Abstract
In epidermal cells, the keratin cytoskeleton interacts with the elements in the basement membrane via a multimolecular junction called the hemidesmosome. A major component of the hemidesmosome plaque is the 230-kDa bullous pemphigoid autoantigen (BP230/BPAG1), which connects directly to the keratin-containing intermediate filaments of the cytoskeleton via its C terminus. A second bullous pemphigoid antigen of 180 kDa (BP180/BPAG2) is a type II transmembrane component of the hemidesmosome. Using yeast two-hybrid technology and recombinant proteins, we show that an N-terminal fragment of BP230 can bind directly to an N-terminal fragment of BP180. We have also explored the consequences of expression of the BP230 N terminus in 804G cells that assemble hemidesmosomes in vitro. Unexpectedly, this fragment disrupts the distribution of BP180 in transfected cells but has no apparent impact on the organization of endogenous BP230 and α6β4 integrin. We propose that the BP230 N terminus competes with endogenous BP230 protein for BP180 binding and inhibits incorporation of BP180 into the cell surface at the site of the hemidesmosome. These data provide new insight into those interactions of the molecules of the hemidesmosome that are necessary for its function in integrating epithelial and connective tissue types.
INTRODUCTION
The hemidesmosome is a complex molecular junction located along the basal aspect of basal epithelial cells, where it forms a connection with the underlying extracellular matrix (Jones et al., 1998; Borradori and Sonnenberg, 1999). It also serves to tether the keratin cytoskeleton to the cell surface and is the conduit for signals from the extracellular matrix to the cytoplasm of the cell (Giancotti, 1996; Jones et al., 1998; Borradori and Sonnenberg, 1999).
A major component of the inner cytoplasmic plaque of the hemidesmosome is a 230-kDa protein termed BP230 (BPAG1) (Klatte et al., 1989; Jones et al., 1998; Borradori and Sonnenberg, 1999). BP230 was originally identified as one of two major antigens that is recognized by autoantibodies present in the serum of patients with bullous pemphigoid (Stanley, 1993). BP230 autoantibodies bind to a specific site within the cytoplasmic plaque of the hemidesmosome to which keratin bundles attach (Klatte et al., 1989). Molecular characterization of BP230 has demonstrated that it belongs to the “plakin” family of proteins and is related to the intermediate filament-binding proteins desmoplakin and plectin (Sawamura et al., 1991; Tanaka et al., 1991; Green et al., 1992; Ruhrberg and Watt, 1997). Like desmoplakin and plectin, BP230 can interact directly with keratin filaments, as shown by Fuchs and coworkers (Guo et al., 1995). Furthermore, hemidesmosomes in mice in which BP230 has been ablated lack well-developed cytoplasmic plaques, and keratin filament bundles fail to interact with the cell surface (Guo et al., 1995). Thus, BP230 is an important connector in the series of molecules that link the extracellular matrix with the cytoskeleton of an epithelial cell. Recently, considerable evidence has been accrued that supports the notion that this connection is essential for tissue integrity. For example, pathogenic autoantibodies against the BP antigens bind to hemidesmosomes and disrupt epidermal cell interaction with the connective tissue, leading to blistering of the skin (Stanley, 1993). Mutations in hemidesmosome components and failure to assemble normal hemidesmosomes by epidermal cells have a similar consequence (Borradori and Sonnenberg, 1999).
There are several isoforms of BP230, termed BPAG1n1, BPAG1n2, and BPAG1n3, that are present primarily in sensory neurons of the nervous system (Yang et al., 1996, 1999). BPAG1n1 has a distinct N-terminal domain that is capable of binding actin, whereas its C terminus interacts with peripherin-type intermediate filaments, thereby cross-linking actin-containing and intermediate filament systems in neurons (Yang et al., 1996; Leung et al., 1999). BPAG1n3 has a microtubule-binding motif at its N terminus and can mediate interaction between the intermediate filament system and the microtubule network of neurons (Yang et al., 1999). There is no actin- or microtubule-binding domain in the N terminus of the BP230 isoform found in epithelial cells (Sawamura et al., 1991; Tanaka et al., 1991). Rather, its N-terminal binding partner is unknown at this time. Two studies have previously provided indirect evidence that BP230 associates with the second BP antigen (BP180, BPAG2, type XVII collagen) (Borradori et al., 1998; Hopkinson et al., 1998). BP180 is a type II transmembrane protein whose extracellular region is composed primarily of collagen-like repeats (Giudice et al., 1991, 1992; Hopkinson et al., 1992; Li et al., 1993). It has already been demonstrated by a number of workers that the N-terminal cytoplasmic domain of BP180 can bind the β4 integrin component of hemidesmosomes (Borradori et al., 1997; Aho and Uitto, 1998; Hopkinson et al., 1998; Schaapveld et al., 1998). In this study, we tested the hypothesis that BP180 binds directly to the N-terminal domain of BP230, thereby mediating the interaction of the cytoskeleton with a transmembrane component of the hemidesmosome.
MATERIALS AND METHODS
Cell Culture and Transfection Procedure
804G cells were cultured as detailed by Riddelle et al. (1991). 804G cells were maintained for 72 h on 22-mm glass coverslips. They were transfected with 4 μg of plasmid DNA by means of the calcium phosphate protocol detailed by Sambrook et al. (1989). At 24 h after transfection, cells were harvested for immunoblotting or processed for immunofluorescence microscopy (see below).
Yeast Two-Hybrid Assay
In brief, cDNAs encoding portions of BP180, BP230, and β4 integrin were amplified with the use of reverse transcription PCR (RT-PCR) from MCF10A mRNA with specific forward and reverse primers containing engineered restriction sites (Figure 1). These fragments were digested with the appropriate enzymes, isolated from an agarose gel with the use of the QIAquick gel extraction kit (Qiagen, Chatsworth, CA), and ligated in frame into the digested yeast expression vector pACT2-1 or pAS1 (Clontech, Palo Alto, CA). All constructs were sequenced to ensure that the cDNAs were in frame and without error with the use of Big Dye automated sequencing reagents (Applied Biosystems, Foster City, CA) on an ABI Prism DNA sequencer (Applied Biosystems). Individual clones containing these constructs were grown in selective medium and DNA prepared from the clones with the use of a Wizard mini prep kit (Promega, Madison, WI).
Figure 1.
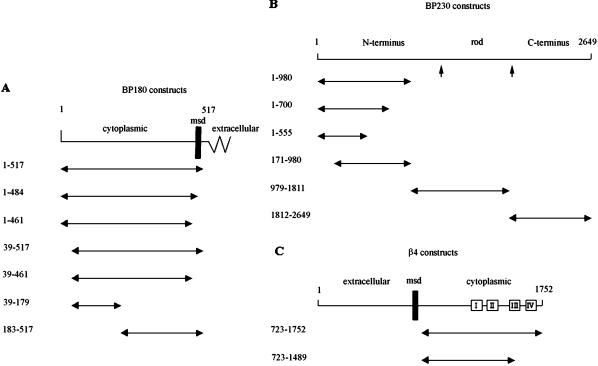
Scheme of the protein fragments encoded by cDNAs that are used in yeast two-hybrid, recombinant protein, and transfection assays. The amino acid residue numbers of each fragment are displayed along the left, and a double-ended arrow depicts the location of each within the hemidesmosome molecule under assay. (A) BP180 is shown minus most of its large extracellular domain. The cytoplasmic and membrane-spanning domains (msd) and a small region of the extracellular domain are marked. (B) Arrowheads demarcate the boundaries of the N terminus, rod, and C terminus (keratin-binding domain) of BP230. (C) The complete β4 protein is shown. Boxes represent its four fibronectin type III repeats (I–IV). The extracellular, membrane-spanning (msd), and cytoplasmic domains are marked.
DNA preparations were used to transform the yeast strain Y190 according to protocols outlined in the Matchmaker 2 two-hybrid system manual (Clontech). Transfected colonies were selected by growth in medium lacking leucine, tryptophan, and histidine (−Leu/−Trp/−His) but containing 25 mM 3-amino-1,2,4-triazole. The latter was used to inhibit low levels of “leaky” expression of His3p in the reporter yeast strain. To monitor transfection efficiency of both plasmids, the transfected yeast was also plated onto −Leu medium, −Trp medium, or −Leu/−Trp medium. At 7 d, the number of colonies growing on both the −Leu/−Trp and −Leu/−Trp/−His media were scored. Yeast colonies growing on both −Leu/−Trp/−His and −Leu/−Trp media were also spotted onto nylon filters and flash frozen in liquid nitrogen. To detect activation of the reporter gene lacZ and the resulting expression of β-galactosidase, the filters were placed on Whatman paper soaked in a solution containing X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). A binding domain plasmid (pVA3-1), containing a cDNA encoding murine p53, and an activation domain plasmid (pTD1-1), containing the SV40 large T-antigen coding sequence, were used as part of our control studies (Clontech).
Recombinant Protein Preparation
The cytoplasmic domain of BP180 (residues 1–461) and the amino one-third of BP230 (residues 1–980) were prepared as 6X His-tagged recombinant fusion proteins in Escherichia coli. To prepare the relevant portion of BP180, RT-PCR was performed on MCF10A mRNA with the use of appropriate forward and reverse primers. The BP230 partial cDNA was excised from a pACT2-1 vector by digesting the plasmid with BglII, which cuts on both sides of the BP230 insert and includes sequence encoding an epitope recognized by a mAb (HA11) against influenza hemagglutinin (HA) (BAbCO, Richmond, CA). Both the BP180 and BP230 cDNA fragments were cloned in frame into the pET32 vector (Novagen, Madison, WI). The vectors were sequenced to confirm that the reading frame was maintained and that the sequences were without error. Both plasmids were transformed into E. coli cells that were subsequently induced to produce recombinant protein by the addition of isopropyl-β-d-thiogalactopyranoside to the medium. The cells were lysed, and extracts were incubated overnight in a 6 M urea buffer. Cell extracts were passed over a His-Bind resin column (Novagen), and bound fusion protein eluted in an imidazole elution buffer in the presence of 6 M urea. The eluent was dialyzed against 10 mM Tris buffer, pH 7.5, overnight at 4°C, concentrated by lyophilization, and resuspended in sterile H2O. The purity of the recombinant polypeptides was assessed by visualizing the protein samples by SDS-PAGE and by Western blotting.
Green Fluorescent Protein and HA-tagged Constructs
cDNAs were generated by RT-PCR from MCF10A mRNA with the use of BP180 and BP230 sequence-specific forward and reverse primers that included a BamHI restriction site. The resulting fragments, encoding residues 1–517 of BP180 and residues 1–980 of the BP230 molecule, were cloned in frame into the BamHI site of the multiple cloning site of the pEGFP (Invitrogen, Carlsbad, CA) expression vector. A cDNA encoding the rod domain of BP230 (residues 979-1811), including an HA tag incorporated at its 5′ end, was cloned into pCR3.1 (Invitrogen).
Antibodies
5E, a human mAb against BP230, was a gift from Dr. Takashi Hashimoto (Keio University, Tokyo, Japan) (Hashimoto et al., 1993). A mouse IgM mAb preparation (1804b) against the N-terminal domain of BP180 was described by Hopkinson et al. (1992) and Riddelle et al. (1991). J17 rabbit antiserum was generated against the same BP180 domain (Hopkinson et al., 1992). A mAb against Green Fluorescent Protein (GFP) was purchased from Clontech. mAb HA11 against the HA epitope tag was obtained from BAbCO. The β4 integrin polyclonal rabbit antiserum was purchased from Chemicon (Temecula, CA).
Gel Electrophoresis, Immunoblotting, and Immunoprecipitation
Recombinant proteins and bacterial and mammalian cell extracts were solubilized in sample buffer (8 M urea, 10% β-mercaptoethanol, 1% SDS, 10% glycerol in 10 mM Tris-HCl, pH 6.8) and were subjected to SDS-PAGE with the use of 7.5% acrylamide gels (Bio-Rad, Hercules, CA) (Laemmli, 1970). For Western immunoblotting, proteins separated on gels were transferred to polyvinylidene difluoride membranes that were then processed with antibody as described elsewhere (Harlow and Lane, 1988).
For immunoprecipitation studies, ∼1 μg each of the recombinant BP230 and BP180 fragments were mixed together in 100 μl of Tris-buffered saline, pH 8.0, containing a cocktail of protease inhibitors. After a 2-h incubation at 4°C, J17 antiserum against BP180 was added to a 1:100 dilution and incubated at 4°C for another 2 h. Subsequently, 20 μl of protein G–agarose (Life Technologies/BRL, Gaithersburg, MD) was added to the mixture for an additional 2 h. The protein G–agarose was collected by centrifugation, washed four times in buffer, and then solubilized in sample buffer. The resulting protein solution was processed for Western immunoblotting as detailed above.
Immunofluorescence Microscopy
Cells, grown on glass coverslips, were fixed for 1 min in 3.7% formaldehyde and then extracted in 0.5% Triton X-100 at 4°C for 8 min. Single- and double-label immunofluorescence was performed as detailed previously (Riddelle et al., 1991). After mounting, coverslips were viewed on a Zeiss (Thornwood, NY) LSM510 confocal microscope fitted with appropriate filters for visualization of GFP as well as fluorescein- and rhodamine-conjugated probes (Zeiss). Controls for immunocytochemistry included omission of primary antibodies or use of irrelevant IgG and IgM probes to determine nonspecific binding of secondary antibodies.
RESULTS
The N Terminus of BP230 Interacts with the N Terminus of BP180
Yeast Two-Hybrid Assays To provide evidence that the hemidesmosome components BP230 and BP180 interact directly, we used the yeast two-hybrid system. A cDNA that encodes residues 1–517 of BP180 (BP1801–517), coupled to the DNA activation domain of the pACT vector, was coexpressed in yeast cells together with a cDNA encoding the first 980 residues of BP230 (BP2301–980), coupled to the DNA-binding domain of the pAS2-1 vector (Figure 1). The transfected yeast was plated onto solid medium either lacking leucine and tryptophan (−Leu/−Trp) or lacking leucine, tryptophan, and histidine (−Leu/−Trp/−His). The number of colonies growing on these two media was compared at 7 d. The yeast show >50% plating efficiency on the −Leu/−Trp/−His medium compared with yeast plated onto −Leu/−Trp medium, implying an interaction between BP180 and BP230. In addition, we observed activation of transcription of the lacZ reporter gene in the transfected yeast clones with the use of a blue/white β-galactosidase assay (Fields and Sternglanz, 1994) (Table 1). Colonies given the grade ++ turned bright blue within 6 h, whereas those showing a less intense blue color were graded + in this assay. In addition, unless indicated, no constructs autoactivated yeast when transfected alone or when cotransfected with a control plasmid (pVA3/pAS2-1 or pTD1/pACT2 as appropriate) (results not shown).
Table 1.
Pairs of yeast vectors containing the various indicated test constructs were cotransfected into Y190 yeast, plated onto both −Leu/−Trp and −Leu/−Trp/−His media, and allowed to grow for 7 d
| Test pairs | Plating efficiency on −Leu/−Trp/−His medium | β-gal activity |
|---|---|---|
| 2301–980 pAS2-1/1801–517 pACT2 | ++ | ++ |
| 2301–700 pAS2-1/1801–517 pACT2 | ++ | ++ |
| 2301–555 pAS2-1/1801–517 pACT2 | − | − |
| 230171–980 pAS2-1/1801–517 pACT2 | − | − |
| 230979–1811 pAS2-1/1801–517 pACT2 | − | − |
| 2301812–2649 pAS2-1/1801–517 pACT2 | − | − |
| 2301–980 pAS2-1/1801–484 pACT2 | ++ | ++ |
| 2301–980 pAS2-1/18039–517 pACT2 | ++ | ++ |
| 2301–980 pAS2-1/18039–461 pACT2 | ++ | ++ |
| 2301–980 pAS2-1/18039–179 pACT2 | − | − |
| 2301–980 pAS2-1/180183–517 pACT2 | ++ | ++ |
| β4723–1752 pAS2-1/1801–517 pACT2 | ++ | ++ |
| β4723–1752 pAS2-1/2301–980 pACT2 | + | + |
| β4723–1752 pAS2-1/230979–1811 pACT2 | − | − |
| β4723–1752 pAS2-1/2301812–2649 pACT2 | ++ | ++ |
| β4723–1489 pAS2-1/1801–517 pACT2 | ++ | ++ |
| β4723–1489 pAS2-1/2301–980 pACT2 | − | − |
| β4723–1489 pAS2-1/230979–1811 pACT2 | − | − |
| β4723–1489 pAS2-1/2301812–2649 pACT2 | − | − |
Plating efficiency on −Leu/−Trp/−His medium is expressed relative to that on −Leu/−Trp medium. ++, +, and − indicate plating efficiencies of >50%, <15%, and 0%, respectively. Colonies that grew on the restrictive plates were transferred to nylon sheets. These sheets were then placed on β-galactosidase-soaked filters, and the ability of the yeast colonies to turn blue was assessed. To assay for β-galactosidase activity in transfected yeast that was unable to develop on −Leu/−Trp/−His medium, colonies that grew on −Leu/−Trp medium were analyzed. ++ indicates colonies that turned intensely blue, whereas + indicates colonies that turned weakly blue at 6 h of development. Each pair was tested at least three times for plating efficiency on the restrictive −Leu/−Trp/−His medium and in the β-galactosidase assay.
To further analyze the interaction between BP230 and BP180, we generated a series of deletions in both the BP230 and BP180 cDNA fragments, cotransformed them into yeast, and assayed for direct binding as described above. The results are shown in Table 1. The BP230 fragment BP2301–700, like BP2301–980, can interact with BP1801–517. However, if 145 residues are removed from the C terminus of this particular BP230 fragment, creating BP2301–555, its ability to bind BP180 is lost (Table 1). Likewise, deletion of the first 170 residues from BP2301–980 produces a BP230 piece (BP230171–980) incapable of interaction with BP1801–517. These results indicate that residues 1–170 and 555–700 of the BP230 protein are both required for BP230–BP180 interactions.
As with BP230, a series of deletions in BP180 was also created by removing residues from either end of BP1801–517 to define further the region of BP180 that interacts with BP230 (Figure 1, Table 1). A BP180 fragment of 484 residues (BP1801–484) that lacks any extracellular residues also binds BP2301–980. Removal of the membrane-spanning domain of the former (residues 462–484) produces a BP180 protein (BP1801–461) that autoactivates in yeast, precluding any analysis (Table 1). However, removal of the first 38 amino acids from this fragment (BP18039–461) prevents such autoactivation. Moreover, this fragment is capable of interaction with BP2301–980 (Table 1). We have shown previously that these residues are necessary for correct targeting of the BP180 molecule to the hemidesmosome in 804G cells (Hopkinson et al., 1995). Thus, the hemidesmosome-targeting sequence of BP180 is distinct from its BP230-binding site. Removal of the first 182 residues of BP1801–517 produces a BP180 fragment (BP180183–517) that is also capable of interaction with BP2301–980 (Table 1). However, a BP180 polypeptide consisting of residues 39–179 (BP18039–179) is incapable of any detectable interaction with BP2301–980 (Table 1). Together, these results suggest that the site of binding of BP180 to BP230 must be contained within the region between residues 180 and 460 of the BP180 cytoplasmic domain.
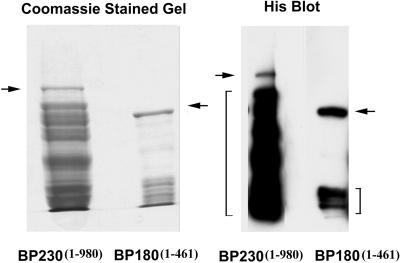
To determine whether domains other than the N-terminal fragment of BP230 can interact directly with BP180, we also generated a pACT vector containing sequences encoding either residues 979-1811 (BP230979–1811), a fragment containing most of the rod domain of the molecule, or residues 1812–2649 (BP2301812–2649), the intermediate filament-binding, C-terminal domain of BP230 (Sawamura et al., 1991; Tanaka et al., 1991; Yang et al., 1996). These were cotransfected with BP1801–517 in the pAS2-1 vector. Neither of these sets of cotransformants is able to grow on the restrictive −Leu/−Trp/−His medium, nor is any color change detected when the cotransfectants grown on −Leu/−Trp medium are processed in the β-galactosidase assay (Table 1). Thus, BP180–BP230 interaction is apparently limited to the N-terminal domain of BP230. Recombinant Protein Assays To confirm the results of our yeast two-hybrid analysis, we used recombinant BP180 and BP230 polypeptides, each tagged with 6X His, in an immunoprecipitation assay. The first 980 amino acids of BP230 tagged with the HA epitope were expressed in bacteria and purified over a His-Bind resin column, as described in MATERIALS AND METHODS. Similarly, the cytoplasmic domain of BP180 (residues 1–461) was also expressed in E. coli and purified by column chromatography. The purified polypeptides were separated by SDS-PAGE and visualized with Coomassie stain or by immunoblotting with the use of a probe recognizing the 6X His tags on each protein fragment (Figure 2). Multiple lower-weight bands are seen in the Coomassie-stained gel profile for BP230. These represent degradation products because these same bands are also detected by the His probe (Figure 2).
Figure 2.
Purification of recombinant BP180 and BP230 fragments. cDNAs encoding the entire cytoplasmic region of BP180 (residues 1–461) and the N terminus of BP230 (residues 1–980 with an HA epitope tag at the N terminus) were cloned into expression vectors that also contained sequences for a 6X His tag. Both fragments were expressed in bacteria and purified from bacterial extracts over His-Bind resin (Novagen). An aliquot of each purified protein was diluted in sample buffer and examined by SDS-PAGE. One such gel was stained with Coomassie to visualize the isolated polypeptides, as indicated. Arrows mark the BP180 fragment of 75 kDa and the BP230 protein fragment of 110 kDa. There are several other lower-molecular-mass polypeptides in both preparations that copurify with the BP180 and BP230 fragments (brackets). These appear to be proteolytic breakdown products because they are recognized by the His probe.
The BP230 and BP180 recombinant proteins were mixed in Tris-buffered saline containing a cocktail of protease inhibitors. Proteins were precipitated by the addition of a 1:100 dilution of antiserum J17, which was generated against the N terminus of BP180 (Hopkinson et al., 1992). Precipitated proteins were separated by SDS-PAGE and transferred to nitrocellulose. The filter was probed with either the J17 antiserum or HA11 antibodies that recognize the HA epitope on the BP230 polypeptide. As shown in Figure 3, the purified BP230 fragment is coprecipitated with the purified BP180 cytoplasmic domain, supporting the idea that these two proteins are capable of interacting directly.
Figure 3.
Immunoprecipitation assays. Approximately 1 μg each of the purified BP180 and HA-tagged BP230 fragments shown in Figure 2 were incubated either together or separately in a 10 mM Tris buffer with 0.1% Tween-20 at 4°C for 2 h. A polyclonal antibody directed against the BP180 cytoplasmic domain was added to the protein mixture for 2 h, followed by the addition of protein G–agarose beads (Life Technologies/BRL). The precipitated proteins were subjected to SDS-PAGE and transferred to nitrocellulose. The latter was either incubated in antibody HA11 directed against the HA tag or rabbit antiserum J17, which recognizes BP180 (as indicated). In lane 1, the HA antibody recognizes the BP230 recombinant polypeptide, indicating that it has been precipitated with BP180. Lanes 2 and 3 show no reactivity with this antibody. The BP180 antibody reacts with the BP180 protein fragment in lanes 1 and 3.
Transfection Analyses
We next assessed the consequences of expressing the BP230 N-terminal domain on hemidesmosome protein organization in cultured cells. For these studies, we used 804G cells because these cells express all of the known hemidesmosome proteins and assemble bona fide hemidesmosomes in vitro (Riddelle et al., 1991). 804G cells were grown to ∼60% confluence and then transfected according to standard procedures (see Hopkinson et al., 1995). After 24 h, the transfected cell populations were fixed and prepared for immunofluorescence. Our initial studies involved attempting to express the various N-terminal domain pieces of BP230 tagged with the HA epitope. Although we have been successful in visualizing the HA-tagged protein products of a number of transgenes in 804G cells, we were unable to detect the expression of HA-tagged BP230 N-terminal fragments in a variety of different epithelial cell types. In contrast, when a GFP-tagged N-terminal fragment of BP230 (BP230GFP1–980) is expressed in 804G cells, we see bright cytoplasmic fluorescence (Figure 4, A, D, and G). We have confirmed that the transgene product of the appropriate molecular weight is expressed in the transfected cell population by immunoblotting with the use of an anti-GFP antibody probe (Figure 5).
Figure 4.
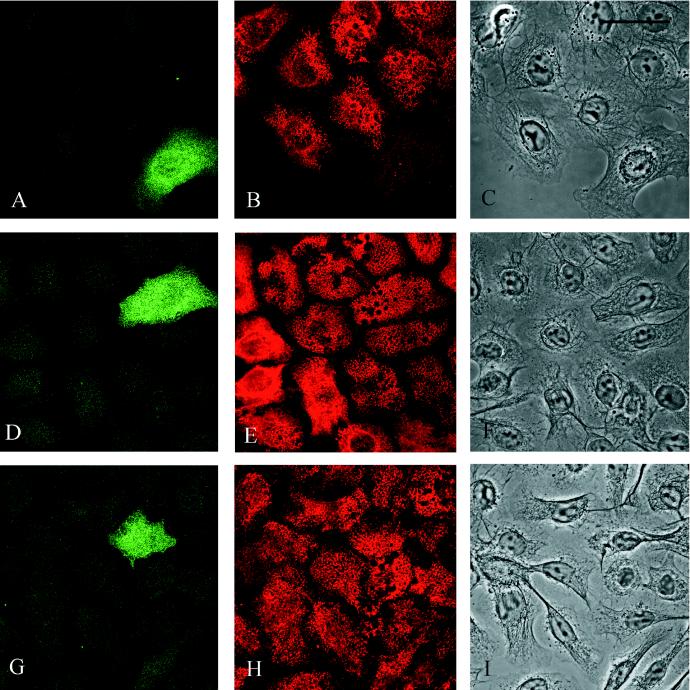
A GFP-tagged BP230 N-terminal fragment (BP230GFP1–980) was expressed in 804G cells. Expression of the transgene is shown in A, D, and G. The fragment shows a diffuse distribution throughout the cytoplasm of the transfected cells. The same transfected cell populations were processed for immunofluorescence with the use of a mAb preparation against BP180 (B), 5E mAbs against BP230 (E), and a rabbit anti-β4 integrin serum (H). BP230 and β4 in E and H show a normal punctate, basal localization in the transfected and nontransfected cells, whereas there is little obvious basal staining for BP180 in the cell expressing BP230GFP1–980 in B. It should be noted that BP180 shows a normal basal localization in cells that fail to express BP230GFP1–980 in B. Cells were viewed in a confocal microscope with the focal plane being at the site of cell–substrate interaction. C, F, and I show phase images of the cells. Bar, 25 μm.
Figure 5.
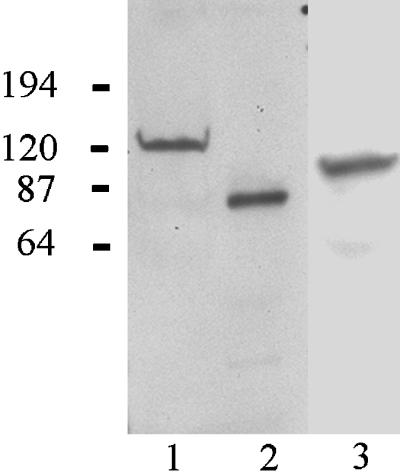
Extracts of 804G cells transfected with vectors encoding BP230GFP1–980 (lane 1), BP180GFP1–517 (lane 2), and BP230HA979–1811 (lane 3) were subjected to SDS-PAGE and then transferred to nitrocellulose. The nitrocellulose was then processed for immunoblotting with the use of either an antibody against GFP (lanes 1 and 2) or an antibody against the HA epitope tag (lane 3).
The BP230GFP1–980 shows no obvious polarization and fails to localize in a hemidesmosome-like staining pattern in transfected 804G cells (Figure 4, A, D, and G) (Riddelle et al., 1991). Furthermore, BP180 fails to localize along the site of cell–substrate interaction in the transfected cells, although “wild-type” BP230 and the β4 integrin subunit show their normal basal, cat-paw localization in 804G cells expressing the BP230 N-terminal transgene product (Figure 4, B, E, and H).
To determine whether the GFP tag might be inhibiting incorporation of the BP230 N-terminal domain into hemidesmosomes, we also expressed a GFP-tagged BP180 fragment in 804G cells (BP180GFP1–517). We have shown previously that this domain is capable of targeting to the hemidesmosomes of cultured cells (Hopkinson et al., 1995). BP180GFP1–517 shows basal staining in transfected 804G cells, as shown in Figure 6A, and it colocalizes with BP230 in a cat-paw pattern (Figure 6B). As an additional control, we also expressed a fragment of BP230 (BP230HA979–1811) that includes a portion of its rod domain in 804G cells. Although this fragment fails to polarize in transfected 804G cells (Figure 7, A and D), it has no impact on the organization of other hemidesmosome elements in the cells, including BP180 and BP230 (Figure 7, B and E). As in the case of BP230GFP1–980, we confirmed by immunoblotting that transfected cell populations express BP180GFP1–517 and BP230HA979–1811 (Figure 5).
Figure 6.
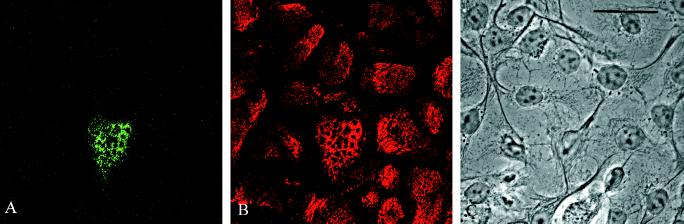
A GFP-tagged BP180 fragment (BP180GFP1–517) was expressed in 804G cells. The cells were subsequently processed for immunofluorescence microscopy with the use of 5E antibodies against BP230. Expression of the transgene is shown in A. The fragment shows a basal, punctate localization and codistributes with staining generated by the BP230 mAb probe in B. Cells were viewed in a confocal microscope with the focal plane being at the site of cell–substrate interaction. C shows a phase image of the cells. Bar, 25 μm.
Figure 7.
An HA epitope–tagged BP230 rod domain fragment (BP230HA979–1811) was expressed in 804G cells. The cells were prepared for double-label indirect immunofluorescence microscopy with the use of HA11 antibody (A and D) in combination with either antibody 5E against BP230 (B) or a mAb against BP180 (E). The BP230HA979–1811 fragment shows a diffuse distribution throughout the cytoplasm of the transfected cells (A and D), whereas both BP180 and BP230 show a basal, punctate stain in transfected and nontransfected cells (B and E). Cells were viewed in a confocal microscope with the focal plane being at the site of cell–substrate interaction. C and F show phase images of the cells. Bar, 25 μm.
Thus, although our yeast data indicate that BP230 and BP180 interact, the results of the transfection studies detailed above provide an indication that BP230 can still associate morphologically with the α6β4 integrin heterodimer in vivo even in the absence of polarized BP180. To determine whether this association is direct, we assayed for interaction between the cytoplasmic domain of the β4 integrin subunit and BP230 in the yeast two-hybrid system with the use of a β4 cytoplasmic domain (β4723–1752) fragment and three different fragments of BP230 (BP2301–980, BP230979–1811, and BP2301812–2649) (Figure 1). In transfected yeast, the C-terminal fragment of BP2301812–2649 shows interaction with the β4 cytoplasmic domain, as indicated by the plating efficiency of the yeast on the restrictive −Leu/−Trp/−His medium. These same colonies turn intensely blue at 6 h in a blue/white β-galactosidase assay. Removal of the C-terminal 263 residues of the β4 cytoplasmic domain produces a fragment incapable of interacting with BP2301812–2549 as well as BP230979–1811 and BP2301812–2649 (Table 1). On the other hand, β4723–1489 shows interaction with BP1801–517. There is no evidence of any association between β4723–1752 and the BP230979–1811 fragment in the yeast two-hybrid assay. Yeast cotransfected with vectors containing cDNAs encoding β4723–1752 and BP2301–980 show limited plating efficiency on −Leu/−Trp/−His medium and turn weakly blue in the β-galactosidase assay. These results suggest that BP230 interacts primarily with β4 via its C-terminal domain, although they do not rule out the possibility of an interaction between the N terminus of BP230 and the β4 cytoplasmic domain in the hemidesmosome.
DISCUSSION
The authors of two publications have speculated that BP180 and BP230 may form a complex at the site of the hemidesmosome (Borradori et al., 1998; Hopkinson et al., 1998). In the first of these studies, it was suggested that BP230 may interact with BP180 based on a study of immortalized keratinocytes derived from a patient with generalized atrophic benign epidermolysis bullosa (GABEB) in which BP180 was not expressed. In the GABEB cells used, BP230 shows a diffuse localization. However, when these GABEB cells are induced to express BP180 protein, both BP180 and BP230 target to hemidesmosome-like structures at sites of cell–substrate interaction, implying that there is a relationship between the two (Borradori et al., 1998). In Hopkinson et al. (1998), we showed that disruption of the association between BP180 and the α6β4 integrin heterodimer does not result in a loss of colocalization of BP230 and BP180, suggesting the possibility that BP230 and BP180 are tightly coupled. In this work, we have provided the first direct evidence that BP180 and BP230 may interact and have shown that this interaction can be mediated by an association between the N-terminal cytoplasmic domain of BP180 and the N-terminal domain of BP230. Furthermore, our data reveal that two regions encompassing amino acid residues 1–171 and 555–700 in the N terminus of BP230 are necessary for this interaction. Conversely, residues 180–460 in the cytoplasmic domain of the BP180 molecule are involved in its interaction with BP230. In this context, it is interesting to note that whereas the C terminus of various BP230 isoforms associate with the intermediate filament cytoskeleton (and the β4 integrin subunit, in the case of the epithelial BP230 isoform, as we discuss below), the specificity of the cytoskeletal and membrane interactions of the isoforms of BP230 is in large part determined by their distinct N-terminal sequences. For example, a neuronal isoform of BP230 (BPAG1n1) possessing an N-terminal actin-binding motif that mediates its interaction with the microfilament system has been identified in certain neurons (Yang et al., 1996, 1999). Recently, a microtubule-binding domain has been characterized in the N terminus of an additional neuronal BP230 isoform (BPAG1n3) (Yang et al., 1999). Based on the latter data, it has been argued that isoforms of BP230 integrate all three cytoskeleton systems, at least in neurons. Here we have added to the list of proteins that may interact with the N-terminal domain of spliced variants of BP230. In epithelial cells, the distinct sequence at the N-terminal domain of BP230 appears to allow it to interact with BP180 at the site of the hemidesmosome, and, unlike its neuronal counterparts, BP230 in epithelial cells shows no obvious association with either microtubules or microfilaments.
To extend our analyses of the protein interactions of the N terminus of BP230, we undertook a molecular genetic study with the use of 804G cells that assemble hemidesmosomes in vitro (Riddelle et al., 1991). This investigation was stymied for a considerable period because we were unsuccessful at detecting expression of the BP230 N-terminal fragment (1–980) not only in transfected 804G but also in a number of human epithelial cells, such as Fgmet2 cells, when the fragment was tagged with either the c-myc or the HA epitope (our unpublished observations). We speculate that the fragment is unstable under these circumstances. This is consistent with our observation that there is breakdown of the same fragment when it is made recombinantly in bacteria. However, when tagged with GFP, we have been able to visualize the BP230 N-terminal fragment in transfected cells. We assume that GFP provides some stability to the protein, and we have been able to detect expression of the transgene in cells viewed in the fluorescence microscope and in extracts of cells processed for immunoblotting.
Once we were able to detect the GFP-tagged N-terminal fragment in transfected cells, we were surprised to observe that its expression appears to have a dominant negative effect on the polarization of BP180 but not on BP230 and the α6β4 integrin transmembrane components of the hemidesmosome. We had assumed that the BP230 N-terminal fragment would most likely compete with the endogenous BP230 for BP180 binding in vivo, resulting in endogenous BP230 failing to localize at the site of hemidesmosomes. We did not expect to see any impact on the distribution of endogenous BP180. However, contrary to our expectations, in those 804G cells expressing the BP230 N terminus we observed an inhibition of BP180 polarization. One explanation for this phenomenon is that the N-terminal BP230 fragment binds BP180 before its incorporation into the cell surface of an epithelial cell. This would occur when the BP180 molecule is still in the Golgi apparatus or in some sort of Golgi transfer vesicle. The interaction of BP180 and the N-terminal BP230 fragment may then either target the protein complex for degradation or simply prevent it from reaching the cell surface. This model also leads to the prediction that during normal hemidesmosome assembly BP180 and BP230 may associate in the cytoplasm before the former is incorporated into the basal cell surface. There is some evidence to support this, because we observed colocalization of BP230 and BP180 in the cytoplasm before hemidesmosome assembly in cells migrating over connective tissue in explanted tissue pieces (Jones, unpublished observations). Alternatively, the N terminus of BP230 may link to a second unknown protein in the transfected 804G cells. The latter protein may prevent a BP180/BP230 N-terminal fragment complex from incorporating into hemidesmosomes.
Although our study indicates that BP180 may play an important role in linking BP230 to the plaque of the hemidesmosome, our transfection studies and previously published data would suggest that BP230 interacts with more than just BP180 to effect its interaction with the basal cell surface of epithelial cells. In particular, we show that in cells in which BP180 organization has been disrupted, endogenous BP230 nonetheless targets to the cell surface in contact with the substrate. Likewise, it has been shown that in the basal keratinocyte layer of the skin of most, if not all, GABEB patients, BP230 is distributed normally along the site of epidermal interaction with the basement membrane zone, despite the fact that epidermal cells in these patients lack BP180 expression (Jonkman et al., 1995; McGrath et al., 1995; Chavanas et al., 1997). Our data provide some evidence that interaction between the cytoplasmic domain of the β4 integrin subunit and the BP230 C-terminal domain, or possibly the N terminus of BP230, can ensure a basal localization of the BP230 molecule even when BP180 fails to polarize in cells. The reason that such an association does not occur in the immortalized GABEB cells described by Borradori et al. (1998) is unclear; this lack of association may reflect a defect induced in the GABEB cells during the immortalization process. Moreover, our results would appear to be more consistent with the observation that GABEB patient hemidesmosomes, although somewhat rudimentary, possess many features of normal hemidesmosomes, including a three-layered plaque, and show extensive association with keratin intermediate filaments (see, for example, Figure 2B in Jonkman et al., 1995). If these GABEB hemidesmosomes were to lack BP230 as well as BP180, then one would assume that they would appear more like hemidesmosomes in the BP230 knockout mouse, which are deficient in their inner cytoplasmic plaque and keratin filament bundle attachment (Guo et al., 1995).
The region of β4 integrin that we have identified as being potentially important in BP230 interaction is contained within residues 1489–1752 of the cytoplasmic domain of the β4 subunit. This domain consists of a portion of the third and all of the fourth fibronectin type III repeat and the very C terminus of the cytoplasmic domain of the β4 molecule (Figure 1). Therefore, the binding site must lie adjacent to the predicted site of BP180 interaction with the β4 integrin subunit, which resides in the third fibronectin type III repeat and the C-terminal portion of the so-called connecting segment (Figure 1) (Schaapveld et al., 1998). Both sites are distinct from the hemidesmosome-targeting sequence of β4 integrin, which extends through the second fibronectin type III repeat and an N-terminal section of the connecting segment (Spinardi et al., 1993, 1995; Niessen et al., 1997; Schaapveld et al., 1998) (Figure 1). The latter region is also involved in β4 binding to HD1/plectin (Niessen et al., 1997).
In summary, we have defined a novel series of molecular interactions by which keratin bundles are tethered to cell surface proteins at the site of the hemidesmosome plaque. Rezniczek et al. (1998) have already shown that plectin mediates the association of keratin-type intermediate filaments with the cytoplasmic domain of the β4 subunit of the α6β4 integrin heterodimer. Here we show that BP230, which, like plectin, binds intermediate filaments, may interact with both BP180 and the α6β4 integrin, thereby permitting a second means by which keratin bundles can associate with transmembrane components of the hemidesmosome. We speculate that these multiple linkage systems play synergistic roles in maintaining the firm anchorage of keratin bundles to the hemidesmosome plaque. This idea is supported by studies that reveal that ablation of either plectin or BP230 in keratinocytes results in loss of keratin bundle association with the plaque of the hemidesmosome (Guo et al., 1995; Gache et al., 1996; McLean et al., 1996; Smith et al., 1996). These complex interconnections may enhance further the stability of keratin interaction with the cell surface and not only modulate cell–matrix association but also facilitate signal transduction at the site of the hemidesmosome (Giancotti, 1996).
ACKNOWLEDGMENTS
We thank Xiang He for technical assistance. We are grateful for grant support from the National Institutes of Health (RO1 GM38470 to J.C.R.J.).
Abbreviations used:
- BP
bullous pemphigoid
- GABEB
generalized atrophic epidermolysis bullosa
- GFP
Green Fluorescent Protein
- HA
hemagglutinin
REFERENCES
- Aho S, Uitto J. Direct interaction between the intracellular domains of bullous pemphigoid antigen 2 (BP180) and β4 integrin, hemidesmosomal components of basal keratinocytes. Biochem Biophys Res Commun. 1998;243:694–699. doi: 10.1006/bbrc.1998.8162. [DOI] [PubMed] [Google Scholar]
- Borradori L, Chavanas S, Schaapveld RQJ, Gagnoux-Placacios L, Calafat J, Meneguzzi G, Sonnenberg A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion: reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp Cell Res. 1998;239:463–476. doi: 10.1006/excr.1997.3923. [DOI] [PubMed] [Google Scholar]
- Borradori L, Koch PJ, Niessen CM, Erkeland S, van Leusden MR, Sonnenberg A. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the β4 integrin subunit. J Cell Biol. 1997;136:1333–1349. doi: 10.1083/jcb.136.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Chavanas S, Gache Y, Tadini G, Pulkkinen L, Uitto J, Ortonne JP, Meneguzzi G. A homozygous in-frame deletion in the collagenous domain of bullous pemphigoid antigen BP180 (type XVII collagen) causes generalized atrophic benign epidermolysis bullosa. J Invest Dermatol. 1997;109:74–78. doi: 10.1111/1523-1747.ep12276614. [DOI] [PubMed] [Google Scholar]
- Fields S, Sternglanz R. The two hybrid system: an assay for protein-protein interactions. Trends Genet. 1994;10:286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- Gache Y, Chavanas S, Lacourmm JP, Wiche G, Owaribe K, Meneguzzi G, Ortonne JP. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J Clin Invest. 1996;97:2289–2298. doi: 10.1172/JCI118671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Signal transduction by the alpha 6 beta 4 integrin: charting the path between laminin binding and nuclear events. J Cell Sci. 1996;109:1165–1172. doi: 10.1242/jcs.109.6.1165. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen, BP180. J Invest Dermatol. 1992;99:243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Giudice GJ, Squiquera HL, Elias PM, Diaz LA. Identification of two collagen domains within the bullous pemphigoid autoantigen, BP180. J Clin Invest. 1991;87:734–738. doi: 10.1172/JCI115054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Virata MLA, Elgart GW, Stanley JR, Parry DAD. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992;14:145–153. doi: 10.1016/s0141-8130(05)80004-2. [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu Q-C, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified squamous epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. pp. 485–510. [Google Scholar]
- Hashimoto T, Amagai M, Ebihara T, Gamou S, Shimizu N, Tsubata T, Hasegawa A, Miki K, Nishikawa T. Further analyses of epitopes for human monoclonal antibasement membrane zone antibodies produced by stable human hybridoma cell lines constructed with Epstein-Barr virus transformants. J Invest Dermatol. 1993;100:310–315. doi: 10.1111/1523-1747.ep12469916. [DOI] [PubMed] [Google Scholar]
- Hopkinson SB, Baker SE, Jones JCR. Molecular genetic studies of a human epidermal autoantigen (the 180 kDa bullous pemphigoid antigen/BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and α6 integrin. J Cell Biol. 1995;130:117–126. doi: 10.1083/jcb.130.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson SB, Findlay K, deHart GW, Jones JCR. Interaction of BP180 (type XVII collagen) and α6 integrin is necessary for stabilization of hemidesmosome structure. J Invest Dermatol. 1998;111:1015–1022. doi: 10.1046/j.1523-1747.1998.00452.x. [DOI] [PubMed] [Google Scholar]
- Hopkinson SB, Riddelle KS, Jones JCR. Cytoplasmic domain of the 180-kDa bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992;99:264–270. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Hopkinson SB, Goldfinger L. Structure and assembly of hemidesmosomes. BioEssays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jonkman MF, de Jong MCJM, Heeres K, Pas HH, van der Meer JB, Owaribe K, Martinez de Velasco AM, Niessen CM, Sonnenberg A. 180-kDa bullous pemphigoid antigen (BP180) is deficient in generalized atrophic benign epidermolysis bullosa. J Clin Invest. 1995;95:1345–1352. doi: 10.1172/JCI117785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte DH, Kurpakus MA, Grelling KA, Jones JCR. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol. 1989;109:3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung CL, Sun D, Liem RKH. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1-n (Dystonin) in neurons. J Cell Biol. 1999;144:435–446. doi: 10.1083/jcb.144.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Tamai K, Tan EML, Uitto J. Cloning of type XVII collagen: complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 5′-end of the gene and the 3′-untranslated region of the mRNA. J Biol Chem. 1993;268:8825–8834. [PubMed] [Google Scholar]
- McGrath JA, Gatalica B, Christiano AM, Li K, Owaribe K, McMillan JR, Eady RAJ, Uitto J. Mutations in the 180-kDa bullous pemphigoid antigen (BP180), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat Genet. 1995;11:83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- McLean WHI, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996;10:1724–1735. doi: 10.1101/gad.10.14.1724. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Hulsman EHM, Oomen LCJM, Kuikman I, Sonnenberg A. A minimal region of the integrin β4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J Cell Sci. 1997;110:1705–1716. doi: 10.1242/jcs.110.15.1705. [DOI] [PubMed] [Google Scholar]
- Rezniczek GA, de Pereda JM, Reiper S, Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the β4 subunit and plectin at multiple molecular sites. J Cell Biol. 1998;141:209–226. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddelle KS, Green KJ, Jones JCR. Formation of hemidesmosomes in vitro by a rat bladder cell line. J Cell Biol. 1991;112:159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Watt FM. The plakin family: versatile organizers of cytoskeletal architecture. Curr Opin Genet Dev. 1997;7:392–397. doi: 10.1016/s0959-437x(97)80154-2. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sanchez-Aparicio P, de Velasco M, Neissen CM, Borradori L, Kuikman L, Hulsman EHM, Faessler R, Owaribe K, Sonnenberg A. The subcellular distribution of the high molecular mass protein, HD1, is determined by the cytoplasmic domain of the integrin β4-subunit. J Cell Sci. 1997;110:169–178. doi: 10.1242/jcs.110.2.169. [DOI] [PubMed] [Google Scholar]
- Sawamura D, Kekua L, Chu ML, Uitto J. Human bullous pemphigoid antigen (BPAG1) amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991;270:17784–17790. [PubMed] [Google Scholar]
- Schaapveld RQJ, Borradori L, Geerts D, van Leusden MR, Kuikman I, Nievers MG, Niessen CM, Steenbergen RDM, Snijders PJF, Sonnenberg A. Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin, and involves a direct interaction between β4 and the bullous pemphigoid antigen BP180. J Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FJD, et al. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat Genet. 1996;13:450–457. doi: 10.1038/ng0896-450. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Einheber S, Cullen T, Milner TA, Giancotti FG. Recombinant tail-less integrin β4 subunit disrupts hemidesmosomes, but does not suppress α6β4-mediated cell adhesion to laminins. J Cell Biol. 1995;129:473–487. doi: 10.1083/jcb.129.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinardi L, Ren Y-L, Sanders R, Giancotti FG. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol Biol Cell. 1993;4:871–884. doi: 10.1091/mbc.4.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley JR. Cell adhesion molecules as targets of autoantibodies in pemphigus and pemphigoid, bullous diseases due to defective epidermal cell adhesion. Adv Immunol. 1993;53:291–325. doi: 10.1016/s0065-2776(08)60503-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Parry DAD, Klaus-Kovton V, Steinert PM, Stanley JR. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin 1 confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991;266:12555–12559. [PubMed] [Google Scholar]
- Yang Y, Bauer C, Strasser G, Wollman R, Julien J-P, Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Dowling J, Yu Q-C, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]