Abstract
Laparoscopic radical nephrectomy has been shown in long-term follow-up to provide shorter patient hospitalization and effective cancer control with no significant difference in survival compared with open radical nephrectomy. The major technical issue for success of laparoscopic partial nephrectomy is hemostatic control, and several techniques have been developed to improve control. Laparoscopic partial nephrectomy continues to evolve along two therapeutic technical avenues: hilar clamping with ischemia versus no hilar clamping. The benefits of laparoscopy for the kidney have clearly been demonstrated in terms of less pain, decreased convalescence, and decreased narcotic requirements. With short-term outcomes demonstrating laparoscopic partial nephrectomy as an efficacious procedure, the role of laparoscopic partial nephrectomy should continue to increase.
Key words: Renal neoplasms, Partial nephrectomy, Hemostasis, Radiofrequency ablation
Laparoscopic radical nephrectomy has established its role as a standard of care for management of renal neoplasms. Long-term follow-up has demonstrated that laparoscopic radical nephrectomy has shorter patient hospitalization and effective cancer control with no significant difference in survival compared with open radical nephrectomy.1 For renal masses of less than 4 cm, partial nephrectomy is indicated for patients with a solitary kidney or who demonstrate impairment of contralateral renal function. Open partial nephrectomy has an overall local recurrence rate of 0%–10%.2,3
The major technical issue for success of laparoscopic partial nephrectomy is hemostatic control. Several techniques have been developed to achieve better hemostatic control, including radiofrequency pretreatment, laparoscopic hilar clamping with bulldog clamps or Satinsky clamp, argon-beam coagulation, electrocautery, harmonic scalpel, fibrin glue, ultrasonic dissection, Surgicel, Avitene, fibrin-soaked Gelfoam activated by thrombin, pledget reinforced sutures, hydrojet dissection, microwave tissue coagulation, and cable ties.4–11 Development of new laparoscopic techniques for partial nephrectomy can be divided into two categories: hilar control with warm ischemia versus no hilar control.
Technique
Preoperative workup includes abdominal computed tomography (CT) scan with intravenous contrast in order to delineate anatomy. Staging workup further includes chest x-ray, electrolyte panel, complete blood count, and liver function tests. If the alkaline phosphatase is increased, a bone scan is necessary to assess metastatic disease. A renal scan determines percent function. If renal function is less than 10%, the patient is better served with radical nephrectomy. A 5F ureteral catheter is cystoscopically placed at the beginning of the case to allow retrograde injection after excision of the mass to determine if the collecting system has been violated. The ureteral catheter is tied to a 16F Foley catheter with a silk tie. A 60 mL syringe with dilute indigo carmine is affixed to the ureteral catheter for subsequent retrograde injection.
Three trocars are used in a transperitoneal approach. After initial insufflation with a Veress needle, an 11-mm trocar is placed under direct visualization using the Optiview trocar (Ethicon, Cincinnati, OH). This trocar has a cutting element, which dissects through fascia under direct visualization to enter the pneumoperitoneum. The second, 12-mm trocar is placed laterally to the rectus in the midclavicular line at the level of the umbilicus. The third, 5-mm trocar is placed halfway between the xiphoid and the umbilicus. After incising along the line of Toldt, the colon is reflected medially. On the right, the lateral colonic peritoneal reflection is incised from the right common iliac artery up to the hepatic flexure. The anterolateral surface of the right kidney is often not entirely behind the ascending colon and is usually covered by the lateral peritoneum. The right triangular and anterior coronary ligaments must be divided. The colorenal attachments must then be sharply divided to allow the ascending colon and hepatic flexure to be rolled medially. The duodenum is exposed and then mobilized medially by means of the Kocher maneuver until the vena cava is clearly visualized. On the left, mobilization must take place from the splenic flexure down to the level of the common iliacs.
Exophytic renal masses can often be localized by mobilizing the kidney within Gerota’s fascia while being conscious to keep a layer of fascia over the mass. Laparoscopic ultrasonography can also aid in identifying the location of the renal mass. Detailed information about tumor size, tumor depth, extension into the parenchyma, distance from the adjacent calyx, and presence of satellite lesions can be determined from real-time ultrasonography (see Figures 1 and 2).
Figure 1.
Laparoscopic ultrasound helps localize the renal mass as well as determine tumor size, extension into the parenchyma, and presence of satellite lesions.
Figure 2.

Exposure of the exophytic renal mass with preservation of Gerota’s fascia over the mass.
The radiofrequency probe (RITA Medical systems, Mountain View, CA) is then percutaneously positioned within the mass and deployed to coagulate a spherical area including both the lesion and a margin of normal parenchyma (margin). Settings for the radiofrequency probe for a 3-cm lesion are temperature-based, with a target temperature of 105°C, 90 watts, treatment time of 5.5 minutes, dual cycle. The energy is delivered at 90 W until the average of the five temperature gauges is above 105°C and then autoregulated to maintain the temperature at this level for 5.5 minutes (per the manufacturer’s recommendations). At the conclusion of the second cycle, the harmonic scalpel (Ethicon, Cincinnati, OH) is used to excise the mass together with a 0.5-cm margin of normal parenchyma (Figure 3). The lesion is placed in an Endocatch bag, and the parenchymal resection margin is biopsied (Figure 4). Argon-beam coagulation is applied to the cut surface. The argon beam is essential for hemostatic control. Argon is an inert gas that does not support combustion and is rapidly cleared from the body.
Figure 3.
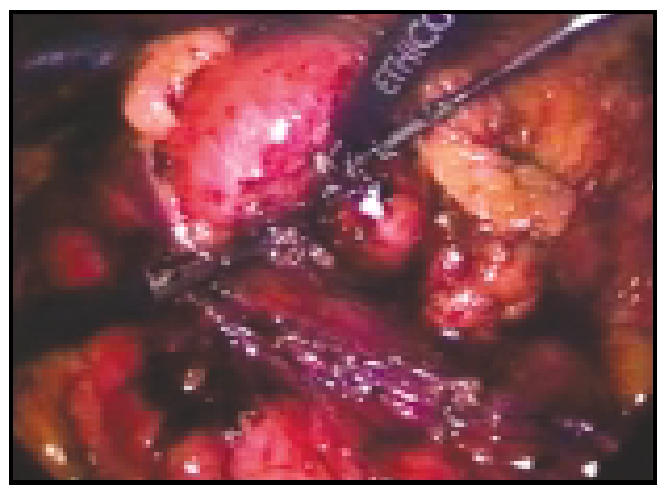
The harmonic scalpel utilizes ultrasonic energy to coagulate as well as ligate vessels as it transects the parenchyma.
Figure 4.

Retrieval of the excised mass. Frozen section is performed on the base of the mass to determine margin status.
Retrograde injection of indigo carmine dye is performed to determine collecting system viability. If the collecting system has been entered, a CT-1 needle with 3-0 polyglactin is used to perform a running suture repair of the collecting system. Oxidized cellulose or fibrin glue can be placed over the resected base to help maintain hemostasis (Figure 5). Follow-up monitoring includes physical examination, serum creatinine, chest x-ray, and abdominal CT scan at 6 months and annually thereafter.
Figure 5.

Surgicel, fibrin-soaked Gelfoam, or fibrin glue can be applied to the base of the mass for maintenance of hemostasis.
Discussion
Laparoscopic partial nephrectomy was first reported in 1993 by Winfield and colleagues7 in a patient with a lower-pole calyceal diverticulum diverticulum containing a calculus. Hemostasis was aided through use of a renal tourniquet cinched down around the lower pole of the kidney. Further application of this concept of parenchymal compression was investigated by Cadeddu and colleagues,11 with application of cable ties circumferentially to the kidney to aid in hemostasis. Reversible regional hypoperfusion was achieved in the porcine model. However, in clinical evaluation of these modalities, consistent adequate hemostasis has been unreliable, with intermittent arterial bleeding from the cut edge of the kidney despite application of the tourniquet. If excessive force is applied as the tourniquet is tightened, cutting and subsequent fracture of the renal parenchyma can occur. Alternatively, if the tourniquet is too loose, significant hemorrhage can occur.
Laparoscopic partial nephrectomy continues to evolve along two therapeutic technical avenues: hilar clamping with ischemia versus no hilar clamping. Development of a laparoscopic Satinsky clamp has achieved en bloc control of the renal hilum to allow cold-knife excision of the mass, with laparoscopic repair of the collecting system if needed. Gill and colleagues6 reported their experience with this technique in 50 patients, mean tumor size 3.0 cm, with warm ischemia time of 23 minutes plus or minus 7.4 minutes (range 9.8–40 minutes). Caliceal entry was demonstrated in 18 patients, with immediate repair of the collecting system performed. Two patients required postoperative transfusion, with a mean hospitalization stay of 2.2 days. Three complications were reported: one intraoperative hemorrhage, one delayed hemorrhage plus nephrectomy, and one urine leak. (Tables 1 and 2 review intraoperative data and results from the experience of Gill and colleagues and others.)
Table 1.
Intraoperative Data-Laparoscopic Partial Nephrectomy
| Author | No. Patients | OR Time | EBL Mean (range) | Warm Ischemia Time |
|---|---|---|---|---|
| Gill et al6 | 50 | 3.0 hrs | 270.4 mL (40-1,500) | 23 min (9.8-40) |
| Gettman et al4 | 10 | 2.8 hrs | 125 mL | - |
| Winfield et al2 | 1 | 6 hrs, 10 min | 300 mL | - |
| Yoshimura et al12 | 6 | 3.1 hrs | minimal | - |
| Janetschek et al14 | 25 | 2.7 hrs | 287 mL | - |
| Rassweiler et al16 | 53 | 3.2 hrs | 725 mL (20-1500) | - |
OR, operating room; EBL, estimated blood loss.
Table 2.
Review of Results with Laparoscopic Partial Nephrectomy
| Author | Hospital Stay (range) | Complications | Pathology | Margins |
|---|---|---|---|---|
| Gill et al6 | 2.2 days (1-9) | 6%: intraoperative hemorrhage (1), | Renal cell carcinoma (34), | 100% negative |
| nephrectomy (1), urine leak (1) | angiomyolipoma (8), | |||
| oncocytoma (5), other (3) | ||||
| Gettman et al4 | - | Intraoperative hemorrhage (1) | Renal cell carcinoma (10) | 100% negative |
| Rassweiler et al16 | 5.4 days | Intraoperative hemorrhage (8%), | Renal cell carcinoma (37), | - |
| conversions (8%), | angiomyolipoma (2), | |||
| reintervention (12%) | oncocytoma (3), multilocular | |||
| cyst (7), adenoma (3), malignant | ||||
| lymphoma (1) | ||||
| Janetschek et al14 | 5.8 days | Pneumothorax (1), fistulae (2) | Renal cell carcinoma (19), | |
| oncocytoma (1), angiomyolipoma (1), | ||||
| cysts (4) | ||||
| Winfield et al7 | 7 days | None | - | - |
This technique is appealing in its goal of duplicating the open surgical technique. Renal function following this procedure was preserved with 100% negative margins.
Combination of laparoscopic partial nephrectomy with ablative techniques has achieved successful excision of renal masses with adequate hemostasis without hilar clamping. In patients undergoing excision without hilar control, combination radiofrequency ablation with immediate excision of the mass has been reported in 10 patients.4 Mean tumor size was 2.1 cm (range 1.0–3.2 cm), mean operative time was 170 minutes, and median blood loss was 125 mL. No perioperative complications were reported, and final diagnoses of renal cell carcinoma (9) and angiomyolipoma (1) with 100% negative margins were reported. The benefit of hemostasis without hilar clamping is that it decreases the risk of warm renal ischemia. Furthermore, excisional partial nephrectomy provides clear pathological analysis and confirmation of clear margins, and a better oncological approach over ablative techniques such as cryosurgery or radiofrequency ablation alone.
Other techniques without hilar control have been investigated. Yoshimura and colleagues12 reported use of a microwave tissue coagulator for laparoscopic partial nephrectomy without hilar clamping. In 6 patients with mean tumor size of 1.7 cm, mean operating time was 186 minutes and blood loss was minimal. In this approach, multiple insertions of the probe (range 5–23 coagulations) 5–8 mm apart were conducted prior to excision of the mass.
The benefits of laparoscopy for the kidney have clearly been demonstrated in terms of less pain, decreased convalescence, and decreased narcotic requirements.1,13 The benchmarks for long-term success of both laparoscopic approaches for radical nephrectomy and partial nephrectomy will be defined by oncologic principles. Five-year outcome data on actuarial disease-free survival will assess the success of these procedures. Janetschek and colleagues14 reported 13.3-month follow-up for laparoscopic radical nephrectomy and 22.2-month follow-up for wedge resection. One patient had distant metastases to the lung and another patient demonstrated multilocular tumor 1 year postoperatively. There were no local recurrences reported. For laparoscopic radical nephrectomy, a multi-institutional study of 157 patients reported an actuarial 5-year, cancer-free rate of 89% for clinical T2 and 100% for clinical T1 disease.15
Chan and colleagues1 recently reported a comparison of laparoscopic nephrectomy for renal cell carcinoma to open nephrectomy. At follow-up of 35.6 months versus 44 months, no statistical difference was determined on the Kaplan-Meier actuarial survival analysis. Patients were matched for age and size, with mean tumor size of 5.1 cm (1–13 cm). Clearly laparoscopic radical nephrectomy nephrectomy for T1/T2 lesions is equivalent to open surgery in both efficiency and efficacy.
Laparoscopic radical nephrectomy for renal cell carcinoma has clearly demonstrated low morbidity and equivalent cancer control to open techniques. The rates for local recurrences and metastatic spread are low, and actuarial survival is high. Furthermore, laparoscopic partial nephrectomy has been demonstrated to be technically feasible and has low morbidity. With short-term outcomes demonstrating laparoscopic partial nephrectomy as an efficacious procedure, the role of laparoscopic partial nephrectomy should continue to increase.
Main Points.
Laparoscopic radical nephrectomy has shorter patient hospitalization and equally effective cancer control with no significant difference in survival compared with open radical nephrectomy.
For renal masses of less than 4 cm, partial nephrectomy is indicated for patients with a solitary kidney or who demonstrate impairment of contralateral renal function.
If renal function is less than 10%, the patient is better served with laparoscopic radical nephrectomy.
Development of new laparoscopic techniques for partial nephrectomy can be divided into two categories: hilar control with warm ischemia versus no hilar clamping.
Development of a laparoscopic Satinsky clamp has achieved en bloc control of the renal hilum in order to allow cold-knife excision of the mass.
Combination of laparoscopic partial nephrectomy with ablative techniques has achieved successful excision of renal masses with adequate hemostasis without hilar clamping.
Excisional partial nephrectomy provides clear pathological analysis and confirmation of clear margins.
References
- 1.Chan DY, Cadeddu JA, Jarrett TW, et al. Laparoscopic radical nephrectomy: cancer control for renal cell carcinoma. J Urol. 2001;166:2095–2210. doi: 10.1016/s0022-5347(05)65513-9. [DOI] [PubMed] [Google Scholar]
- 2.Uzzo RG, Novick AC. Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol. 2001;166:6–18. [PubMed] [Google Scholar]
- 3.Montie JE, Novick AC. Partial nephrectomy for renal cell carcinoma. J Urol. 1992;148:1835. doi: 10.1016/s0022-5347(17)41505-9. [DOI] [PubMed] [Google Scholar]
- 4.Gettman MT, Bishoff JT, Lu LM. Hemostatic laparoscopic partial nephrectomy: initial experience with the radiofrequency coagulationassisted technique. Urology. 2001;58:8–11. doi: 10.1016/s0090-4295(01)01086-x. [DOI] [PubMed] [Google Scholar]
- 5.Corwin TS, Cadeddu JA. Radio frequency coagulation to facilitate laparoscopic partial nephrectomy. J Urol. 2001;165:175–176. doi: 10.1097/00005392-200101000-00042. [DOI] [PubMed] [Google Scholar]
- 6.Gill IS, Desai MM, Kaouk JH, et al. Laparoscopic partial nephrectomy for renal tumor: duplicating open surgical techniques. J Urol. 2002;167:469–476. doi: 10.1016/S0022-5347(01)69066-9. [DOI] [PubMed] [Google Scholar]
- 7.Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993;7:521–526. doi: 10.1089/end.1993.7.521. [DOI] [PubMed] [Google Scholar]
- 8.Jackman SV, Cadeddu JA, Chen RN, et al. Utility of the harmonic scalpel for laparoscopic partial nephrectomy. J Endourol. 1998;12:441–444. doi: 10.1089/end.1998.12.441. [DOI] [PubMed] [Google Scholar]
- 9.McDougall EM, Clayman RV, Stone AM. Laparoscopic partial nephrectomy: preliminary laboratory studies. Abstract presented at the Tenth World Congress of Endourology and SWL; September 3–6, 1992; Singapore. [Google Scholar]
- 10.Elashry OM, Wolf JS, Rayala JH. Recent advances in laparoscopic partial nephrectomy: comparative study of electrosurgical snare electrode and ultrasonic dissection. J Endourol. 1997;11:15. doi: 10.1089/end.1997.11.15. [DOI] [PubMed] [Google Scholar]
- 11.Cadeddu JA, Corwin TS, Traxer O, et al. Hemostatic laparoscopic partial nephrectomy: cable-tie compression. Urology. 2001;57:562–566. doi: 10.1016/s0090-4295(00)01009-8. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura K, Okubo K, Ichioka K, et al. Laparoscopic partial nephrectomy with a microwave tissue coagulator for small renal tumor. J Urol. 2001;165:1893–1896. doi: 10.1097/00005392-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Clayman RV, Kavoussi LR, Soper JN. Laparoscopic nephrectomy. N Engl J Med. 1991;324:1370. doi: 10.1056/NEJM199105093241917. [DOI] [PubMed] [Google Scholar]
- 14.Janetschek G, Jeschke K, Peschel R. Laparoscopic surgery for stage 1 renal cell carcinoma: radical nephrectomy and wedge resection. Eur Urol. 2000;38:131. doi: 10.1159/000020269. [DOI] [PubMed] [Google Scholar]
- 15.Cadeddu JA, Ono Y, Clayman RV, et al. Laparoscopic nephrectomy for renal cell carcinoma: evaluation of efficacy and safety. A multicenter experience. Urology. 1998;52:773–777. doi: 10.1016/s0090-4295(98)00391-4. [DOI] [PubMed] [Google Scholar]
- 16.Rassweiler JJ, Abbou C, Janetschek G, Jeschke K. Laparoscopic partial nephrectomy. The European experience. Urol Clin North Am. 2000;27:721. doi: 10.1016/s0094-0143(05)70121-x. [DOI] [PubMed] [Google Scholar]



