Abstract
Since the early 20th century, radical prostatectomy has been used in the treatment of prostate cancer. However, before the widespread acceptance of prostate-specific antigen screening, the majority of cancers were clinically advanced and not amenable to cure, so relatively few men were candidates for this procedure. Modern advances have contributed dramatically to the reduction of complications and morbidity associated with radical prostatectomy. As a result, the procedure has become the most common treatment selected by men with localized prostate cancer. This article reviews several issues regarding radical prostatectomy, including surgical techniques, cancer control, intraoperative localization of the cavernous nerves, patient selection, and laparoscopic versus robotic approaches.
Key words: Laparoscopy, Nerve-sparing technique, Prostate cancer, Radical prostatectomy, Robotic surgery
Radical perineal prostatectomy was first described by Hugh Hampton Young1 in 1905 for the treatment of prostate cancer. In 1947, Millin2 described a retropubic approach for radical prostatectomy. Historically, both radical retropubic prostatectomy and radical perineal prostatectomy were associated with significant intraoperative and postoperative morbidity.3 Major complications included rectal injury, ureteral injury, massive hemorrhage, pulmonary embolus, anastomotic leaks, lymphoceles, wound infections, and incontinence. Virtually all men were rendered impotent. Therefore, many potential surgical candidates opted for radiation therapy introduced because of the lower risk of erectile dysfunction.
The advantage of the retropubic approach was the ability to perform a simultaneous staging pelvic lymphadenectomy through a single lower-abdominal incision. In addition, urologists were generally more familiar with the retropubic approach. The primary disadvantage of a retropubic approach was that significant bleeding was often encountered while attempting to control the dorsal venous complex.4 The dorsal venous complex is not encountered during perineal dissection because the mobilization of the prostate is performed entirely within the endopelvic fascia.
In 1979, Reiner and Walsh5 described the anatomy of the dorsal venous complex and a technique for its early ligation during radical retropubic prostatectomy. This maneuver greatly reduced blood loss during radical retropubic prostatectomy and allowed for the dissection of the prostate to be performed in a relatively bloodless surgical field.
Before the widespread acceptance of prostate-specific antigen (PSA) screening for prostate cancer, the overwhelming majority of diagnosed prostate cancers were clinically advanced and not amenable to cure.6 Therefore, relatively few men were candidates for a radical prostatectomy. Jewett, one of the renowned prostate cancer surgeons of his time, performed only 160 radical prostatectomies between 1951 and 1963.7 PSA screening has resulted in a dramatic stage migration, favoring the detection of localized disease.8 This has greatly increased the demand for radical prostatectomy. Advances in surgical technique, anesthetic agents, intraoperative monitoring, and greater case volumes have contributed to dramatically decreasing complications and morbidity associated with the procedure. In 2004, radical prostatectomy will represent the most common treatment selected by men with localized prostate cancer.
Development of the Anatomic Nerve-Sparing Radical Retropubic Prostatectomy
In 1979, Reiner and Walsh5 described a technique for controlling the dorsal venous complex during radical retropubic prostatectomy. This advancement in surgical technique provided the opportunity to perform radical retropubic prostatectomy in a relatively bloodless surgical field. Ultimately, this modification of surgical technique would prove to be essential for developing the anatomic nerve-sparing radical prostatectomy.
Two years after reporting the technique for controlling the dorsal venous complex, Walsh visited the laboratory of Peter Donker, who was performing dissections in the human fetus in order to gain insights into the continence mechanism. Walsh observed nerves emanating from the pelvic plexus that coursed along the prostate and ultimately innervated the corpus cavernosum.9 He hypothesized that these nerves were likely injured during radical prostatectomy, possibly resulting in erectile dysfunction.
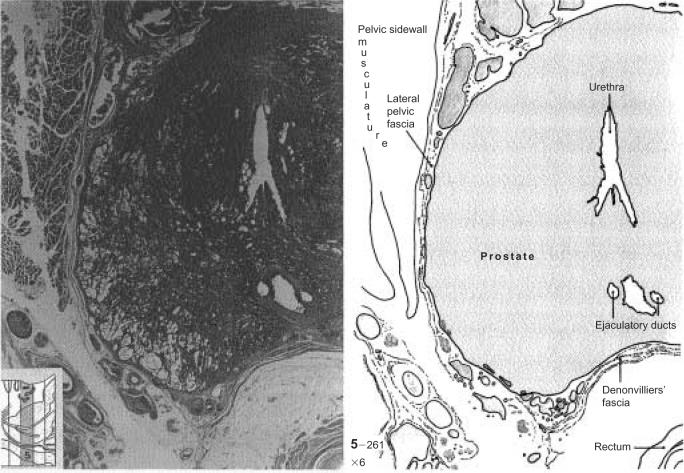
Upon returning to the United States, Walsh challenged the author to identify the precise pathway of the cavernous nerves in the human. In 1981, an autopsy specimen was obtained from a male that included the prostate and adjacent soft tissues.10 A cross-section of this autopsy specimen suggested that the autonomic innervation to the penis courses posterolateral to the prostate (Figure 1). These nerves are located outside the prostatic capsule and within the visceral layer of the pelvic fascia. Walsh proposed that the neural innervation to the corpus cavernosum could be preserved during radical retropubic prostatectomy without entering the prostatic capsule. Walsh and colleagues10 reported his initial results of the nerve-sparing radical prostatectomy, confirming that preservation of the cavernous nerves and of potency could be achieved without compromising cancer control.
Figure 1.
Cross-section of an adult prostate demonstrating the anatomic relationships between the lateral pelvic fascia, Denonvilliers’ fascia, and the neurovascular bundle. From Walsh PC et al.10 © 1983 Alan R. Liss, Inc. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
In 1983, the author identified a human male cadaver that was perfused with Bouin’s solution shortly after death.11 The penis, prostate, bladder, rectum, and adjacent soft tissue were removed en bloc. Working in collaboration with Dr. Fathollah Mostolfi of the Armed Forces Institute of Pathology, the specimen was serially step-sectioned using a specialized dermatome. Every tenth step-section was stained with hematoxylin and eosin and an anastomotic three-dimensional reconstruction was performed.11 The precise pathway of the cavernous nerves and its relationship to the pelvic viscera and fascia were delineated. The precise pathway of the cavernous nerves allowed for further refinements of the surgical technique designed to preserve these nerves during radical retropubic prostatectomy.
Does the Nerve-Sparing Technique Compromise Cancer Control?
Several skeptics of the Walsh anatomic nerve-sparing radical retropubic prostatectomy referred to the procedure as “cancer-sparing” surgery. Walsh responded to these critics with a compelling comparative pathologic study demonstrating that more prostatic soft tissue was removed during a nerve-sparing radical retropubic prostatectomy than with a standard radical perineal prostatectomy.10 Wider surgical margins are achieved with the nerve-sparing radical retropubic prostatectomy because the perineal dissection is performed completely within the lateral pelvic fascia. Eggleston and associates12 subsequently examined 100 surgical specimens obtained during nerve-sparing radical prostatectomy and demonstrated that there were no cases without seminal vesicle invasion that had isolated positive margins at the site of the neurovascular bundle. These rigorous, pathologic studies silenced the critics claiming that nerve-sparing surgery compromised cancer control.
Selecting Candidates for Nerve-Sparing Surgery
The goal of nerve-sparing radical retropubic prostatectomy is to maximally preserve neurovascular bundles without compromising cancer control. When is it appropriate to perform nerve-sparing versus wide excision of the neurovascular bundle?
Pettus and coworkers13 reviewed the Mayo Clinic experience suggesting that in men with extracapsular extension, nerve-sparing status was not predictive of margin status. This study may be interpreted to suggest that the Mayo surgeons were instinctively properly selecting candidates for nerve-sparing surgery or that wide excision of the neurovascular bundle does not improve margin status. Although I believe the former is the case, the question remains unanswered by this study. The authors did not establish prospective criteria for preserving the neurovascular bundles, so the review provides no insight into when it is appropriate to spare the nerves.
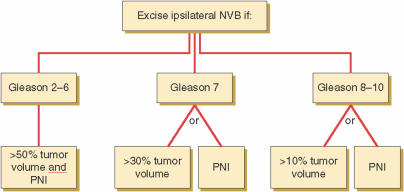
It is reasonable to assume that in men with macroscopic extracapsular extension, the nerve-sparing technique compromises cancer control. The likelihood of extracapsular extension is related to Gleason score, tumor volume, and perineural invasion in the biopsy specimen.14–17 Shah and colleagues18 reported an algorithm for guiding surgeons on when it is appropriate to deliberately widely excise the neurovascular bundle based on these predictive factors of extracapsular extension. The ipsilateral neurovascular bundles were excised for Gleason score 6 cancers with both perineural invasion and >50% of the biopsy specimen involved with cancer. The ipsilateral neurovascular bundles were excised for Gleason 7 cancers with perineural invasion or >30% of the biopsy specimen involved with the tumor and for Gleason 8 cancers with perineural invasion or >10% of the biopsy specimen involved with the tumor (Figure 2). Using this algorithm, the investigators decreased positive surgical margins from 14% to 8% while increasing the proportion of nerves spared from 85% to 92%.
Figure 2.
New York University nerve-sparing algorithm based on Gleason score, percent tumor volume, and evidence of perineural invasion (PNI) in biopsy specimens. The algorithm for decision regarding wide excision versus preservation of the neurovascular bundles (NVB) is presented. Reprinted with permission from Shah O et al.18
Surgical Technique
At the level of the prostate, the autonomic innervation to the prostate and penis is not a single nerve but rather a neurovascular bundle comprising many microscopic nerve branches (Figure 3). Therefore, meticulous hemostasis facilitates identification of anatomic landmarks that allow for preservation of the neurovascular bundle because the actual nerve is not visible. We have previously reported our technique for radical retropubic prostatectomy.19 Control of the dorsal venous complex involves both proximal and distal suture ligation before dividing the structure. After controlling the dorsal venous complex, the anterior component of the prostatourethral junction is sharply incised. The tissue immediately lateral to the prostatovesical junction should not be disturbed because this is the location of the neurovascular bundle as it courses through the membranous urethra. Six anastomotic sutures are positioned into the urethra, with meticulous care taken not to entrap the adjacent nerve, which is located at the 3 and 9 o’clock positions. The posterior urethra is then sharply divided. The anterior layer of Denonvilliers’ fascia overlying the rectum is sharply incised, and the prostate is bluntly mobilized off the rectum in the midline. The visceral layer of the endopelvic fascia overlying the prostate and neurovascular bundle can be incised using 2 different techniques. In the absence of significant biopsy artifact, dense adhesions, or a large apical tumor, I prefer to incise the fascia beginning at the apex. The visceral layer of the endopelvic fascia can be incised beginning at the bladder neck in the presence of significant apical adhesions or a large apical tumor. In cases of low-volume, low-grade disease, the fascia is divided anteriorly on the prostate.
Figure 3.
Representative tissue section showing location of neurovascular bundle in relationship to midportion of prostate. Reprinted with permission from Lepor H et al.11
After incising the lateral pelvic fascia, a finger is insinuated in the midline and the prostate is retracted medially as the neurovascular bundle is sharply and bluntly mobilized off the prostate. Surgical clips or sutures are not routinely used until the prostatic pedicle is encountered. Specific arterial bleeders are controlled with ligature clips. The Denonvilliers’ fascia overlying the seminal vesicles and vas deferens is incised, and the rectum is mobilized off these structures. The pedicle to the prostate is divided beginning at the lateral margin of the seminal vesicle, staying close but not entering the prostate. The surgical specimen is inspected to ensure that the prostate has not been inadvertently incised. In cases in which the dissection is felt to be too close to the prostate, additional soft tissue may be excised.
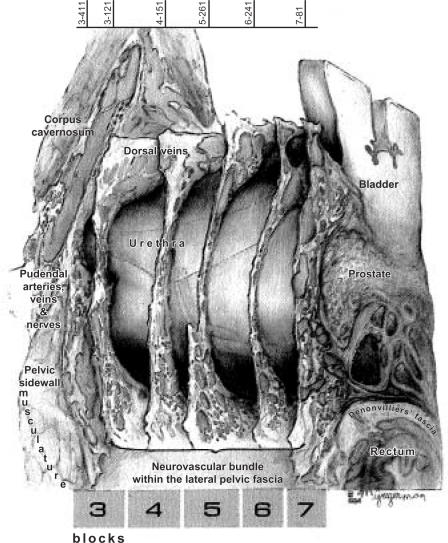
Intraoperative Localization of the Cavernous Nerves
The autonomic nerves innervating the prostate course posterolateral to the prostate (Figure 4). These nerves typically cannot be visualized, even with the magnification provided by an intraoperative microscope. The nerves are preserved by carefully dividing the prostatourethral junction and performing the dissection in very close proximity to the prostate.
Figure 4.
Anatomic reconstruction of prostate, ejaculatory ducts, pelvic sidewall fascia, bladder, urethra, rectum, and neurovascular structures. Reprinted with permission from Lepor H et al.11
The CaverMap™ (Blue Torch Medical Technologies, Inc., Ashland, MA) is a device that was designed to aid the surgeon in identifying the precise localization of the cavernous nerves intraoperatively.20 The device consists of a hand-held nerve stimulator and a ringlike tumescence-monitoring gauge, which is positioned at the base of the penis and is very sensitive to changes in the circumference of the penis. A recorder monitors changes in the circumference of the penis in response to an electrical current delivered by the handset. I was 1 of the 5 investigators who studied the utility of the CaverMap device in the United States.21
One of the primary limitations of the hand-held nerve stimulator is its relatively large size, which precludes identifying small components of the neurovascular bundle. The device allows for gross localization, which is of limited benefit to the skilled surgeon. Another limitation of the CaverMap is its lack of specificity. In 50% of cases, stimulation of the bladder elicited a penile response consistent with stimulation of the cavernous nerve.21 Finally, there is no uniform definition of a nerve tumescence response. In some cases, penile elongation is associated with a decrease in penile circumference. Therefore, a sustained increase or decrease in the penile circumference was ultimately considered a positive response.
Although the concept for developing the CaverMap was appealing, its lack of specificity, the large size of the stimulator, and the inconsistent response have limited the clinical utility of this device.
Laparoscopic Versus Robotic Radical Prostatectomy
Laparoscopic radical prostatectomy performed with or without robotic assistance has become an accepted treatment for localized prostate cancer. The primary theoretic advantage of laparoscopic technique is magnification. The use of loupes can provide the open surgeon with a similar magnification advantage if it is deemed helpful. Because the nerves are microscopic and the critical landmarks for preserving these nerves are grossly visible, I do not consider magnification an advantage for nerve sparing.
One of the inherent advantages of open over laparoscopic radical prostatectomy is propioception. I rely on palpation of the prostate while sharply and bluntly mobilizing the neurovascular bundle off the prostate to avoid inadvertently incising the prostate.
A disadvantage of laparoscopic radical prostatectomy is that the lateral pedicle and the branches to the prostate are controlled with electrocautery. Electrocautery has the propensity to cause adjacent tissue damage. I feel that laparoscopic radical prostatectomy places the nerves innervating the penis at an unnecessary risk for thermal injury.
Nerves during open radical prostatectomy may be traumatized as a result of traction. This traction is minimized during laparoscopic approaches. The potential problems attributable to traction on the cavernous nerves are only theoretic.
Thus, there is no compelling theoretic or clinical evidence that laparoscopic techniques with or without robotic assistance represent any advantage for preserving potency during radical prostatectomy. The claim of superiority by open or laparoscopic surgeons must ultimately be based on prospective and unbiased outcome studies using similar outcome measures and definitions of potency.
Main Points.
Prostate-specific antigen screening has resulted in a dramatic stage migration, favoring the detection of localized disease. This has greatly increased the demand for radical prostatectomy.
Walsh and colleagues reported initial results of nerve-sparing radical prostatectomy, confirming that preservation of the cavernous nerves and of potency could be achieved without compromising cancer control.
It is reasonable to assume that in men with macroscopic extracapsular extension, the nerve-sparing technique compromises cancer control. The likelihood of extracapsular extension is related to Gleason score, tumor volume, and perineural invasion in the biopsy specimen.
Laparoscopic radical prostatectomy performed with or without robotic assistance has become an accepted treatment for localized prostate cancer.
There is no compelling theoretic or clinical evidence that laparoscopic techniques with or without robotic assistance represent any advantage for preserving potency during radical prostatectomy.
References
- 1.Young HH. The early diagnosis and radical cure of carcinoma of the prostate: being a study of 40 cases and presentation of a radical operation which was carried out in four cases. Bull Johns Hopkins Hosp. 1905;16:315. [Google Scholar]
- 2.Millin T. Retropubic Urinary Surgery. London: Livingston; 1947. [Google Scholar]
- 3.Catalona WJ, Scott WW. Carcinoma of the prostate. In: Walsh PC, Gittes RF, Perlmutter AD, Stamey TA, editors. Campbell’s Urology. vol 5. Philadelphia: WB Saunders Company; 1986. pp. 1463–1534. [Google Scholar]
- 4.Walsh PC, Lepor H. The role of radical prostatectomy in the management of prostatic carcinoma. Cancer. 1987;60(3 suppl):526–537. doi: 10.1002/1097-0142(19870801)60:3+<526::aid-cncr2820601515>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Reiner WG, Walsh PC. An anatomical approach to the surgical management of the dorsal vein and Santorini’s plexus during radical retropubic surgery. J Urol. 1979;212:198–200. doi: 10.1016/s0022-5347(17)56718-x. [DOI] [PubMed] [Google Scholar]
- 6.Newcomer LM, Stanford JL, Blumenstein BA, et al. Temporal trends in rates of prostate cancer: declining incidence of advanced stage disease, 1974 to 1994. J Urol. 1997;158:1427–1430. doi: 10.1016/s0022-5347(01)64231-9. [DOI] [PubMed] [Google Scholar]
- 7.Elder JS, Jewett HJ, Walsh PC. Radical perineal prostatectomy for clinical stage B2 carcinoma of the prostate. J Urol. 1982;127:704–706. doi: 10.1016/s0022-5347(17)54005-7. [DOI] [PubMed] [Google Scholar]
- 8.Taneja SS. The rationale for early detection of prostate cancer. In: Lepor H, editor. Prostatic Diseases. Philadelphia: WB Saunders Company; 2000. pp. 371–380. [Google Scholar]
- 9.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–497. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 10.Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473–485. doi: 10.1002/pros.2990040506. [DOI] [PubMed] [Google Scholar]
- 11.Lepor H, Gregerman M, Crosby R, et al. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosal: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207–212. doi: 10.1016/s0022-5347(17)48885-9. [DOI] [PubMed] [Google Scholar]
- 12.Eggleston JC, Walsh PC. Radical prostatectomy with preservation of sexual function: pathologic findings in the first 100 cases. J Urol. 1985;134:1146–1148. doi: 10.1016/s0022-5347(17)47661-0. [DOI] [PubMed] [Google Scholar]
- 13.Pettus JA, Weight CJ, Thompson CJ, et al. Biochemical failure in men following radical retropubic prostatectomy: impact of surgical margin status and location. J Urol. 2004;173:129–132. doi: 10.1097/01.ju.0000132160.68779.96. [DOI] [PubMed] [Google Scholar]
- 14.Ravery V, Schmid HP, Toublane M, Boccon-Gibod L. Is the percentage of cancer in biopsy cores predictive of extracapsular disease in T1–T2 prostate carcinoma? Cancer. 1996;78:1079–1084. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1079::AID-CNCR18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Cheng L, Slezak J, Bergstralh EJ, et al. Preoperative prediction of surgical margin status in patients with prostate cancer treated by radical prostatectomy. J Clin Oncol. 2000;18:2862–2868. doi: 10.1200/JCO.2000.18.15.2862. [DOI] [PubMed] [Google Scholar]
- 16.de la Taille A, Katz A, Bagiella E, et al. Perineural invasion on prostate needle biopsy: an independent predictor of final pathologic stage. Urology. 1999;54:1039–1043. doi: 10.1016/s0090-4295(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JI. The role of perineural invasion and other biopsy characteristics as prognostic markers for localized prostate cancer. Semin Urol Oncol. 1998;16:124–128. [PubMed] [Google Scholar]
- 18.Shah O, Robbins DA, Melamed J, Lepor H. The New York University nerve sparing algorithm decreases the rate of positive surgical margins following radical retropubic prostatectomy. J Urol. 2003;169:2147–2152. doi: 10.1097/01.ju.0000057496.49676.5a. [DOI] [PubMed] [Google Scholar]
- 19.Lepor H. Radical retropubic prostatectomy. Urol Clin North Am. 2001;28:509–519. doi: 10.1016/s0094-0143(05)70159-2. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L, Herschorn S. Early experience with intraoperative cavernous nerve stimulation with penile tumescence monitoring to improve nerve-sparing during radical prostatectomy. Urology. 1998;52:537–542. doi: 10.1016/s0090-4295(98)00319-7. [DOI] [PubMed] [Google Scholar]
- 21.Walsh PC, Marschke P, Catalona WJ, et al. Efficacy of first-generation CaverMap to verify location and function of cavernous nerves during radical prostatectomy: a multi-institutional evaluation by experienced surgeons. Urology. 2001;57:491–494. doi: 10.1016/s0090-4295(00)01067-0. [DOI] [PubMed] [Google Scholar]