Abstract
Injectable materials of various types have been used for decades as an alternative to surgery for the treatment of stress urinary incontinence. Their success stems from their ability to improve intrinsic sphincter function, and patients with hypermobility may benefit as well. Nevertheless, the ideal agent has yet to be discovered, and surgery still may be necessary after treatment in some patients. Results vary among the different materials used, and safety, durability, and cost-effectiveness are important areas of concern in which more research is needed.
Key words: Stress incontinence, Intrinsic sphincter deficiency, Injectables, Collagen, Silicone, Teflon®
In 1938, Murless1 first reported on the injection of sodium morrhuate around the urethra for urinary incontinence, and since then various materials have been injected as an alternative to surgery. Quackels2 reported the use of paraffin wax in 1955, and Sachse3 used sclerosing agents in 1963. The initial results were poor, and significant complications such as pulmonary emboli and urethral sloughing were seen. Polytetrafluoroethylene (Teflon®) paste was first introduced by Berg4 and then popularized by Politano and associates5 in the 1970s. Shortliffe and coworkers6 published the first report on glutaraldehyde cross-linked collagen, and more recently, autologous fat injection was described.7 Newer agents, such as silicone microparticles8 and carbon beads,9 have also been reported.
This article will summarize the techniques of administration, properties, published results, and complications of the various injectable agents as well as outline some of the controversies. Some of the unmet needs associated with injectable agents will also be discussed.
Mechanism of Action of Injectables
It is generally agreed that these agents improve intrinsic sphincter function. Collagen injections have been reported to augment urethral mucosa10,11 and improve coaptation and intrinsic sphincter function, as evidenced by an increase in post-treatment abdominal leak pressure.12,13,14 Initial investigators using collagen postulated obstruction as a mechanism of action,15,16 but Monga and associates11 showed that successfully treated patients have an increased area and pressure transmission ratio in the first quarter of the urethra. They suggested that placement of the injectable agent at the bladder neck or proximal urethra prevents bladder neck opening under stress. Proper placement of the injectable, possibly just below the bladder neck, rather than the actual quantity of the agent, improves intrinsic sphincter deficiency (ISD).17
The ideal injectable agent should be easily injectable and conserve its volume over time. If unsuccessful, it should not interfere with subsequent surgical intervention. It should also be biocompatible, nonantigenic, noncarcinogenic, and nonmigratory.18 To date, no substance has met all of these requirements.
Patient Selection
Patients with ISD and normal detrusor function are candidates for injectable agents.19 McGuire and coworkers20 identified these patients with the use of abdominal leak pressures to measure the strength of the intrinsic sphincter. Low leak pressures (<65 cm water) correlate well with type 3 videourodynamic findings, that is, a poorly functioning bladder neck and proximal urethra (ISD), and higher leak pressures correlated with type 1 or 2 hypermobility.
The presence of ISD is the primary indication for the use of injectable agents in patients with stress urinary incontinence.10 Because ISD can coexist with hypermobility,21 injectables have been administered to patients with hypermobility, to improve the ISD component of their incontinence. Furthermore, elderly women with hypermobility, who are poor operative risks, have also received injectable agents.22
Injection Techniques
The materials can be administered under local anesthesia with cystoscopic control as an outpatient procedure. Both the periurethral and transurethral methods are done to implant the agent within the urethral wall, preferably into the submucosa or lamina propria. It is thought that the implant should be positioned at the bladder neck or proximal urethra. Different sites can be chosen, such as 3 and 9 o’clock or 4 and 8 o’clock positions, although Defreitas and colleagues23 recently demonstrated that patients with circumferentially distributed implants had better results than those with random deposits. The actual needle size required depends on the viscosity of the injectable. Pre- and postoperative antibiotics are usually administered.
With the periurethral approach, perimeatal blebs are raised with 1% or 2% lidocaine at the 3 and 9 o’clock or 4 and 8 o’clock positions approximately 3 to 4 mm lateral to the urethral meatus. A 20F urethroscope with a 30° telescope is inserted into the urethra after instillation of topical urethral lidocaine. The periurethral needle is introduced and advanced parallel to the endoscope sheath until its position can be seen cystoscopically just below the bladder neck within the mucosa. Care must be taken to prevent the needle from getting too close to or entering the urethral lumen to avoid rupture of the mucosa and extravasation. Rocking the needle will confirm the position of the tip. If penetration of the mucosa occurs, the needle should be removed and repositioned. The substance is injected either unilaterally or bilaterally to create the appearance of “prostatic” lobes. The patient is asked to cough or strain in the supine and then the upright position. If leakage still occurs, more agent may be given. If no leakage is seen, the procedure may be ended. The patient then voids and can be discharged. Acute retention can be treated by insertion of a fine 8F catheter.
The implant can also be injected transurethrally through the cystoscope with flexible needle-tipped catheters or with specially designed injection scopes with rigid needles. Silicone microparticles, Teflon, and fat, because of their high viscosity, may require the use of injection guns. A recent modification of the transurethral technique has been reported by Tamanini and coworkers.24 They used a device that fits into the urethra, and through needle-guiding channels, injected Macroplastique® (Uroplasty BV, Geleen, The Netherlands) into the 2, 6, and 10 o’clock positions.
Collagen
Glutaraldehyde cross-linked collagen, or GAX collagen, is a highly purified suspension of bovine collagen in normal saline containing at least 95% type I collagen and 1% to 5% type III collagen.25 This cross-linking makes the GAX collagen resistant to the fibroblast-secreted collagenase. As a result, the GAX collagen is only very slightly resorbed. The implant causes no inflammatory reaction or granuloma formation and is colonized by host fibroblasts and blood vessels. It is not known to migrate. However, it does degrade over time and is replaced by host collagen, which explains its persistence.25
Because 2% to 5% of patients26 are sensitized to collagen through dietary exposure, all patients must undergo a skin test at the volar aspect of the forearm 30 days before treatment. Those who show a positive response should not undergo the procedure.
Collagen Results
Numerous reports of its efficacy, safety, ease of administration, and relative lack of associated morbidity have appeared since the first description of collagen injection for urinary incontinence. Table 1 lists various reported series.
Table 1.
Comparison of Collagen Parameters and Results
| Patients | Patients | |||||
|---|---|---|---|---|---|---|
| Type of | Follow-up | Patients Dry | Improved | Failed | ||
| Study | Patients | Incontinence | (mo) | (%) | (%) | (%) |
| Stricker | 50 | ISD | Mean: 11 | 21 (42) | 20 (40) | 7 (14) |
| and Haylen70 | (range, 1–21) | |||||
| Kieswetter | 16 | Not specified | 9 | 7 (44) | 7 (44) | 2 (12) |
| et al71 | ||||||
| Eckford and | 25 | Not specified | 3 | 16 (64) | 4 (16) | 5 (20) |
| Abrams15 | ||||||
| O’Connell | 44 | 42 with ISD | 1–2 | 20 (45) | 8 (18) | 16 (37) |
| et al32 | 2 hypermobile | (longest, 7) | ||||
| Moore et al31 | 11 | Types 1 and 3 | 2 | 1 (9) | 7 (63) | 2 (18) |
| Winters and | 50 | ISD | > 12 | 48 (96) dry or socially | 2 (4) | |
| Appell13 | continent | |||||
| McGuire and | 17 | Mobile | > 12 | 8 (47) | 3 (17) | 6 (35) |
| Appell10 | 137 | ISD | > 12 | 63 (46) | 47 (34) | 29 (19) |
| Faerber22 | 12 | Type 1 | 10.3 | 10 (83) | 2 (17) | 0 |
| (range, 3–24) | ||||||
| Monga et al11 | 60 | Some | 3 (N = 59) | 27 (46) | 22 (40) | |
| hypermobile | 12 (N = 54) | 22 (40) | 20 (37) | |||
| 24 (N = 29) | 14 (48) | 6 (20) | ||||
| Richardson et al14 | 42 | ISD | 46 (10–66 | 17 (40) | 18 (43) | 7 (17) |
| after first | ||||||
| injection) | ||||||
| Herschorn and Radomski29 | 181 | Type 1: 54 | Mean: 22 (range, | 42 (23) | 94 (52) | 45 (25) |
| Type 2: 67 | 4–69) | |||||
| Type 3: 60 | ≥24 (N = 62) | 27 (43.5) | 29 (46.8) | 6 (9.7) | ||
| ≥36 (N = 25) | 13 (52) | 8 (32) | 4 (16) | |||
| Smith et al72 | 94 | Type 3 | Median: 14 | 36 (38.3) | 27 (28.7) | 31 (33) |
| Khullar et al17 | 21 | Not specified | 24 (minimum) | 10 (48) | 2 (9) | 9 (43) |
| Swami et al73 | 107 | Some | 24 (minimum) | 27 (25) | 43 (40) | 37 (35) |
| hypermobile | ||||||
| Cross et al28 | 103 | Type 3 | Median: 18 | Substantially | 29 (20) | 7 (6) |
| (range, 6–36) | Improved | |||||
| 103 (74) | ||||||
| Groutz et al74 | 63 | Type 3 | 6 | 8 (13) | 44 (70) | |
| Bent et al33 | 90 | Types 1 and 2 | 12 | 19 (21) | 19 (21) | 11 (17) |
| Corcos and Fournier75 | 40 | Type 1 (8) | 52 | 12 (30) | 16 (40) | 62 (58) |
| Type 2 (20) | ||||||
| Type 3 (12) |
ISD, intrinsic sphincter deficiency.
Persistence of the implant itself has been demonstrated with magnetic resonance imaging (MRI) of the urethra at intervals of up to 22 months after injection, although the measured volume was less than that injected.27 Early results are generally good, with success rates of 72% to 100% (Table 1). Maintenance of good results in the long term may occur with durability of the initial procedure itself or with reinjections with additional collagen. It is important for authors to differentiate the durability of the original procedure(s) from that resulting from reinjections or top-ups by reporting the follow-up period starting from after the last injection.
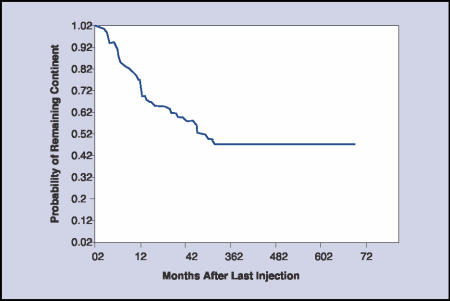
Longer-term (more than 1 to 2 years) cure and improvement17 varies from 57% to 94%.28 Most patients need 1 or 2 treatment sessions with means of 5.6 to 15 cc of collagen used. Because patients are treated at different times and because durations of follow-up vary, the Kaplan-Meier curve can be useful to display the persistence of a good result. In our series, the probability of a patient remaining dry was 72% at 1 year, 57% at 2 years, and 45% at 3 years (Figure 1).29 Winters and Appell13 also reported a similar rate of complete continence (50%) in their multicenter trial after 2 years. Additional administration of collagen has usually resulted in restoration of continence, and this has to be factored into the reporting.
Figure 1.
Kaplan-Meier curve showing durability of cure of incontinence after the last collagen injection in 78 patients. (Reproduced with permission from Herschorn and Radomski.29)
Berman and Kreder30 analyzed the cost-effectiveness of collagen versus sling cystourethropexy for type 3 incontinence. They concluded that surgery was more cost effective than collagen injection.
The use of collagen in patients with hypermobility has been reported. Moore and associates31 included patients with both type 1 and type 3 abnormalities. Faerber22 treated elderly patients with type 1 abnormality. In the report by McGuire and Appell,10 the results at more than 1 year in women with ISD were similar to those in women with hypermobility, although there were far more women with ISD. However, Appell19 subsequently reported that these patients with hypermobility all required bladder neck surgery within 2 years. Monga and associates11 included patients with hypermobility and found that cure rates were not reduced for women with up to 2.5 cm of movement. In our series of 181 patients, there was no significant difference in outcome with or without hypermobility.32 Furthermore, in a prospective trial of patients with hypermobility, Bent and colleagues33 reported a good outcome after 1 year.
Collagen Complications
Treatment-related morbidity has been minimal. Urinary retention ranges from 1% to 21%12,13,19 and can be managed with intermittent catheterization or short-term Foley catheterization. Urinary tract infection occurs in 1% to 25%.12,13,19 Extravasation resolves quickly with flushing away of the dilute collagen suspension and sealing over of the small needle insertion site. Hematuria occurs in 2% of patients.19 Other rare complications include periurethral abscess formation.34
Other reported complications include de novo detrusor overactivity (instability), which was seen in 11 (39%) of 28 elderly women treated by Khullar and colleagues.17 Stothers and coworkers35 reported de novo urgency with urgency incontinence in 43 (12.9%) of 337 patients, 21% of whom did not have response to anticholinergics.
Another rare complication is a reaction at the previously negative skin test site following a urethral collagen injection. In one study, this occurred in 3 patients (1.9%) and was associated with arthralgias in 2.26 This reaction has been reported before in the dermatologic literature,36 and 2 negative pretreatment skin tests have been suggested to prevent it. The potential for hypersensitivity reactions is present because antibody production is stimulated by collagen injection.37
Silicone Microparticles
Silicone microparticles are solid polydimethylsiloxane (silicone rubber) particles suspended in a nonsilicone carrier gel that is absorbed by the reticuloendothelial system and excreted unchanged in the urine.8 Because 99% of the particles are between 100 μm and 450 μm in diameter, the likelihood of migration is low. Henly and associates38 demonstrated distant migration of small particles less than 70 μm in diameter but no migration of particles greater than 100 μm in diameter. Although there was a typical histiocytic and giant-cell reaction within the injection site, there was no granuloma formation in response to the larger particles. Because the substance is quite viscous, it must be injected with an injection gun and a transurethral needle with 16-gauge tip.
Silicone Microparticles Results
The results from reported series using this injectable are shown in Table 2. Harriss and coworkers8 reported on 40 patients who were followed for a minimum of 3 years, at which time 16 (40%) were dry, 7 (18%) were improved, and 17 (42%) had failure. Twelve of the 16 required 1 injection and 4 needed 2 injections to achieve dryness.
Table 2.
Results of Macroplastique Injections
| Patients | |||||
|---|---|---|---|---|---|
| Follow-up | Patients | Improved | Patients | ||
| Study | Patients | (y) | Dry (%) | (%) | Failed (%) |
| Harriss et al.8 | 40 | 3 | 16 (40) | 7 (18) | 17 (42) |
| Sheriff et al39 | 34 | 2 | 16 (48) | 18 (52) | |
| Koelbl et al40 | 32 | 1 | 19 (60) | 13 (40) | |
| Radley et al41 | 60 | 1.5 | 12 (20) | 25 (41) | 23 (39) |
| Barangger et al42 | 21 | 2.5 | 4 (19) | 6 (29) | 11 (52) |
| Tamanini et al24 | 21 | 1 | 12 (57) | 4 (19) | 5 (24) |
Sheriff and colleagues39 reported an overall success rate of 48% in 34 patients after unsuccessful stress incontinence surgery, and Koelbl and colleagues40 reported a 60% success rate in 32 women after 12 months but noted a time-dependent decrease in success. Radley and coworkers41 reported a success rate of 61% (19.6% cured and 41.1% improved) in 60 women after a mean of 19 months. Barranger and associates42 reported a dryness rate of 19%, improvement rate of 29%, and failure rate of 52% in a group of 21 patients at a median follow-up of 31 months. Interestingly, they did not observe a time-dependent decrease in results.
Similar success was shown by Tamanini and coworkers24 using an implant system that allows a transurethral injection without cystoscopy.
Silicone Microparticles Complications
Self-limited side effects of hematuria, dysuria, frequency, and retention have been reported in a minority of patients. The lack of a granulomatous reaction and migration of the large silicone particles may provide some benefit over Teflon, although long-term data are not yet available. Despite the laboratory and clinical evidence of safety with the large particles, concerns still exist about small silicone particle migration and long-term tissue response to the injections.43
Carbon Beads (Durasphere)
A newer injectable bulking agent designed to be biocompatible, which is composed of nonmigratory and nonabsorbable pyrolytic carbon-coated zirconium oxide beads suspended in a carrier gel (Durasphere®; Boston Scientific, Natick, MA), was reported by Lightner and colleagues.9 The bead size ranges from 251 to 300 μm, more than 3 times larger than the 80-μm threshold for particle size associated with migration in tissue.44 The absorbable carrier gel is 2.8% glucan—a polysaccharide used in wound healing. The agent is prepackaged in 1.0-mL syringes and administered transurethrally through an 18-gauge needle.
In a multicenter, prospective, randomized, double-blind trial with collagen as control, at 1 year after the last treatment, 49 (80.3%) of 61 women treated with Durasphere showed improvement of 1 continence grade or more, compared with 47 (69.1%) of 68 women treated with collagen (P = .162).9 The difference was not statistically significant. There was also no difference in number of injections or pad weight test. However, the initial and repeated injection volumes of Durasphere were significantly less than those of collagen. The adverse events were similar in both groups, but the Durasphere group had an increased short-term risk of urgency and urinary retention. Pelvic x-rays obtained at 1 and 2 years after injection showed stability of the bulking agents at the injection site. This suggests potential durability.
In another small series of 13 women and 7 men treated with Durasphere injections, Pannek and coworkers45 reported an overall 12-month success rate of 33%. Furthermore, they demonstrated bead migration on plain radiographs in 2 asymptomatic patients.
Long-term results with particular attention to migration risk are required to assess the benefit of this injectable agent.
Polytetrafluoroethylene Paste (PTFE, Teflon, Urethrin)
Polytetrafluoroethylene paste (Teflon) is composed of equal parts Teflon paste and glycerine with polysorbate 20.46 Teflon is a resin polymer with a very high molecular weight and high viscosity that is composed of small particles (40 μm in diameter). It is inert, stable, and does not induce an allergic response. However, it does cause a local inflammatory response, with histiocytes phagocytizing the particles and coalescing to form foreign-body giant cells and a granuloma. There is also fibrous tissue ingrowth that adds to the bulk formed by the Teflon. Malizia and colleagues44 also showed distant migration of Teflon particles to pelvic nodes, lung, brain, and kidneys of experimental animals, due to the small particle size.
Teflon paste has been used to treat urinary incontinence since 1964, but it was not reported until 1975, by Berg.4 Since that time, numerous reports relating to its use in treating incontinence have appeared in the literature. It may be injected via the periurethral route; volumes of up to 10 to 20 cc are reported. The procedure is done under local or spinal anesthesia and repeated injections may be done after 6 months. Small amounts (2.5 cc) can be injected via the periurethral approach with local anesthesia.46 Heating the Teflon reduces its viscosity and allows injection without a gun.
Teflon Results
Table 3 lists various series investigating the use of Teflon. The outcomes are wide ranging, with longer-term series showing poorer results (33%–76% cure and improved) than short-term series (57%–86%).
Table 3.
Teflon Results for Female Stress Incontinence
| Patients | |||||
|---|---|---|---|---|---|
| Follow-up | Patients | Improved | Patients | ||
| Study | Patients | (mo) | Dry (%) | (%) | Failed (%) |
| Politano83 | 51 | 6 | 26 (51) | 10 (20) | 15 (29) |
| Lim et al49 | 28 | - | 6 (21) | 9 (33) | 13 (46) |
| Schulman et al48 | 56 | 3 | 39 (70) | 9 (16) | 8 (14) |
| Deane et al76 | 28 | 3–24 | 9 (32) | 8 (28) | 11 (40) |
| Beckingham et al77 | 26 | 36 | 2 (7) | 7 (27) | 17 (66) |
| Harrison et al78 | 36 | 61 | 4 (11) | 8 (22) | 24 (67) |
| Lotenfoe et al79 | 21 | 11 | 8 (38) | 4 (19) | 9 (43) |
| Lopez et al80 | 74 | 31 | 41 (56) | 15 (20) | 18 (24) |
| Vesey et al81 | 36 | 9 (3–36) | 20 (56) | 4 (11) | 12 (33) |
| Herschorn and Glazer46 | 46 | 12 | 14 (31) | 19 (41) | 13 (28) |
Teflon Complications
Because relatively large volumes of Teflon have been injected with the patient under general anesthesia, the incidence of urinary retention (25%)47 is higher than that of collagen. Irritative voiding symptoms have also been seen transiently in 20%.48 Urinary infection is rare at 2%.49 Perineal discomfort may occur in 5%47 and transient fever in 10% to 15% of patients. Perforation and extravasation can occur, and if this is recognized at the time of injection, the Teflon should be removed.
Although Teflon particles can migrate,44 only 1 case of clinical significance has been reported in the literature in humans. Claes and coworkers50 described a woman previously treated with large volumes of periurethral Teflon for urinary incontinence who later presented with lymphocytic alveolitis and fever. Light microscopy showed Teflon particles in the lungs. She was treated successfully with corticosteroids. Mittleman and Marraccini51 reported an incidental finding of postmortem interstitial pulmonary granulomas in a previously asymptomatic man who had received Teflon injections. Kiilholma and coworkers52 reported 3 complications among 22 women: a sterile periurethral abscess, a urethral diverticulum, and a urethral granuloma that all required surgical intervention. In another case, the material migrated into the bulbar corpus spongiosum, causing perineal pain for 3 months and necessitating medication for pain relief.53
Although neoplastic transformation was hypothesized,44 there has never been a clinical occurrence reported. Furthermore, in a long-term rat study, Dewan and associates54 demonstrated no increase in tumor risk and no tumors found at the injection site.
Despite the potential for complications with Teflon, the actual rate of reported problems is low. However, Teflon is rarely used now as an injectable.
Autologous Fat
Autologous fat has been used for aesthetic and defect reconstruction since the 1980s.55 Although fat is biocompatible and readily available, 50% to 90% of the transferred adipose tissue graft may not survive.43 Graft survival depends on minimal handling, low suction pressure during liposuction, and the use of large-bore needles. Smaller grafts survive better than larger ones.56
The procedure involves harvesting abdominal wall fat by liposuction either under local57 or general anesthesia.58 The injection is usually carried out via the periurethral route with a 16- or 18-gauge needle. Postprocedure care may involve intermittent catheterization or even a suprapubic tube.58
Autologous Fat Results
A number of reports of urethral fat injections have been published and appear in Table 4. Most of the series report short-term results with success apparently lower than that of other injectables, apart from the study of Su and associates,58 which had a follow-up of more than 12 months. Palma and colleagues59 showed that repeated injections improved the cure rate from 31% to 64%. Haab and associates60 reported a comparative study with collagen: after a mean of 7 months, 13% of the women with fat injection experienced cure, versus 24% of the women who received collagen injections. The subjective improvement rate was also higher with the collagen.
Table 4.
Results of Autologous Fat Injection
| Patients | Patients | ||||
|---|---|---|---|---|---|
| Follow-up | Patients | Improved | Failed | ||
| Study | Patients | (mo) | Dry (%) | (%) | (%) |
| Cervigni and Panei7 | 14 | 9.7 | 8 (57) | 4 (29) | 2 (14) |
| Santarosa and | 12 | 11 | 7 (58) | 5 (42) | |
| Blaivas82 | |||||
| Trockman and | 32 | 6 | 4 (12) | 14 (44) | 14 (44) |
| Leach57 | |||||
| Haab et al60 | 45 | 7 | 6 (13) | 13 (29) | 26 (58) |
| Su et al58 | 26 | Mean: 17.4 | 13 (50) | 4 (15) | 9 (35) |
| (range, | |||||
| 12–30) | |||||
| Palma et al59 | 30 | 12 | 1 injection: | ||
| 4/13 (31) | |||||
| 2 injections: | |||||
| 11/17 (64) |
Lee and colleagues61 reported a randomized double-blind study of autologous fat versus saline injection. At 3 months, 6 (22.2%) of 27 and 6 (20.7%) of 29 women had cure or improvement in the fat and saline groups, respectively. In this study, periurethral fat injection did not appear to be more efficacious than placebo in treating stress urinary incontinence.
Autologous Fat Complications
Reported complications are similar to those with other injectables, with urinary infection, retention, hematuria, and extravasation reported. Additional problems at the donor site (the abdominal wall), such as pain, hematomas, and infection, may also be seen. Other noteworthy complications are urethral pseudolipoma62 and fat embolism,34 1 of which was fatal.
Autologous Chondrocytes
A bulking agent composed of autologous chondrocytes has been used to treat children with vesicoureteral reflux.64 Animal studies of the implant demonstrated stability and lack of migration over time.65,66 The injectable material consists of autologous chondrocytes in a calcium alginate gel administered endoscopically through a 22-gauge needle. The chondrocytes, obtained from biopsy of the external pinna of the patient’s ear, are expanded in tissue culture and combined with a carrier gel that degrades after injection.
Bent and coworkers67 reported 12- month results in 32 women after a single outpatient injection in a multicenter trial. Incontinence grading indicated 16 patients were dry and 10 improved, for a total of 26 (81.3%). Side effects were minimal.
Calcium Hydroxyl Apatite
This normal constituent of bone can be synthesized and formulated in a size to resist migration. It can be used for soft tissue augmentation and does not form heterotopic bone.68
Mayer and colleagues69 reported results in 10 women at 1 year. The substance used had a mean particle size of 100 μm and was injected transurethrally through a 7F catheter with a 21-gauge needle tip. Seven women were substantially improved, 2 used fewer pads, and 1 had no change. No significant complications were seen.
Current Unmet Needs
The unmet needs surrounding injectable agents for stress incontinence can be grouped into those regarding agent, technique, and scientific characteristics.
The ideal agent has been described and mentioned above.18 We still require an agent that is biocompatible, that causes little or no inflammation, and that is nonantigenic. It should retain its bulk and not be molded by the movement of the urethra and pelvic muscles. It should not interfere with the closure mechanism or with the mucosal vascularity. It should also be durable.
The injection technique has undergone modifications over time, yet the ideal technique has yet to be described. It should be reproducible and consistent. The optimal sites along the urethra, the radial location(s), and the injection volume have to be identified. Ideally, the technique should involve a small volume, small needle, no extrusion of the agent, and no need for reinjections.
Histologic studies in experimental animal models showing the site of the deposit in the urethral wall as well as the effect on surrounding structures would be helpful. In addition, long-term data from prospective studies as well as randomized prospective trials comparing injectables with other treatments are needed. These studies should include economic discussions of cost and cost-effectiveness.
Conclusions
Considerable progress has been made since the introduction of collagen injections. Injectable agents are used for buttressing the ISD component of the incontinence, but patients with concomitant hypermobility may benefit as well. They have also been administered to elderly patients who are not surgical candidates. However, there are still a number of important areas in which further study is needed.
Durability is an ongoing concern. Although long-term successes have been reported with collagen and Teflon, the results of both deteriorate over time. Similarly, autologous fat and silicone microparticles yield poorer long-term than short-term results. There are no long-term results reported with any of the other injectable agents. Comparisons between injectables and between injectables and surgery have been done to a limited degree, and prospective studies have yet to be reported. Despite the ease of the technique and the attractiveness to patients of an outpatient procedure that can be repeated if necessary, the cost-effectiveness of injectable agents relative to other treatments, such as newer minimally invasive surgical procedures, still has to be addressed.
Safety of the material is another concern. All of the injectables have excellent safety profiles, although the risk of migration and granuloma formation with Teflon has prevented its widespread use. Rare but serious complications have also been reported with collagen and autologous fat. The long-term risks of silicone microparticles are unknown and the actual migration risk of Durasphere has yet to be elucidated.
Longer-term and comparative studies may settle many of these issues.
Main Points.
Patients with intrinsic sphincter deficiency with or without hypermobility, along with normal detrusor function, are candidates for the use of injectable agents for stress urinary incontinence. However, the search continues for the ideal, cost-effective agent and technique.
Injectables are administered under local anesthesia with cystoscopic control, either periurethrally or transurethrally, with the agent implanted within the urethral wall, ideally at the bladder neck or proximal urethra.
Injection of GAX collagen (after proper skin testing) generally is associated with no inflammatory response, granuloma formation, or migration, and complications are rare. Short-term success rates are 72% to 100%, but re-injection is often necessary to maintain results longer-term.
The diameter of most silicone microparticles makes migration unlikely, and associated side effects (hematuria, dysuria, frequency, and retention) are usually self-limited and not common; still, concern remains over long-term tissue response to this injectable and the durability of the results.
Carbon beads have demonstrated results similar to those of collagen, but more studies are needed to determine the pros and cons of this agent. Teflon, used since the 1960s, is no longer popular; although generally safe, Teflon can migrate to distant sites and offers no durability advantage over other agents.
Autologous fat offers a biocompatible alternative for injection, but a significant portion of the graft may not survive, and results have not been impressive compared with other agents.
References
- 1.Murless BC. The injection treatment of stress incontinence. J Obstet Gynaecol Br Emp. 1938;45:67–73. [Google Scholar]
- 2.Quackels R. Deux incontinences après adénectomie guéries par injection de paraffine dans la périnée. Acta Urol Belg. 1955;23:259–262. (Fre). [PubMed] [Google Scholar]
- 3.Sachse H. Treatment of urinary incontinence with sclerosing solutions. Indications, results, complications. Urol Int. 1963;15:225–244. doi: 10.1159/000279016. [in German] [DOI] [PubMed] [Google Scholar]
- 4.Berg S. Polytef augmentation urethroplasty. Correction of surgically incurable urinary incontinence by injection technique. Arch Surg. 1973;107:379–381. doi: 10.1001/archsurg.1973.01350210017006. [DOI] [PubMed] [Google Scholar]
- 5.Politano VA, Small MP, Harper JM, Lynne CM. Periurethral Teflon injection for urinary incontinence. J Urol. 1974;111:180–183. doi: 10.1016/s0022-5347(17)59921-8. [DOI] [PubMed] [Google Scholar]
- 6.Shortliffe LM, Freiha FS, Kessler R, et al. Treatment of urinary incontinence by the periurethral implantation of glutaraldehyde cross-linked collagen. J Urol. 1989;141:538–541. doi: 10.1016/s0022-5347(17)40885-8. [DOI] [PubMed] [Google Scholar]
- 7.Cervigni M, Panei M. Periurethral autologous fat injection for type III stress urinary incontinence. J Urol. 1993;149:403A. (pt 2) [Google Scholar]
- 8.Harriss DR, Iacovou JW, Lemberger RJ. Peri-urethral silicone microimplants (Macroplastique) for the treatment of genuine stress incontinence. Brit J Urol. 1996;78:722–728. doi: 10.1046/j.1464-410x.1996.17510.x. [DOI] [PubMed] [Google Scholar]
- 9.Lightner D, Calvosa C, Andersen R, et al. A new injectable agent for treatment of stress urinary incontinence: results of a multicenter, randomized, double-blind study of Durasphere. Urology. 2001;58:12–15. doi: 10.1016/s0090-4295(01)01148-7. [DOI] [PubMed] [Google Scholar]
- 10.McGuire EJ, Appell R. Transurethral collagen injection for urinary incontinence. Urology. 1994;43:413–415. doi: 10.1016/0090-4295(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 11.Monga AK, Robinson D, Stanton SL. Periurethral collagen injections for genuine stress incontinence: a 2-year follow-up. Brit J Urol. 1995;76:156–160. doi: 10.1111/j.1464-410x.1995.tb07664.x. [DOI] [PubMed] [Google Scholar]
- 12.Herschorn S, Radomski SB, Steele DJ. Early experience with intraurethral collagen injections for urinary incontinence. J Urol. 1992;148:1797–1800. doi: 10.1016/s0022-5347(17)37032-5. [DOI] [PubMed] [Google Scholar]
- 13.Winters JC, Appell R. Periurethral injection of collagen in the treatment of intrinsic sphincter deficiency in the female patient. Urol Clin North Am. 1995;22:673–678. [PubMed] [Google Scholar]
- 14.Richardson TD, Kennelly MJ, Faerber GJ. Endoscopic injection of glutaraldehyde cross-linked collagen for the treatment of intrinsic sphincter deficiency in women. Urology. 1995;46:378–381. doi: 10.1016/S0090-4295(99)80223-4. [DOI] [PubMed] [Google Scholar]
- 15.Eckford SD, Abrams P. Para-urethral collagen implantation for female stress incontinence. Br J Urol. 1991;68:586–589. doi: 10.1111/j.1464-410x.1991.tb15420.x. [DOI] [PubMed] [Google Scholar]
- 16.Appell RA. New developments: injectables for urethral incompetence in women. Int Urogynecol J. 1990;1:117–119. [Google Scholar]
- 17.Khullar V, Cardozo LD, Abbott D, Anders K. GAX collagen in the treatment of urinary incontinence in elderly women: a two year follow up. Brit J Obstet Gynaecol. 1997;104:96–99. doi: 10.1111/j.1471-0528.1997.tb10657.x. [DOI] [PubMed] [Google Scholar]
- 18.Kershen RT, Atala A. New advances in injectable therapies for the treatment of incontinence and vesicoureteral reflux. Urol Clin North Am. 1999;26:81–94. doi: 10.1016/s0094-0143(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 19.Appell R.A. Periurethral injection therapy. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 7th ed. Philadelphia: WB Saunders Company; 1998. pp. 1109–1120. [Google Scholar]
- 20.McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452–1454. doi: 10.1016/s0022-5347(17)35806-8. [DOI] [PubMed] [Google Scholar]
- 21.Raz S, Little N, Juma S. Female urology. In: Walsh PC, Retik AB, Stamey TA, Vaughan ED, editors. Campbell’s Urology. 6th ed. Philadelphia: WB Saunders Company; 1992. pp. 2782–2828. [Google Scholar]
- 22.Faerber GJ. Endoscopic collagen injection therapy in elderly women with type I stress urinary incontinence. J Urol. 1996;155:512–514. [PubMed] [Google Scholar]
- 23.Defreitas GA, Wilson TS, Zimmern PE, Forte TB. Three-dimensional ultrasonography: an objective outcome tool to assess collagen distribution in women with stress urinary incontinence. Urology. 2003;62:232–236. doi: 10.1016/s0090-4295(03)00353-4. [DOI] [PubMed] [Google Scholar]
- 24.Tamanini JT, D’ancona CA, Tadini V, Netto NR., Jr Macroplastique implantation system for the treatment of female stress urinary incontinence. J Urol. 2003;169:2229–2233. doi: 10.1097/01.ju.0000067472.94016.e8. [DOI] [PubMed] [Google Scholar]
- 25.Remacle M, Bertrand B, Eloy P, Marbaix E. The use of injectable collagen to correct velopharyngeal insufficiency. Laryngoscope. 1990;100:269–274. doi: 10.1288/00005537-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Stothers L, Goldenberg SL. Delayed hypersensitivity and systemic arthralgia following transurethral collagen injection for stress urinary incontinence. J Urol. 1998;159:1507–1509. doi: 10.1097/00005392-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Carr LK, Herschorn S, Leonhardt C. Magnetic resonance imaging after intraurethral collagen injected for stress urinary incontinence. J Urol. 1996;155:1253–1255. [PubMed] [Google Scholar]
- 28.Cross CA, English SF, Cespedes RD, McGuire EJ. A followup on transurethral collagen injection therapy for urinary incontinence. J Urol. 1998;159:106–108. doi: 10.1016/s0022-5347(01)64027-8. [DOI] [PubMed] [Google Scholar]
- 29.Herschorn S, Radomski SB. Collagen injections for genuine stress urinary incontinence: Patient selection and durability. Int Urogyn J. 1997;8:18–24. doi: 10.1007/BF01920289. [DOI] [PubMed] [Google Scholar]
- 30.Berman CJ, Kreder KJ. Comparative cost analysis of collagen injection and fascia lata sling cystourethropexy for the treatment of type III incontinence in women. J Urol. 1997;157:122–124. [PubMed] [Google Scholar]
- 31.Moore KN, Chetner MP, Metcalfe JB, Griffiths DJ. Periurethral implantation of glutaraldehyde cross-linked collagen (Contigen) in women with type I or type III stress incontinence: quantitative outcome measures. Br J Urol. 1995;75:359–363. doi: 10.1111/j.1464-410x.1995.tb07349.x. [DOI] [PubMed] [Google Scholar]
- 32.O’Connell HE, McGuire EJ, Aboseif S, Usui A. Transurethral collagen therapy in women. J Urol. 1995;154:1463–1465. [PubMed] [Google Scholar]
- 33.Bent AE, Foote J, Siegel S, et al. Collagen implant for treating stress urinary incontinence in women with urethral hypermobility. J Urol. 2001;166:1354–1357. [PubMed] [Google Scholar]
- 34.Sweat SD, Lightner DJ. Complications of sterile abscess formation and pulmonary embolism following periurethral bulking agents. J Urol. 1999;161:93–96. [PubMed] [Google Scholar]
- 35.Stothers L, Goldenberg SL, Leone EF. Complications of periurethral collagen injection for stress urinary incontinence. J Urol. 1998;159:806–807. [PubMed] [Google Scholar]
- 36.Elson ML. The role of skin testing in the use of collagen injectable materials. J Dermatol Surg Oncol. 1986;15:301–303. doi: 10.1111/j.1524-4725.1989.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 37.McClelland M, DeLustro F. Evaluation of antibody class in response to bovine collagen treatment in patients with urinary incontinence. J Urol. 1996;155:2068–2073. [PubMed] [Google Scholar]
- 38.Henly DR, Barrett DM, Weiland TL, et al. Particulate silicone for use in periurethral injections: local tissue effects and search for migration. J Urol. 1995;153:2039–2043. [PubMed] [Google Scholar]
- 39.Sheriff MK, Foley S, McFarlane J, et al. Endoscopic correction of intractable stress incontinence with silicone micro-implants. Eur Urol. 1997;32:284–288. [PubMed] [Google Scholar]
- 40.Koelbl H, Saz V, Doerfler D, et al. Transurethral injection of silicone microimplants for intrinsic sphincter deficiency. Obstet Gynecol. 1998;92:332–336. doi: 10.1016/s0029-7844(98)00215-4. [DOI] [PubMed] [Google Scholar]
- 41.Radley SC, Chapple CR, Mitsogiannis IC, Glass KS. Transurethral implantation of macroplastique for the treatment of female stress urinary incontinence secondary to urethral sphincter deficiency. Eur Urol. 2001;39:383–389. doi: 10.1159/000052474. [DOI] [PubMed] [Google Scholar]
- 42.Barranger E, Fritel X, Kadoch O, et al. Results of transurethral injection of silicone micro-implants for females with intrinsic sphincter deficiency. J Urol. 2000;164:1619–1622. [PubMed] [Google Scholar]
- 43.Horl HW, Feller AM, Biemer E. Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg. 1991;26:248–258. doi: 10.1097/00000637-199103000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Malizia AA, Reiman HM, Myers RP, et al. Migration and granulomatous reaction after periurethral injection of polytef (Teflon) JAMA. 1983;251:3277–3281. [PubMed] [Google Scholar]
- 45.Pannek J, Brands FH, Senge T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J Urol. 2001;166:1350–1353. [PubMed] [Google Scholar]
- 46.Herschorn S, Glazer AA. Early experience with small volume periurethral polytetrafluoroethylene for female stress urinary incontinence. J Urol. 2000;163:1838–1842. [PubMed] [Google Scholar]
- 47.Diagnostic and Therapeutic Technology Assessment (DATTA), authors Use of Teflon preparations for urinary incontinence and vesicoureteral reflux. JAMA. 1993;269:2975–2980. [PubMed] [Google Scholar]
- 48.Schulman CC, Simon J, Wespes E, et al. Endoscopic injections of Teflon to treat urinary incontinence in women. Brit Med J (Clin Res Ed) 1984;288:192. doi: 10.1136/bmj.288.6412.192. [in French] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim KB, Ball AJ, Feneley RC. Periurethral Teflon injection: A simple treatment for urinary incontinence. Br J Urol. 1983;55:208–210. doi: 10.1111/j.1464-410x.1983.tb06558.x. [DOI] [PubMed] [Google Scholar]
- 50.Claes H, Stroobants D, Van Meerbeek J, et al. Pulmonary migration following periurethral polytetrafluoroethylene injection for urinary incontinence. J Urol. 1989;142:821–822. doi: 10.1016/s0022-5347(17)38903-6. [DOI] [PubMed] [Google Scholar]
- 51.Mittleman RE, Marraccini JV. Pulmonary Teflon granulomas following periurethral Teflon injection for urinary incontinence. Arch Pathol Lab Med. 1983;107:611–612. [PubMed] [Google Scholar]
- 52.Kiilholma PJ, Chancellor MB, Makinen J, et al. Complications of Teflon injection for stress urinary incontinence. Neurourol Urodyn. 1993;12:131–137. doi: 10.1002/nau.1930120206. [DOI] [PubMed] [Google Scholar]
- 53.Stanisic TH, Jennings CE, Miller JI. Polytetrafluoroethylene injection for post-prostatectomy incontinence: experience with 20 patients during 3 years. J Urol. 1991;146:1575–1577. doi: 10.1016/s0022-5347(17)38170-3. [DOI] [PubMed] [Google Scholar]
- 54.Dewan PA, Owen AJ, Byard RW. Long-term histological response to subcutaneously injected Polytef and Bioplastique in a rat model. Br J Urol. 1995;76:161–164. doi: 10.1111/j.1464-410x.1995.tb07665.x. [DOI] [PubMed] [Google Scholar]
- 55.Billings E, Jr, May JW., Jr Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconst Surg. 1989;83:368–381. doi: 10.1097/00006534-198902000-00033. [DOI] [PubMed] [Google Scholar]
- 56.Bircoll M, Novack BH. Autologous fat transplantation employing liposuction techniques. Ann Plast Surg. 1987;18:327–329. doi: 10.1097/00000637-198704000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Trockman BA, Leach GE. Surgical treatment of intrinsic urethral dysfunction: injectables (fat) Urol Clin North Am. 1995;22:665–671. [PubMed] [Google Scholar]
- 58.Su TH, Wang KG, Hsu CY, et al. Periurethral fat injection in the treatment of recurrent genuine stress incontinence. J Urol. 1998;159:411–414. doi: 10.1016/s0022-5347(01)63935-1. [DOI] [PubMed] [Google Scholar]
- 59.Palma PC, Riccetto CL, Herrmann V, Netto NR., Jr Repeat lipoinjections for stress urinary incontinence. J Endourol. 1997;11:67–70. doi: 10.1089/end.1997.11.67. [DOI] [PubMed] [Google Scholar]
- 60.Haab F, Zimmern PE, Leach GE. Urinary stress incontinence due to intrinsic sphincteric deficiency: experience with fat and collagen periurethral injections. J Urol. 1997;157:1283– 1286. [PubMed] [Google Scholar]
- 61.Lee PE, Kung RC, Drutz HP. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol. 2001;165:153–158. doi: 10.1097/00005392-200101000-00037. [DOI] [PubMed] [Google Scholar]
- 62.Palma PC, Riccetto CL, Netto Junior NR. Urethral pseudolipoma: a complication of periurethral lipo-injection for stress urinary incontinence in a woman. J Urol. 1996;155:646. doi: 10.1016/s0022-5347(01)66480-2. [DOI] [PubMed] [Google Scholar]
- 63.Currie I, Drutz HP, Deck J, Oxorn D. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: case report and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:377–380. doi: 10.1007/BF02765599. [DOI] [PubMed] [Google Scholar]
- 64.Diamond DA, Caldamone AA. Endoscopic correction of vesicoureteral reflux in children using autologous chondrocytes: preliminary results. J Urol. 1999;162:1185–1188. doi: 10.1016/S0022-5347(01)68124-2. [DOI] [PubMed] [Google Scholar]
- 65.Atala A, Kim W, Paige KT, et al. Endoscopic treatment of vesicoureteral reflux with a chondrocyte- alginate suspension. J Urol. 1994;152:641–643. doi: 10.1016/s0022-5347(17)32671-x. [DOI] [PubMed] [Google Scholar]
- 66.Cozzolino DJ, Cendron M, DeVore DP, Hoopes PJ. The biological behavior of autologous collagen- based extracellular material matrix injected into the rabbit bladder wall. Neurourol Urodyn. 1999;18:487–495. doi: 10.1002/(sici)1520-6777(1999)18:5<487::aid-nau11>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 67.Bent AE, Tutrone RT, McLennan MT, et al. Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. Neurourol Urodyn. 2001;20:157–165. doi: 10.1002/1520-6777(2001)20:2<157::aid-nau18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 68.Pettis GY, Kaban LB, Glowacki J. Tissue response to composite ceramic hydroxyapatite/demineralized bone implants. J Oral Maxillofac Surg. 1990;48:1068–1074. doi: 10.1016/0278-2391(90)90291-9. [DOI] [PubMed] [Google Scholar]
- 69.Mayer R, Lightfoot M, Jung I. Preliminary evaluation of calcium hydroxylapatite as a transurethral bulking agent for stress urinary incontinence. Urology. 2001;57:434–438. doi: 10.1016/s0090-4295(00)01098-0. [DOI] [PubMed] [Google Scholar]
- 70.Stricker P, Haylen B. Injectable collagen for type 3 female stress incontinence: the first 50 Australian patients. Med J Aust. 1993;158:89–91. doi: 10.5694/j.1326-5377.1993.tb137530.x. [DOI] [PubMed] [Google Scholar]
- 71.Kieswetter H, Fischer M, Wîber L, Flamm J. Endoscopic implantation of collagen (GAX) for the treatment of urinary incontinence. Br J Urol. 1992;69:22–25. doi: 10.1111/j.1464-410x.1992.tb15451.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith DN, Appell RA, Winters JC, Rackley RR. Collagen injection therapy for female intrinsic sphincteric deficiency. J Urol. 1997;157:1275–1278. [PubMed] [Google Scholar]
- 73.Swami S, Batista JE, Abrams P. Collagen for female genuine stress incontinence after a minimum 2-year follow-up. Br J Urol. 1997;80:757–761. doi: 10.1046/j.1464-410x.1997.00465.x. [DOI] [PubMed] [Google Scholar]
- 74.Groutz A, Blaivas JG, Kessler SS, et al. Outcome results of transurethral collagen injection for female stress incontinence: assessment by urinary incontinence score. J Urol. 2000;164:2006–2009. [PubMed] [Google Scholar]
- 75.Corcos J, Fournier C. Periurethral collagen injection for the treatment of female stress urinary incontinence: 4-year follow-up results. Urology. 1999;54:815–818. doi: 10.1016/s0090-4295(99)00269-1. [DOI] [PubMed] [Google Scholar]
- 76.Deane AM, English P, Hehir M, et al. Teflon injection in stress incontinence. Br J Urol. 1985;57:78–80. doi: 10.1111/j.1464-410x.1985.tb08990.x. [DOI] [PubMed] [Google Scholar]
- 77.Beckingham IJ, Wemyss-Holden G, Lawrence WT. Long-term follow-up of women treated with periurethral Teflon injections for stress incontinence. Br J Urol. 1992;69:580–583. doi: 10.1111/j.1464-410x.1992.tb15626.x. [DOI] [PubMed] [Google Scholar]
- 78.Harrison SC, Brown C, O’Boyle PJ. Periurethral Teflon for stress urinary incontinence: medium-term results. Br J Urol. 1993;71:25–27. doi: 10.1111/j.1464-410x.1993.tb15874.x. [DOI] [PubMed] [Google Scholar]
- 79.Lotenfoe R, O’Kelly JK, Helal M, Lockhart JL. Periurethral polytetrafluoroethylene paste injection in incontinent female subjects: surgical indications and improved surgical technique. J Urol. 1993;149:279–282. doi: 10.1016/s0022-5347(17)36056-1. [DOI] [PubMed] [Google Scholar]
- 80.Lopez AE, Padron OF, Patsias G, Politano VA. Transurethral polytetrafluoroethylene injection in female patients with urinary incontinence. J Urol. 1993;150:856–858. doi: 10.1016/s0022-5347(17)35632-x. [DOI] [PubMed] [Google Scholar]
- 81.Vesey SG, Rivett A, O’Boyle PJ. Teflon injection in female stress incontinence. Effect on urethral pressure profile and flow rate. Br J Urol. 1988;62:39–41. doi: 10.1111/j.1464-410x.1988.tb04262.x. [DOI] [PubMed] [Google Scholar]
- 82.Santarosa RP, Blaivas JG. Periurethral injection of autologous fat for the treatment of sphincteric incontinence. J Urol. 1994;151:607–611. doi: 10.1016/s0022-5347(17)35029-2. [DOI] [PubMed] [Google Scholar]
- 83.Politano VA. Periurethral polytetrafluoroethylene injection for urinary incontinence. J Urol. 1982;127:439–442. doi: 10.1016/s0022-5347(17)53852-5. [DOI] [PubMed] [Google Scholar]