Abstract
Acute urinary retention (AUR) secondary to benign prostatic hyperplasia has in the past represented an immediate indication for surgery, and today most patients failing to void after an attempt at catheter removal still undergo surgery. The concept that this disease is in fact progressive in nature is slowly being accepted. Descriptive and analytical epidemiological data have shown that the incidence rate per 1000 person-years is less variable in the community than previously assumed; however, the risk is cumulative and increases with advancing age. The risk for patients diagnosed with benign prostatic hyperplasia is naturally higher, and analytical epidemiology has identified several strong risk factors, the most important one being serum prostate-specific antigen (PSA). In addition, prostate volume, maximum flow rate, and symptom severity should be considered when counseling patients presenting with lower urinary tract symptoms and clinical benign prostatic hyperplasia who are considering a course of watchful waiting. Efforts toward primary prevention of AUR should be directed to patients at increased risk, ie, those who are older and have more severe symptoms, larger glands, and higher PSA values. Risk reduction with finasteride has been demonstrated, and α-blockers have been shown to aid patients in achieving spontaneous voiding after an episode of AUR.
Key words: Acute urinary retention, Benign prostatic hyperplasia, Transurethral resection of the prostate, Trial without catheter, Lower urinary tract symptoms, Alpha-blockers
Acute urinary retention (AUR) is one of the most significant complications or long-term outcomes of benign prostatic hyperplasia (BPH). It has in the past represented an immediate indication for surgery. Between 25% and 30% of men who underwent transurethral resection of the prostate (TURP) had AUR as their main indication in older series,1 and today most patients failing to void after an attempt at catheter removal still undergo surgery. From an economical viewpoint, AUR is a significant event, and from the viewpoint of the patient, often a feared one. For the patient it presents as the inability to urinate, with increasing pain, a visit to the emergency room, catheterization, follow-up visits to the physician, an attempt at catheter removal, and eventually recovery or surgery—a painful and time-consuming process. In older literature, the risk of recurrent AUR was cited as being 56%–64% within 1 week of the first episode and 76%–83% in men with diagnosed BPH.2–4
Etiology of AUR
The etiology of AUR is poorly understood. Prostate infection, bladder overdistension,5 excessive fluid intake, alcohol consumption, sexual activity, debility, and bed-rest have all been mentioned.6
Prostatic infarction has been suggested as an underlying event causing AUR.7 Spiro and colleagues found evidence for infarction in 85% of prostates removed for AUR versus 3% in prostates of men having surgery for symptoms only.8 In contrast, there was no evidence of infarction in 6 prostatectomy specimens removed from men who had surgery for AUR.9 Anjum and colleagues found fundamentally similar rates of infarction in 35 men with and without AUR (1.9% vs 3.0%) undergoing prostatectomy.10
From a clinical and prognostic point of view, spontaneous AUR should be separated from precipitated AUR, although this is by no means consistently done in the literature. Precipitated AUR refers to the inability to urinate following a triggering event, such as non-prostate-related surgery, catheterization, anesthesia, or ingestion of medications with sympathomimetic or anticholinergic effects, antihistamines, or others. All other AUR episodes are classified as spontaneous.11,12 The importance of differentiating the two types of AUR becomes clear when evaluating the ultimate outcomes of patients. Following spontaneous AUR, 15% of patients had another episode of spontaneous AUR, and a total of 75% underwent surgery, whereas after precipitated AUR, only 9% had an episode of spontaneous AUR, and 26% underwent surgery.11
Epidemiology of AUR in BPH
Data are available on the incidence of AUR from population-based studies as well as from observational studies and placebo-control groups of BPH studies. These data can be analyzed in terms of descriptive and analytical epidemiological approaches.
Descriptive Epidemiology
Older estimates of occurrence of AUR range from 4–15 to as high as 130 per 1000 person-years (calculated by Jacobsen and colleagues13 based on studies by Birkhoff and colleagues,14 Ball and colleagues,15 and Craigen and colleagues,16 giving 10-year cumulative incidence rates ranging from 4% to 73%). The self-reported rate of AUR in a cross-sectional study of 2002 Spanish men was 5.1%.17
More recent data from carefully controlled studies in better-defined populations shed additional light on the incidence rates in community-dwelling men and clinical BPH populations. AUR occurred in the VA Cooperative study in 1 man after TURP and in 8 of 276 men in the watchful waiting arm who were followed for 3 years, yielding an incidence rate of 9.6 per 1000 person-years.18 Barry and colleagues reported outcomes of 500 men diagnosed by urologists with BPH who were candidates for prostatectomy by established criteria but elected to be followed conservatively.19 In 1574 person-years, 40 episodes of AUR occurred at a constant rate throughout the 4 years of follow-up, for an incidence rate of 25 per 1000 person-years.
During 15,851 person-years of follow-up in the Physician's Health Study, 82 men reported an episode of AUR, for an incidence rate of 4.5 per 1000 person-years (95% CI, 3.1–6.2).20 Of the 2115 men aged 40–79 years in the Olmsted County Study, 57 had a first episode of AUR during 8344 person-years of follow-up (incidence 6.8/1000 person-years; 95% CI, 5.2–8.9).13
The best data from men diagnosed with BPH stem from the Proscar Long-Term Efficacy and Safety Study (PLESS).21 In PLESS, 1376 placebo-treated men with enlarged prostates and moderate symptoms had complete follow-up over 4 years, and of these, 99 experienced an episode of AUR, for a calculated incidence rate of 18 per 1000 person-years. The placebo treatment groups from three 2-year studies with a similar patient population were meta-analyzed by Andersen and colleagues.22 Of 2109 patients, 57 experienced AUR over the 2 years, with a constant hazard for an incidence rate of 14 per 1000 person-years (Table 1).
Table 1.
Descriptive Studies of the Incidence of Acute Urinary Retention
| Description | Years of | Percent | Percent | IR per | ||||
|---|---|---|---|---|---|---|---|---|
| Reference | of Cohort | Cases | Cohort | Follow-Up | Overall | per Year | 1000 pt-yrs | 95% CI |
| Ball et al15 | Watchful | 2 | 107 | 5 | 1.9% | 0.37% | 3.7 | |
| waiting study | ||||||||
| Craigen et al16 | Watchful | 15.0 | ||||||
| waiting study | ||||||||
| Birkhoff et al14 | Watchful | 10 | 26 | 3 | 39.0% | 13.0% | 130.0 | |
| waiting study | ||||||||
| Wasson et al18 | *TURP vs | 8 | 276 | 3 | 2.8% | 0.9% | 9.6 | |
| watchful waiting | ||||||||
| VA-COOP | ||||||||
| Hunter et al17 | Self-reported | 102 | 2002 | — | 5.1% | 50.9 | ||
| prior events | ||||||||
| in Spanish men | ||||||||
| Barry et al19 | Prostatectomy | 40 | 500 | 4 | 8.0% | 2.5% | 25.0 | |
| candidates | ||||||||
| Meigs et al20 | Physicians’ | 82 | 6100 | 3 | 1.3% | 4.5 | 3.1–6.2 | |
| Health Study, | ||||||||
| self-reported | ||||||||
| Jacobsen et al13 | Community | 57 | 2115 | 4 | 6.8 | 5.2–8.9 | ||
| cohort 40–49 | ||||||||
| years old | ||||||||
| McConnell et al21 | Placebo group | 99 | 1376 | 4 | 7.2% | 1.8% | 18.0 | |
| of PLESS study | ||||||||
| Andersen et al22 | Placebo groups | 57 | 2109 | 2 | 2.7% | 1.35% | 13.5 | |
| of 2-year | ||||||||
| BPH studies |
Numbers represent watchful waiting arm only.
IR, incidence rate; TURP, transurethral resection of the prostate; VA-COOP, Veterans Affairs Cooperative Study Group; PLESS, Proscar Long-Term
Efficacy and Safety Study; BPH, benign prostatic hyperplasia.
Analytical Epidemiology
Several well-controlled studies provided considerable insights into the risk factors for AUR. In the Physician's Health Study, rates increased with age and baseline symptom severity retention.20 In men with mild symptoms, the incidence of AUR increased from 0.4 per 1000 person-years for those 45–49 years old to 7.9 per 1000 person-years for those 70–83 years old. In men with symptom scores of 8 to 35, rates increased from 3.3 per 1000 person-years for those 45–49 years old to 11.3 per 1000 person-years for those 70–83 years old. Men with a clinical diagnosis of BPH and a symptom score of 8 or greater had the highest rates (age-adjusted incidence 13.7/1000 person-years). All 7 lower urinary tract symptoms (LUTS) comprising the American Urological Association symptom index individually predicted AUR. The sensation of incomplete bladder emptying, having to void again after less than 2 hours, and a weak urinary stream were the best independent symptom predictors. Use of medications with adrenergic or anticholinergic side effects also predicted AUR.
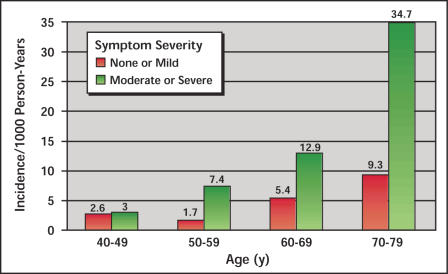
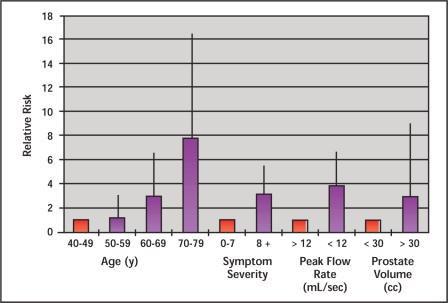
In the Olmsted County Study, analyses focused on age, symptom severity, maximum flow rate, and prostate volume13 (Figure 1 and Figure 2). Incidence rates per 1000 person-years increased from 2.6 to 9.3 for men in their 40s to 70s if they had mild symptoms, and from 3.0 to 34.7 if they had more than mild symptoms (Figure 1). The relative risk (RR) increased for older men, men with moderate to severe symptoms (3.2×), those with a flow rate under 12 mL/sec (3.9×), and those with a prostate volume above 30 mL by transrectal ultrasound (3.0×), all compared to a baseline risk of 1.0× for the corresponding groups (Figure 2). The highest relative risk by proportional hazard models exists for 60–69-year-old men with more than mild symptoms and a flow rate of less than 12 mL/sec (10.3×), and for 70–79-year-old men unless they had mild symptoms and a flow rate of over 12 mL/sec. All other stratification of men over 70 years had a RR ranging from 12.9× to 14.8× (all compared to men 40–49 years old with mild symptoms and a flow rate over 12 mL/sec, for which the base risk is 1.0×).
Figure 1.
Incidence rates of acute urinary retention in Olmsted County Study by age and symptom severity. Data from Jacobsen et al13.
Figure 2.
Relative risk of acute urinary retention in Olmsted County Study by age, symptom severity, peak flow rate, and prostate volume (purple); the red columns represent baseline and a relative risk of 1.0. Vertical lines represent the 95% CI. Data from Jacobsen et al13.
A questionnaire-based study of 5404 men aged 50–80 years from Germany yielded a 1.9% incidence of AUR within a 12-month period overall.23 The incidence was correlated with age and symptom severity. For men 50–59 years, the incidence rate was 0.8%, for those 60–69 years, 1.0%, and for those over 70 years, 2.5%. For those with mild, moderate, and severe symptoms, rates were found of 0.7%, 2.4%, and 13%, respectively.
In an observational study, 331 men presented with BPH to a single clinic, of whom 64 were in AUR at the time of presentation.24 There were no significant differences between the two groups in terms of age, symptoms, and quality of life. However, total and transition zone volume were significantly greater in the AUR group, and the most striking difference was found regarding the ratio of transition zone to total prostate volume (transition zone index or TZI), which was 0.716 versus 0.416 for the AUR and non-AUR groups, respectively (P < .001). By comparing the amount of tissue resected in 90 men presenting in AUR versus 87 men not in AUR, a significant difference was detected by Saboorian and colleagues (30.0 ± 29.8 SD vs 22.8 ± 26.7 SD cc; P < .01).25 Serum prostate-specific antigen (PSA) prior to surgery was also significantly different (6.5 ± 5.5 SD vs 4.5 ± 4.6 SD; P < .001).
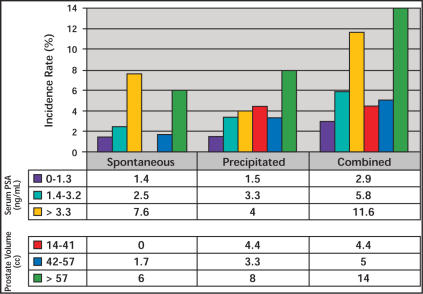
In the placebo groups of three 2-year studies26 and a 4-year study (PLESS),11,21,27 prostate volume, serum PSA, and symptom severity were all predictors of AUR episodes. The incidence increased from 5.6% to 7.7% in men with a serum PSA of under 1.4 ng/mL from mild to severe symptoms, and from 7.8% to 10.2% for those with a serum PSA of over 1.4 ng/mL over 4 years in PLESS.27 In the 2-year studies, the rate of AUR was 8-fold higher in those with a serum PSA of over 1.4 ng/mL (0.4% vs 3.9%), and 3-fold higher if the prostate volume was over 40 mL (1.6% vs 4.2%).26 A detailed analysis showed a near-linear increase in risk for AUR with increasing thresholds of serum PSA in PLESS, an observation that applies to both spontaneous and precipitated AUR.12 The risk for both types of AUR increased with increasing serum PSA as well as prostate volume stratified by tertiles (Figure 3). An analysis of over 100 possible outcome predictors alone or in combination revealed a combination of serum PSA, urinating more than every 2 hours, symptom problem index, maximum urinary flow rate, and hesitancy as being only slightly superior to PSA alone in predicting AUR episodes.28
Figure 3.
Spontaneous, precipitated, and combined acute urinary retention incidence over 4 years in PLESS, stratified by tertiles of serum prostate-specific antigen (PSA) or prostate volume at baseline.
Management of Acute Urinary Retention
Trial Without Catheter
A trial without catheter (TWOC) is recommended in nearly all patients presenting with AUR, although precise estimates regarding the success or failure of such strategy have been lacking. Hastie and colleagues reported a retrospective study of 94 patients presenting with AUR.3 Following a TWOC in 76 of these patients, 53 had a further episode of AUR and 5 were offered definitive treatment for unsatisfactory voiding. The remaining 18 patients (24%) voided spontaneously. Within 1–2 years of follow-up, 82% of all patients underwent surgery. Klarskov and colleagues reported a prospective study of 228 men presenting with AUR who were treated with either indwelling catheter or in-and-out catheterization.4 Re-retention occurred in 56% within 1 week, 62% within 1 month, and 68% within 1 year. After 1 year of follow-up, 85% of the patients had undergone surgery. These authors identified a peak flow rate of over 5 mL/sec, a voided volume of over 150 mL, and residual urine of under 100 mL as predictors of a successful TWOC.
In a prospective study of patients admitted with AUR as emergencies to a single institution, 22 of 40 (55%) were able to void spontaneously after TWOC. The main differences between those able to void and those who were not were the digital rectal examination (DRE)-estimated prostate volume (15.9 g vs 27.5 g) and the catheterized residual urine volume (814 mL vs 1062 mL).29
Djavan and colleagues randomized 114 patients presenting with AUR to either in-and-out catheterization or 2- or 7-day catheterization.30 Successful voiding was achieved in 44% of the in-and-out catheterization, 51% of the 2-day, and 62% of the 7-day catheter groups. Patients under 75 years of age with retention volumes under 1000 mL and a detrusor pressor at peak flow (Pdet at Qmax) of over 35 cm H20 had a better chance of successful voiding independent of length of catheterization. In those patients with retention volumes of over 1300 mL, however, longer catheterization was associated with better outcomes.
In the placebo-treated patients in the PLESS Study, 17% of 52 patients experiencing spontaneous AUR had a subsequent episode of AUR, versus 21% of 47 patients experiencing precipitated AUR.11 However, following spontaneous AUR, 75% of patients underwent prostate surgery, versus only 26% of those who had precipitated AUR.
α-Blocker and Trial Without Catheter—Secondary Prevention
Several trials have been conducted assessing the ability of α-blockers to improve the outcomes of TWOC. Caine and colleagues treated 8 patients in AUR with intravenous phentolamine 10 mg and/or phenoxybenzamine 5 mg q.i.d., and 5 of the 8 were able to void spontaneously.31 Chan and colleagues reported a success rate of 75%–87% in men treated with 5 or 10 mg terazosin versus 15% in those treated with placebo in a small study enrolling 30 patients presenting with AUR.32
McNeill and colleagues, enrolled 81 patients presenting with AUR into a randomized trial comparing placebo with slow-release (SR) alfuzosin 5 mg b.i.d.33 Following catheter removal 24 hours after initiation of therapy, 55% of alfuzosin-treated patients voided successfully, versus 29% of placebo-treated patients (P = .03). Of the 34 patients voiding successfully, 68% required no further therapy within a 7-month follow-up period. Age was an important factor affecting outcome, and the alfuzosin-treated patients were younger than the placebo-treated patients. After adjusting for age, the difference in the intention to treat (ITT) analysis was no longer significant but remained significant in the perprotocol analysis.
The largest randomized study reported to date examing the role of α1-blockers for men with AUR was conducted in Britain. Of the 149 patients enrolled, 75 received tamsulosin 0.4 mg and 74 placebo for 3–7 days prior to TWOC (Lucas, unpublished data). According to strict definition of success (peak flow rate > 5 mL/sec, voided volume > 100 mL, post-void residual < 200 mL), 84 patients (60.3%) required recatheterization. Successful voiding was achieved by 33.8% of tamsulosin and 24.3% of placebo-treated patients. Recatheterization was required in 70% on placebo and 40% on tamsulosin (P = .011).
Surgical Outcomes and Outcome Predictors in Patients With Acute Urinary Retention
Despite the number of patients with successful TWOC and the improved odds of such intervention when treating the patients with α-blockers, a significant proportion of patients presenting in AUR will ultimately require a surgical procedure, usually TURP. Morbidity and mortality have been reported to be higher in those patients undergoing TURP for AUR compared to those treated for symptoms only, at least in older series.34 Furthermore, a sizable number of patients fail to void spontaneously after undergoing TURP for AUR. This review does not aim to analyze the outcomes of TURP in general, but several relevant reports assessing and trying to predict the outcomes of TURP for men in AUR are worth reviewing.
Radomski and colleagues evaluated 50 patients presenting with AUR before and following TURP.35 Ninety percent of the patients were able to void spontaneously at 3-month follow-up. Poor sensation, large retention volumes, lack of instability, and absence of voluntary detrusor contractions were more common in those patients not able to void postoperatively.
Djavan and colleagues assessed 81 men with a mean age of 72 years presenting with AUR before and after TURP.36 AT 6-month follow-up, 13% of them were unable to void spontaneously and were classified as treatment failure. Treatment failures were significantly older and had higher retention volumes and lower maximal detrusor pressures prior to TURP. The authors identified patients over the age of 80 years with retention volumes over 1500 mL, no evidence of instability, and a maximal detrusor pressure of less than 28 cm H20 to be at high risk for surgical failure.
In a very similar study, Dubey and colleagues followed 58 patients presenting in AUR for TURP, and found that 16% of them failed to void spontaneously.37 Old age, absence of instability, and a maximal detrusor pressure of less than 20 cm H20 were associated with a poor surgical outcome and failure to void.
A report by the National Prostatectomy Audit Steering Group offers a unique look at the outcomes of prostate surgery in men with AUR.38 In five UK health care regions, 3966 prostatectomies were performed in 56 hospitals by 103 surgeons. The 1242 patients presenting with AUR were older and had larger glands and more comorbidities. Although the final symptomatic outcomes of the prostatectomies were similar to those of treatment for symptoms alone, the patients with AUR had an excess risk of death at 30 days (RR 26.6; 3.5–204.5 CI) and at 90 days after surgery (RR 4.4; 2.5–7.6 CI). In addition, the risk of intra- and perioperative complications was higher in the AUR group (Table 2).
Table 2.
Selected Outcomes Following TURP for AUR Versus Symptoms Only
| Outcome | Relative (Risk 95 % CI) | AUR | Symptoms | Chi-Square |
|---|---|---|---|---|
| Intraoperative | 18 (CI 1.3–2.5) | 61 (5.0%) | 76 (2.9%) | <.001 |
| complications | ||||
| Bleeding | 26 (2.1%) | 33 (1.2%) | ||
| Transfusion > 2 units | 2.5 (CI 1.8–3.3) | 93 (7.7%) | 87 (3.3%) | <.001 |
| Return to OR | 2.5 (CI 1.8–3.3) | 39 (3.1%) | 38 (1.4%) | <.001 |
| Postoperative | 1.6 (CI 1.2–2.0) | 124 (10.2%) | 177 (6.8%) | <.001 |
| complications | ||||
| Postoperative | 2.1 (CI 1.8–2.4) | 605 (50.0%) | 845 (32.6%) | <.001 |
| antibiotics | ||||
| In-Hospital | 3.3 (CI 1.2–9.3) | 9 (0.7%) | 6 (0.2%) | .024 |
| mortality | ||||
| Mortality | 26.6 (CI 3.5–205) | 12 (1.0%) | 1 (< 0.1%) | <.001 |
| 30-day OPCS | ||||
| Mortality | 4.4 (CI 2.5–7.6) | 37 (3.0%) | 19 (0.7%) | <.001 |
| 90-day OPCS |
Data from Pickard et al.38
TURP, transurethral resection of the prostate; AUR, acute urinary retention; OR, operating room; OPCS, Office of Population Census and Surveys
Primary Prevention of Acute Urinary Retention
Given the pain and suffering involved in an episode of AUR, the health care cost associated with its treatment, and the increased risks of surgical treatment of AUR, primary prevention of AUR should be a desirable goal of preventive medicine. To this date, only one major study has been carried out to assess the incidence of AUR as a predefined outcome parameter with sufficient power and sample size to provide a statistically valid answer.21 At the present time, at least two additional studies are under way to study the primary prevention of AUR under different therapeutic regimens. These studies are the ARIA studies—comparing the dual 5α reductase inhibitor dutasteride to placebo in more than 3000 men over 2 years; and a study comparing alfuzosin 10 mg versus placebo (personal communication).
The MTOPS study compared doxazosin, finasteride, and combination therapy in 3047 men 50 years of age or older and with baseline AUA symptom scores greater than or equal to 8 who were followed for a mean of 4.5 years. Combination therapy decreased the risk for AUR by 81% versus placebo (P < .001), and the risk reduction with finasteride alone was 68% (P < .001). Doxazosin monotherapy did not significantly decrease the risk for AUR versus placebo (34% risk reduction, P = NS).39
PLESS was a 4-year study of finasteride 5 mg daily versus placebo in over 3000 men with enlarged prostate glands and moderate to severe LUTS.21 The risk of AUR was found to be linear and cumulative over the 4 years of the study, and overall 6.6% of placebo (versus 2.8% of finasteride-treated patients) experienced AUR, for a risk reduction of 57%. Many of the patients experiencing AUR underwent subsequent surgery, and the overall risk for either AUR or surgery was reduced by 51% from 13.2% to 6.6%, respectively. It is of interest that fewer finasteride-treated patients underwent surgery subsequent to an episode of either spontaneous or precipitated retention compared to placebo-treated patients, despite the fact that the treating physicians at the time of the decision for or against surgery were blinded as to the treatment allocation (Table 3). The risk reduction regarding both spontaneous and precipitated AUR induced by finasteride is dependent on serum PSA and prostate volume at baseline (Table 4). Stratified by PSA tertiles, the reduction ranges from 7% to 77% for spontaneous and from 35% to 66% for both types of AUR as a result of the increased risk in the placebo-treated patients.
Table 3.
Ultimate Outcomes Following Spontaneous and Precipitated Acute Urinary Retention (AUR) Episodes Stratified by Finasteride Versus Placebo Treatment Groups
| Risk Reduction | ||||
|---|---|---|---|---|
| Finasteride n (%) | Placebo n (%) | P value | with Finasteride | |
| Spontaneous AUR | 20 | 52 | <.001 | 62% |
| Subsequent spontaneous AUR | 3 (15) | 8(15) | .999 | |
| Subsequent precipitated AUR | 1 (5) | 1(2) | .481 | |
| Any subsequent AUR | 4(20) | 9(17) | .746 | |
| Subsequent BPH-related surgery | 8 (40) | 39 (75) | .011 | |
| Precipitated AUR | 22 | 47 | <.001 | 52% |
| Subsequent spontaneous AUR | 0 | 4(9) | .299 | |
| Subsequent precipitated AUR | 3 (14) | 6(13) | .999 | |
| Any subsequent AUR | 3(14) | 10 (21) | .528 | |
| Subsequent BPH-related surgery | 3 (14) | 12 (26) | .355 |
AUR, acute urinary retention; BPH, benign prostatic hyperplasia.
Table 4.
Rates of Spontaneous and Spontaneous/Precipitated AUR in PLESS Stratified by PSA and Prostate Volume Tertiles for Placebo- and Finasteride-Treated Patients and Calculated Risk Reduction
| Placebo | Finasteride | Risk Reduction | |||
|---|---|---|---|---|---|
| Spontaneous | PSA | 0–1.3 | 1.4 | 1.3 | 7% |
| AUR | tertiles | 1.4–3.2 | 2.5 | 1.3 | 48% |
| (ng/mL) | 3.3–10.0 | 7.6 | 1.4 | 77% | |
| Volume | 14–41 | 0.0 | 0.0 | ||
| tertiles | 42–57 | 1.7 | 0.0 | 100% | |
| (cc) | 58–222 | 6.0 | 1.9 | 67% | |
| Spontaneous | PSA | 0–1.3 | 2.9 | 1.9 | 35% |
| and | tertiles | 1.4–3.2 | 5.8 | 2.6 | 54% |
| precipitated | (ng/mL) | 3.3–10.0 | 11.6 | 3.8 | 66% |
| AUR | Volume | 14–41 | 4.4 | 0.0 | 100% |
| tertiles | 42–57 | 5.0 | 0.0 | 100% | |
| (cc) | 58–222 | 14.0 | 1.9 | 81% |
AUR, acute urinary retention; PLESS, Proscar Long-Term Efficacy and Safety Study; PSA, prostate-specific antigen.
Although none of the α-blocker studies done to date were designed to show reduction in the incidence of AUR, the observed incidence rates appear to suggest a reduction with alfuzosin, doxazosin, and tamsulosin.
Study US527.2 represents the α-blockers study with the longest reported follow up. In this open-label, extension study of patients originally enrolled in several controlled studies comparing tamsulosin 0.4 mg versus placebo (unpublished communication). 109 subjects were available for follow-up up to 6 years. The overall incidence of AUR was 1.8%, again lower than the expected rate in similar populations without active therapeutic intervention.
The incidence of AUR in a 6-month study of alfuzosin 2.5 mg t.i.d. involving 518 patients was 0.4% versus 2.4% (P = .04) in the alfuzosin versus the placebo arm.40 A pooled analysis of 11 studies of alfuzosin versus placebo suggests an incidence rate of 0.3% versus 1.4%.41 Of 2829 patients treated over 1 year in an open-label study, 1.2% experienced AUR.42 Lastly, in a phase IV study of 7093 patients treated in general practice with alfuzosin up to 3 years, incidence rates were reported for AUR of 0.77% in the first year, 0.16% in the second, and 0.11% in the third year.43 These last two studies, although not placebo-controlled, suggest an incidence rate lower than might be expected without active therapy in a cohort of men with moderate to severe symptoms and clinical BPH.
A trial randomizing 1089 men with moderate to severe symptoms and an enlarged prostate by DRE to doxazosin, finasteride, combination, and placebo demonstrated superiority of doxazosin over placebo and finasteride in terms of symptoms and flow rate improvement.44 Although the trial was not powered to demonstrate significant differences, the incidence rates for AUR were 0.0%, 1.1%, 0.0%, and 1.5% for the doxazosin, finasteride, combination, and placebo arms, respectively. The incidence rates for TURP were 0.4%, 1.1%, 0.0%, and 2.6%, respectively.
Michel and Goepel undertook a meta-analysis of four α-blocker studies and compared the calculated incidence rates of AUR in the pooled placebo (2.49/100 patient-years), finasteride (0.94/100 patient-years), and α-blocker (0.51/100 person-years) groups.45
At this time caution is advised against direct comparison of AUR incidence rates across different trials, because differences in study design, inclusion and exclusion criteria, baseline characteristics such as prostate volume and PSA, discontinuation rates, and follow-up after discontinuation may have as important an impact on AUR rates as the intervention under consideration.
Conclusions
AUR is one of the most significant events in the course of the natural history of BPH. The concept that this disease is in fact progressive in nature is slowly being accepted. Whereas in the past uncertain estimates regarding the incidence rates of AUR existed, and almost one third of patients presented with AUR for TURP, nowadays better estimates are available from population-based studies of community-dwelling men as well as from patients diagnosed with BPH (placebo-control groups). Descriptive and analytical epidemiological data have shown that the incidence rate per 1000 patient-years is less variable in the community than previously assumed. The bestcontrolled studies allow for an estimate of 5–25 per 1000 person-years, or 0.5%–2.5% per year. However, this risk is cumulative and increases with advancing age. Even at these seemingly low rates, the cumulative risk for a man in his 50s, with more than mild symptoms, to experience AUR if he lives to be 80 is about 20%, for a man in his 60s who lives another 20 years, about 23%, and for a man in his 70s who lives another 10 years, 30%. The risk for patients diagnosed with BPH is naturally higher, and analytical epidemiology has identified several strong risk factors for AUR, the most important one being serum PSA. In addition, prostate volume, maximum flow rate, and symptom severity should be considered when counseling patients presenting with LUTS and clinical BPH who are considering a course of watchful waiting.
Surgical treatment carries a higher rate of morbidity and mortality in men presenting with AUR compared to those presenting with symptoms alone. Efforts toward primary prevention of AUR are therefore justified and should logically be directed to patients at increased risk, ie, those who are older and have more severe symptoms, larger glands, and higher PSA values. Risk reduction with finasteride has been demonstrated, and data for α-blockers suggest a similar reduction in risk, although conclusive studies are pending.
Main Points.
Following spontaneous acute urinary retention (AUR), 15% of patients had another episode of spontaneous AUR, and a total of 75% underwent surgery, whereas after precipitated AUR, only 9% had an episode of spontaneous AUR, and 26% underwent surgery.
The sensation of incomplete bladder emptying, having to void again after less than 2 hours, and a weak urinary stream are the best independent symptom predictors; use of medications with adrenergic or anticholinergic side effects also predicts AUR.
Men with a clinical diagnosis of benign prostatic hyperplasia and a symptom score of 8 or greater had the highest rates of AUR, and all 7 lower urinary tract symptoms (LUTS) comprising the American Urological Association symptom index individually predicted AUR.
The cumulative risk for a man in his 50s with more than mild symptoms to experience AUR if he lives to be 80 is about 20%, for a man in his 60s who lives another 20 years, about 23%, and for a man in his 70s who lives another 10 years, 30%.
Most patients should be offered trial without catheter, although a significant number of patients will fail and require surgery within the first year of follow-up.
Morbidity and mortality have been reported in older series to be higher in those patients undergoing transurethral resection of the prostate for AUR compared to those treated for symptoms only.
In the PLESS study of finasteride versus placebo in men with enlarged prostate glands and moderate to severe LUTS, fewer finasteride- treated patients underwent surgery subsequent to an episode of either spontaneous or precipitated retention compared to placebo-treated patients.
Editor's Summary of Meeting Presentation
Dr. Roehrborn delivered a comprehensive discussion on the risks of acute urinary retention and its prevention with medical therapy.
There was consensus among the experts that the risk of developing acute urinary retention is related to age, symptom severity, and prostate volume, and that in men with “larger” prostates finasteride reduces the risk of acute urinary retention. The majority of the experts offer finasteride with the intent to reduce the risk of developing acute urinary retention. The criteria for initiating finasteride therapy varied among the experts. Dr. Roehrborn offers finasteride to men with PSA levels above 3.2 ng/dL and prostate volumes > 50 cm3. According to Dr. Roehrborn, approximately 75% of men in this group will pursue medical therapy. Dr. Lowe indicated that he usually offers finasteride with the intent to reduce the risk of acute urinary retention to men with prostate volumes > 70 cm3. Dr. Lepor indicated that he offers finasteride to men with prostate volumes > 50 cm3, and approximately 40% of these men will embark on preventative therapy.
At the present time, there have been no randomized, double-blind, placebo-controlled studies determining the role of α1-blockers in the prevention of acute urinary retention. The long-term studies with tamsulosin have shown that the risk of developing acute urinary retention is significantly less than the placebo group reported in the PLESS study. This is indirect evidence that α1-blockers may also prevent the development of acute urinary retention. At the present time, MTOPS is the only published long-term, placebo-controlled study to assess the effectiveness of an α1-adrenoceptor antagonist in preventing AUR. The results of MTOPS indicate that treatment with an α1-adrenoceptor antagonist does not significantly reduce the risk for AUR in men with benign prostatic hyperplasia.
All of the panel agreed that α1-blockers are effective at preventing recurrent episodes of acute retention. The primary advantage of tamsulosin is that no titration is required, and therefore an effective dose can be achieved immediately, which allows for a shorter interval of time for the voiding trial.
References
- 1.Holtgrewe HL, Mebust WK, Dowd JB, et al. Transurethral prostatectomies: practical aspects of the dominant operation in American urology. J Urol. 1989;141:248–253. doi: 10.1016/s0022-5347(17)40732-4. [DOI] [PubMed] [Google Scholar]
- 2.Breum L, Klarskov P, Munck LK, et al. Significance of acute urinary retention due to infravesical obstruction. Scand J Urol Nephrol. 1982;16:21–24. doi: 10.3109/00365598209179635. [DOI] [PubMed] [Google Scholar]
- 3.Hastie KJ, Dickinson AJ, Ahmad R, Moisey CU. Acute retention of urine: is trial without catheter justified? J R Coll Surg Edinb. 1990;35:225–227. [PubMed] [Google Scholar]
- 4.Klarskov P, Andersen JT, Asmussen CF, et al. Symptoms and signs predictive of the voiding pattern after acute urinary retention in men. Scand J Urol Nephrol. 1987;21:23–28. doi: 10.3109/00365598709180285. [DOI] [PubMed] [Google Scholar]
- 5.Powell PH, Smith PJ, Feneley RC. The identification of patients at risk from acute retention. Br J Urol. 1980;52:520–522. doi: 10.1111/j.1464-410x.1980.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 6.Stimson JB, Fihn SD. Benign prostatic hyperplasia and its treatment [review] J Gen Intern Med. 1990;5:153–165. doi: 10.1007/BF02600518. [DOI] [PubMed] [Google Scholar]
- 7.Graversen PH, Gasser TC, Wasson JH, et al. Controversies about indications for transurethral resection of the prostate [review] J Urol. 1989;141:475–481. doi: 10.1016/s0022-5347(17)40864-0. [DOI] [PubMed] [Google Scholar]
- 8.Spiro LH, Labay G, Orkin LA. Prostatic infarction. Role in acute urinary retention. Urology. 1974;3:345–347. doi: 10.1016/s0090-4295(74)80119-6. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 10.Anjum I, Ahmed M, Azzopardi A, Mufti GR. Prostatic infarction/infection in acute urinary retention secondary to benign prostatic hyperplasia. J Urol. 1998;160(3 pt 1):792–793. doi: 10.1016/S0022-5347(01)62788-5. [DOI] [PubMed] [Google Scholar]
- 11.Roehrborn CG, Bruskewitz R, Nickel GC, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. Characterization of patients and ultimate outcomes. The PLESS Study Group. Eur Urol. 2000;37:528–536. doi: 10.1159/000020189. [DOI] [PubMed] [Google Scholar]
- 12.Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology. 1999;53:473–480. doi: 10.1016/s0090-4295(98)00654-2. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 14.Birkhoff JD, Wiederhorn AR, Hamilton ML, Zinsser HH. Natural history of benign prostatic hypertrophy and acute urinary retention. Urology. 1976;7:48–52. doi: 10.1016/0090-4295(76)90560-4. [DOI] [PubMed] [Google Scholar]
- 15.Ball AJ, Feneley RC, Abrams PH. The natural history of untreated “prostatism.”. Br J Urol. 1981;53:613–616. doi: 10.1111/j.1464-410x.1981.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 16.Craigen AA, Hickling JB, Saunders CR, Carpenter RG. Natural history of prostatic obstruction. J R Coll Gen Pract. 1969;18:226–232. [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ, Berra-Unamuno A, Martin-Gordo A. Prevalence of urinary symptoms and other urological conditions in Spanish men 50 years old or older. J Urol. 1996;155:1965–1970. See comments. [PubMed] [Google Scholar]
- 18.Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med. 1995;332:75–79. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]
- 19.Barry MJ, Fowler FJ, Bin L, et al. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. J Urol. 1997;157:10–15. [PubMed] [Google Scholar]
- 20.Meigs JB, Barry MJ, Giovannucci E, et al. Incidence rates and risk factors for acute urinary retention: the health professionals follow-up study. J Urol. 1999;162:376–382. [PubMed] [Google Scholar]
- 21.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 22.Andersen JT, Nickel JC, Marshall VR, et al. Finasteride significantly reduces acute urinary retention and need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology. 1997;49:839–845. doi: 10.1016/s0090-4295(97)00185-4. [DOI] [PubMed] [Google Scholar]
- 23.Berges R, Pientka L, Hofner K, et al. Herner LUTS Study: incidence of acute urinary retention in the elderly men in Germany. J Urol. 2000;163(4 suppl):252. [Google Scholar]
- 24.Kurita Y, Masuda H, Terada H, et al. Transition zone index as a risk factor for acute urinary retention in benign prostatic hyperplasia. Urology. 1998;51:595–600. doi: 10.1016/s0090-4295(97)00685-7. [DOI] [PubMed] [Google Scholar]
- 25.Saboorian M, Gurevitch E, Salinger F, et al. Morphometric analysis of pathological specimens in men undergoing prostate surgery for acute retention or symptoms of BPH only. J Urol. 1998;159:108. [Google Scholar]
- 26.Marberger MJ, Andersen JT, Nickel JC, et al. Prostate volume and serum prostate-specific antigen as predictors of acute urinary retention. Combined experience from three large multinational placebo-controlled trials. Eur Urol. 2000;38:563–568. doi: 10.1159/000020356. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan S, Garvin D, Gilhooly P, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia-related outcomes and long-term response to finasteride. The PLESS Study Group. Urology. 2000;56:610–616. doi: 10.1016/s0090-4295(00)00724-x. [DOI] [PubMed] [Google Scholar]
- 28.Roehrborn C, Malice M-P, Cook T, Girman C. Clinical predictors of spontaneous acute urinary retention in men with LUTS and clinical BPH: a comprehensive analysis of the pooled placebo groups of several large clinical trials. Urology. 2001;58:210–216. doi: 10.1016/s0090-4295(01)01155-4. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V, Marr C, Bhuvangiri A, Irwin P. A prospective study of conservatively managed acute urinary retention: prostate size matters. BJU Int. 2000;86:816–819. doi: 10.1046/j.1464-410x.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- 30.Djavan B, Shahrokh S, Musbah O, et al. Does prolonged catheter drainage improve the chance of recovering voluntary voiding after acute urinary retention (AUR)? Eur Urology. 1998;33(suppl 1):110. [Google Scholar]
- 31.Caine M, Pfau A, Perlberg S. The use of alpha-adrenergic blockers in benign prostatic obstruction. Br J Urol. 1976;48:255–263. doi: 10.1111/j.1464-410x.1976.tb03013.x. [DOI] [PubMed] [Google Scholar]
- 32.Chan P, Wong W, Chan L, Cheng C. Can terazosin relieve acute urinary retention and obviate the need for an indwelling urethral catheter? Br J Urol. 1996;77:7. [Google Scholar]
- 33.McNeill SA, Daruwala PD, Mitchell ID, et al. Sustained-release alfuzosin and trial without catheter after acute urinary retention: a prospective, placebo-controlled. BJU Int. 1999;84:622–627. doi: 10.1046/j.1464-410x.1999.00277.x. [DOI] [PubMed] [Google Scholar]
- 34.Mebust WK, Holtgrewe HL, Cockett ATK, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3885 patients. J Urol. 1989;141:243–247. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- 35.Radomski SB, Herschorn S, Naglie G. Acute urinary retention in men: a comparison of voiding and nonvoiding patients after prostatectomy. J Urol. 1995;153(3 pt 1):685–688. [PubMed] [Google Scholar]
- 36.Djavan B, Madersbacher S, Klingler C, Marberger M. Urodynamic assessment of patients with acute urinary retention: is treatment failure after prostatectomy predictable? J Urol. 1997;158:1829–1833. doi: 10.1016/s0022-5347(01)64139-9. [DOI] [PubMed] [Google Scholar]
- 37.Dubey D, Kumar A, Kapoor R, et al. Acute urinary retention: defining the need and timing for pressure-flow studies. BJU Int. 2001;88:178–182. doi: 10.1046/j.1464-410x.2001.02273.x. [DOI] [PubMed] [Google Scholar]
- 38.Pickard R, Emberton M, Neal DE. The management of men with acute urinary retention. National Prostatectomy Audit Steering Group. Br J Urol. 1998;81:712–720. doi: 10.1046/j.1464-410x.1998.00632.x. [DOI] [PubMed] [Google Scholar]
- 39.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 40.Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy [see comments]. The BPH-ALF Group. Lancet. 1991;337:1457–1461. doi: 10.1016/0140-6736(91)93140-5. [DOI] [PubMed] [Google Scholar]
- 41.McNeill SA, Hargreave TB, Geffriaud-Ricouard C, et al. Postvoid residual urine in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: pooled analysis of eleven controlled studies with alfuzosin. Urology. 2001;57:459–465. doi: 10.1016/s0090-4295(00)01021-9. [DOI] [PubMed] [Google Scholar]
- 42.Lukacs B, Grange JC, Comet D. One-year follow-up of 2829 patients with moderate to severe lower urinary tract symptoms treated with alfuzosin in general practice according to IPSS and a health-related quality-of-life questionnaire. BPM Group in General Practice. Urology. 2000;55:540–546. doi: 10.1016/s0090-4295(99)00539-7. [DOI] [PubMed] [Google Scholar]
- 43.Lukacs B, Grange JC, Comet D, McCarthy C. History of 7,093 patients with lower urinary tract symptoms related to benign prostatic hyperplasia treated with alfuzosin in general practice up to 3 years. Eur Urol. 2000;37:183–190. doi: 10.1159/000020116. [DOI] [PubMed] [Google Scholar]
- 44.Kirby R, Boyle P, Roehrborn C. Results of PREDICT (Prospective Randomized European Doxazosin and Combination) study of medical therapy for BPH. Br J Urol. 1999;83(suppl 4):83. [Google Scholar]
- 45.Michel M, Goepel M. Meta-analysis of alpha blocker effects on acute urinary retention (AUR) Eur Urol. 2001;39(suppl 5):97. [Google Scholar]