests that autophagy and autophagic vacuole maturation are involved in MLB biogenesis in multiple cell types. If so, our data may have significant implications for the mechanism of MLB accumulation in lysosomal storage diseases.
The formation of the multiple membrane lamella of the MLB requires a vast amount of cellular lipids, and autophagy may constitute the most efficient means of accumulating the necessary molecules within a single organelle. Select resistance of the contents of the autophagic vacuole to lysosomal degradation, possibly because of to β1–6-branched N-glycans in this GlcNAc-TV-transfected cellular model and other mechanisms in various cell types and pathological states, results in the localized formation of membrane lamellae, which give rise to the concentric membrane whorls of the MLB.
ACKNOWLEDGMENTS
We thank Anne Guenette for assistance with the quantification of the electron microscopy and Jean Leveillé for the photographic reproductions. This study was supported by the Medical Research Council of Canada.
Abbreviations used:
- AVd
degradative autophagic vacuole
- AVi
nascent or immature autophagic vacuole
- GlcNAc-TV
β1–6-N-acetylglucosaminyl transferase V
- LAMP-2
lysosomal-associated membrane protein-2
- 3-MA
3-methyladenine
- L-PHA
phaseolis vulgaris leucoagglutinin
- MLB
multilamellar body
- PBS/CM
PBS supplemented with 0.1 mM Ca2+ and 1 mM Mg2+
REFERENCES
- Allegranza A, Tredici G, Marmirolli P, di Donato S, Franceschetti S, Mariani C. Sialidosis type I: pathological study in an adult. Clin Neuropathol. 1989;8:266–271. [PubMed] [Google Scholar]
- Alroy J, Orgad U, Ucci AA, Schelling SH, Scunk KL, Warren CD, Raghavan SS, Kolodny EH. Neurovisceral and skeletal gangliosidosis in dogs with beta-galactosidase deficiency. Science. 1985;229:470–472. doi: 10.1126/science.3925555. [DOI] [PubMed] [Google Scholar]
- Amano N, Yokoi S, Akagi M, Sakai M, Yagashita S, Nakata K. Neuropathological findings of an autopsy case of adult β-galactosidase and neuraminidase deficiency. Acta Neuropathol. 1983;61:283–290. doi: 10.1007/BF00691999. [DOI] [PubMed] [Google Scholar]
- Amos B, Lotan R. Modulation of lysosomal-associated membrane glycoproteins during retinoic acid-induced embryonal carcinoma cell differentiation. J Biol Chem. 1990;265:19192–19198. [PubMed] [Google Scholar]
- Balis JU, Conen PE. The role of alveolar inclusion bodies in the developing lung. Lab Invest. 1964;13:1215–1229. [PubMed] [Google Scholar]
- Brockhausen I, Romero PA, Herscovics A. Glycosyltransferase changes upon differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res. 1991;51:3136–3142. [PubMed] [Google Scholar]
- Caro LH, Plomp PJ, Wolvetang EJ, Kerkhof C, Meijer AJ. 3-Methyladenine, an inhibitor of autophagy, has multiple effects on metabolism. Eur J Biochem. 1988;175:325–329. doi: 10.1111/j.1432-1033.1988.tb14200.x. [DOI] [PubMed] [Google Scholar]
- Chevalier G, Collet AJ. In vivo incorporation of choline-3H, leucine-3H and galactose-3H in alveolar type II pneumocytes in relation to surfactant synthesis. A quantitative radioautographic study in mouse by electron microscopy. Anat Rec. 1972;174:289–310. doi: 10.1002/ar.1091740303. [DOI] [PubMed] [Google Scholar]
- de Vries ACJ, Schram AW, Tager JM, Batenburg JJ, van Golde LMG. A specific α-glucosidase in lamellar bodies of the human lung. Biochim Biophys Acta. 1985;837:230–238. doi: 10.1016/0005-2760(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Demetriou M, Nabi IR, Capaleno M, Dedhar S, Dennis JW. Serum-independent growth, apoptosis and reduced substrate adhesion in epithelial cells expressing GlcNAc-transferase V. J Cell Biol. 1995;130:383–392. doi: 10.1083/jcb.130.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAugustine RP. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974;249:584–593. [PubMed] [Google Scholar]
- Diglio CA, Kikkawa Y. The type II epithelial cells of the lung. IV. Adaptation and behavior of isolated type II cells in culture. Lab Invest. 1977;37:622–631. [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta. 1985;846:155–166. doi: 10.1016/0167-4889(85)90121-1. [DOI] [PubMed] [Google Scholar]
- Dunn WA. Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–142. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Dunn WAJ. Studies on the mechanism of autophagy: formation of the autophagic vacuole. J Cell Biol. 1990;110:1923–1935. doi: 10.1083/jcb.110.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson JLE. Studies on induced cellular autophagy. I. Electron microscopy of cells with in vivo labeled lysosomes. Exp Cell Res. 1969;55:95–106. doi: 10.1016/0014-4827(69)90462-5. [DOI] [PubMed] [Google Scholar]
- Flaks B, Flaks A. Electron-microscope observations on the formation of the cytoplasmic lamellar inclusion bodies in murine pulmonary tumors induced in vitro. J Pathol. 1972;108:211–217. doi: 10.1002/path.1711080307. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780:119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Furuno K, Ishikawa T, Akasaki K, Lee S, Nishimura Y, Tsuji H, Himeno M, Kato K. Immunocytochemical study of the surrounding envelope of autophagic vacuoles in cultured rat hepatocytes. Exp Cell Res. 1990;189:261–268. doi: 10.1016/0014-4827(90)90245-6. [DOI] [PubMed] [Google Scholar]
- Furuno K, Ishikawa T, Kato K. Isolation and characterization of autolysosomes which appeared in rat liver after leupeptin treatment. J Biochem. 1982;91:1943–1950. doi: 10.1093/oxfordjournals.jbchem.a133888. [DOI] [PubMed] [Google Scholar]
- Goldfischer S, Kikkawa Y, Hoffman L. The demonstration of acid hydrolase activities in the inclusion bodies of type II alveolar cells and other lysosomes in the rabbit lung. J Histochem Cytochem. 1968;16:102–109. doi: 10.1177/16.2.102. [DOI] [PubMed] [Google Scholar]
- Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun. 1988;151:40–47. doi: 10.1016/0006-291x(88)90556-6. [DOI] [PubMed] [Google Scholar]
- Haagman HP, van Golde LMG. Synthesis and assembly of lung surfactant. Annu Rev Physiol. 1991;53:441–464. doi: 10.1146/annurev.ph.53.030191.002301. [DOI] [PubMed] [Google Scholar]
- Hakamori SI. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Hatasa K, Nakamura T. Electron microscopic observations of lung alveolar epithelial cells of normal young mice, with special reference to formation and secretion of osmophilic lamellar bodies. Z Zellforsch. 1965;68:266–277. doi: 10.1007/BF00342433. [DOI] [PubMed] [Google Scholar]
- Heath MF, Gandy G, Jacobson W. Lysosomes in the lung. In: Dingle JT, Dean RT, editors. Lysosomes in Biology and Pathology. Amsterdam: Elsevier/North Holland; 1976. pp. 33–58. [PubMed] [Google Scholar]
- Heffernan M, Yousefi S, Dennis JW. Molecular characterization of P2B/LAMP-1, a major protein target of a metastasis-associated oligosaccharide structure. Cancer Res. 1989;49:6077–6084. [PubMed] [Google Scholar]
- Hoffman L. Isolation of inclusion bodies from rabbit lung parenchyma. J Cell Physiol. 1972;79:65–72. doi: 10.1002/jcp.1040790107. [DOI] [PubMed] [Google Scholar]
- Holmes EH, Hakomori S, Ostrander GK. Synthesis of type 1 and 2 lacto series glycolipid antigens in human colonic adenocarcinoma and derived cell lines is due to activation of a normally unexpressed β1–3N-acetylglucosaminyltransferase. J Biol Chem. 1987;262:15649–15658. [PubMed] [Google Scholar]
- Hook GER, Gilmore LB. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982;257:9211–9220. [PubMed] [Google Scholar]
- Kopitz J, Kisen GØ, Gordon PB, Bohley P, Seglen PO. Nonselective autophagy of cytosolic enzymes in isolated rat hepatocytes. J Cell Biol. 1990;111:941–953. doi: 10.1083/jcb.111.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs AL, Reith A, Seglen PO. Accumulation of autophagosomes after inhibition of hepatocytic protein degradation by vinblastine, leupeptin, or a lysomotropic amine. Exp Cell Res. 1982;137:191–201. doi: 10.1016/0014-4827(82)90020-9. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Brown WJ. Autophagic vacuoles rapidly fuse with preexisting lysosomes in cultured hepatocytes. J Cell Sci. 1992;102:515–526. doi: 10.1242/jcs.102.3.515. [DOI] [PubMed] [Google Scholar]
- Lee N, Wang W-C, Fukuda M. Granulocytic differentiation of HL-60 cells is associated with increase of poly-N-acetyllactosamine in Asn-linked oligosaccharides attached to human lysosomal membrane glycoproteins. J Biol Chem. 1990;265:20476–20487. [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Geelen JH, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuole. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Murphy RF, Lanni F, Taylor DL. A method for incorporating macromolecules in adherent cells. J Cell Biol. 1984;98:1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Dennis JW. The extent of polylactosamine glycosylation of MDCK LAMP-2 is determined by its Golgi residence time. Glycobiology. 1998;8:947–953. doi: 10.1093/glycob/8.9.947. [DOI] [PubMed] [Google Scholar]
- Nabi IR, Le Bivic A, Fambrough D, Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol. 1991;115:1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Rodriguez-Boulan E. Increased LAMP-2 polylactosamine glycosylation is associated with its slower Golgi transit during establishment of a polarized MDCK epithelial monolayer. Mol Biol Cell. 1993;4:627–635. doi: 10.1091/mbc.4.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, et al. α-Galactosidase A deficient mice: a model for Fabry disease. Proc Natl Acad Sci USA. 1997;94:2540–2544. doi: 10.1073/pnas.94.6.2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen E-L, Marjomäki VS, Reunanen H. 3-Methyladenine inhibits transport from late endosomes to lysosomes in cultured rat and mouse fibroblasts. Eur J Cell Biol. 1994;65:14–25. [PubMed] [Google Scholar]
- Ryan US, Ryan JW, Smith DS. Alveolar type II cells: studies on the mode of release of lamellar bodies. Tissue Cell. 1975;7:587–599. doi: 10.1016/0040-8166(75)90028-2. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Schlesinger PH, Steinberg TH, Mangel WF, Kobayashi T, Russel DG. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cytosol via two independent routes. J Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- Schmitz G, Müller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- Seglen PO, Bohley P. Autophagy and other vacuolar protein degradation mechanisms. Experientia. 1992;48:158–172. doi: 10.1007/BF01923509. [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: a specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin SP. A morphologic and cytochemical study of the great alveolar cell. J Histochem Cytochem. 1967;14:884–897. doi: 10.1177/14.12.884. [DOI] [PubMed] [Google Scholar]
- Spillmann D, Finne J. Poly-N-acetyllactosamine glycans of cellular glycoproteins: predominance of linear chains in mouse neuroblastoma and rat pheochromocytoma cell lines. J Neurochem. 1987;49:874–883. doi: 10.1111/j.1471-4159.1987.tb00975.x. [DOI] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? Bioessays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- Stratton CJ. The ultrastructure of multilamellar bodies and surfactant in the human lung. Cell Tissue Res. 1978;193:219–229. doi: 10.1007/BF00209036. [DOI] [PubMed] [Google Scholar]
- Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsiani DR, Touster O. Evidence that swainsonine pretreatment of rats leads to the formation of autophagic vacuoles and endosomes with decreased capacity to mature to, or fuse with, active lysosomes. Arch Biochem Biophys. 1992;296:556–561. doi: 10.1016/0003-9861(92)90610-9. [DOI] [PubMed] [Google Scholar]
- Tuo XH, Itai S, Nishikata J, Mori T, Tanaka O, Kannagi R. Stage-specific expression of cancer-associated type 1 and type 2 chain polylactosamine antigens in the developing pancreas of human embryos. Cancer Res. 1992;52:5744–5751. [PubMed] [Google Scholar]
- Ueno T, Muno D, Kominami E. Membrane markers of endoplasmic reticulum preserved in autophagic vacuolar membranes isolated from leupeptin-administered rat liver. J Cell Biol. 1991;266:18995–18999. [PubMed] [Google Scholar]
- Voorhut WF, Veenendahl T, Haagsman HP, Weaver TE, Whitsett JA, Van Golde LMG, Geuze HJ. Intracellular processing of pulmonary surfactant protein B in an endosomal/lysosomal compartment. Am J Physiol. 1992;263:L479–L486. doi: 10.1152/ajplung.1992.263.4.L479. [DOI] [PubMed] [Google Scholar]
- Voorhut WF, Weaver TE, Haagsman HP, Geuze HJ, van Golde LMG. Biosynthetic routing of pulmonary surfactant proteins in alveolar type II cells. Microsc Res Tech. 1993;26:366–373. doi: 10.1002/jemt.1070260504. [DOI] [PubMed] [Google Scholar]
- Wang W-C, Lee N, Aoki D, Fukuda M, Fukuda M. The poly-N-acetyllactosamines attached to lysosomal membrane glycoproteins are increased by prolonged association with the Golgi complex. J Biol Chem. 1991;266:23185–23190. [PubMed] [Google Scholar]
- Williams MC. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977;72:260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita KY, Tachibana Y, Ohkura T, Kobata A. Enzymatic basis for the structural changes of asparagine-linked sugar chains of membrane glycoproteins of baby hamster kidney cells induces by polyoma virus. J Biol Chem. 1985;260:3963. [PubMed] [Google Scholar]
- Yokota S, Himeno M, Kato K. Formation of autophagosomes during degradation of excess peroxisomes induced by di-(2-ethylhexyl)-phthalate treatment. III. Fusion of early autophagosomes with lysosomal compartments. Eur J Cell Biol. 1995;66:15–24. [PubMed] [Google Scholar]
- Youakim A, Romero PA, Yee K, Carlsson SR, Fukuda M, Herscovics A. Decrease in polylactosamines associated with lysosomal membrane glycoproteins during differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res. 1989;49:6889–6895. [PubMed] [Google Scholar]
- Yousefi S, Higgins E, Daoling Z, Pollex-Kruger A, Hindsgaul O, Dennis JW. Increased UDP-GlcNAc:Galβ1–3GalNAc-R (GlcNAc to GalNAc) β-1,6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991;266:1772–1782. [PubMed] [Google Scholar]