Abstract
Bladder outlet obstruction (BOO) is a common cause of lower urinary tract symptoms (LUTS) in men and women. By definition, BOO is determined urodynamically, assessing the pressure-flow relation during voiding. Since the 1960s much work has been done to standardize the urodynamic definitions of obstruction in men and more recently women. Today, urodynamic testing voiding pressure-flow analysis remains the gold standard for the diagnosis of BOO and the etiology of LUTS. The pressure-flow relation is much better defined in men than in women, but recent work suggests that although the definition of obstruction may differ between men and women, the concept of the pressure-flow relation to diagnose obstruction holds true for both genders.
Key words: Lower urinary tract symptoms, Postvoid residual urine volume, Bladder outlet obstruction, Urodynamic studies, Detrusor contractility
For years it was assumed that lower urinary tract symptoms (LUTS) in men were caused by obstruction by an “enlarged prostate.” The terms benign prostatic hyperplasia (BPH) and prostatic obstruction were used interchangeably. Over the past 2 decades, we have developed both a better understanding of bladder and prostate function and its relationship to symptoms and new terminology.1,2 Although symptoms usually cause patients to seek treatment, several studies have shown that there is no correlation between symptoms and the presence of obstruction.3–6 Therefore although a diagnosis of obstruction is important, as in cases of failed empiric treatment or before surgical intervention, further diagnostic testing is necessary.
Uroflowmetry and postvoid residual urine volume (PVR) are simple tests that can raise or lower the suspicion of bladder outlet obstruction (BOO), but neither can make a definitive diagnosis. Urinary flow rate is one of the simplest urodynamic tests available, serving as a general indicator of normal or abnormal voiding. Most men with BOO have diminished flow rates,7 and 90% of men with a maximum flow rate (Qmax) of less than 10 mL/sec are obstructed.8 Conversely, 25% to 30% of men with decreased flow are not obstructed.8 Decreased uroflow can result from impaired detrusor contractility or obstruction. Without the synchronous measurement of detrusor pressure (Pdet), uroflow is unable to distinguish between these 2 entities.9–11
Furthermore, there are no features of the uroflow curve that allow a definitive distinction between outlet obstruction and impaired detrusor contractility.9 Similarly, a normal uroflow does not exclude outlet obstruction.10 Elevated PVR has been shown to be more indicative of detrusor failure than of outlet obstruction.12 One study found that 50% of unobstructed men with LUTS had an “elevated” PVR, and that up to one fourth of severely obstructed men did not.8 Elevated PVR is only weakly related to BOO 13 and cannot be used with certainty in the diagnosis of obstruction.
Urodynamics with pressure flow studies remain the gold standard for diagnosing BOO and other voiding and storage abnormalities responsible for LUTS and voiding dysfunction. Urodynamic studies are most useful when their results will affect treatment and therefore should be used judiciously. Under these circumstances, we believe that the pros of urodynamic studies (generation of well-defined parameters, providing a precise diagnosis leading to specific treatment with improved outcomes, and reproducible findings) outweigh the potential cons. The latter include invasiveness, time, consumption, expense, patient discomfort and anxiety, and the fact that symptoms are not always reproduced. We recently showed the excellent tolerability of urodynamic studies in men and women of all ages, with 95% of the patients saying they would repeat urodynamic studies if medically necessary.
Urodynamic Studies
Urodynamic studies are the most definitive tests available to determine the etiology of voiding dysfunction and lower urinary tract symptoms. The urodynamic study can be divided into 2 parts, the filling and storage phase (cystometrogram) and the voiding phase (voiding pressure flow study). The voiding phase allows one to definitively make a diagnosis of obstruction, as detrusor pressure and urinary flow rate can be measured and outlet resistance calculated. However the filling and storage phase measured by the cystometrogram (CMG) can provide useful information in the patient in whom obstruction is suspected, for example, detrusor over-activity, or involuntary contractions, may be present (with or without obstruction) and may account for symptoms. Sensation and capacity also can be determined.
Another overlooked urodynamic parameter is impaired compliance. Normally the bladder should hold increasing volumes of urine at low pressures indicating a highly compliant structure (compliance = change in volume/change in pressure). Impaired compliance may result from several conditions including neurogenic voiding dysfunction, radiation cystitis, tuberculosis, and chronic bladder outlet obstruction. In the case of obstruction, compliance appears to deteriorate as a result of high intravesical pressure generated by bladder muscular activity opposed by inappropriately high outlet resistance.14 Prolonged high-storage pressures are known to be detrimental to renal function.15
Renal deterioration associated with chronic BOO is usually connected to impaired compliance and high-storage pressures, and the finding of significantly impaired compliance with BOO is an absolute indication for intervention. Thus the CMG, as well as the voiding pressure flow study, is important in the evaluation of the potentially obstructed patient. The simultaneous measurement of detrusor pressure and urinary flow rate during voluntary voiding is one of the best ways currently available to access 2 critical parameters of bladder and outlet function: detrusor contractility (normal vs impaired) and outlet resistance (obstructed vs unobstructed). In general, pressure-flow studies will identify 3 fundamental voiding states:
Low detrusor pressure and high flow rate (unobstructed)
High detrusor pressure and low flow rate (obstructed)
Low detrusor pressure with low flow rate (poor detrusor contractility).
Although it is important to understand these 3 fundamental patterns, it is equally important to realize the limitations of such categorization. Unfortunately, pressure-flow studies do not always allow for an absolute classification into one distinct category. Borderline cases exist as well as cases in which there is a combination of impaired contractility and obstruction.
Measures of Outlet Resistance and Obstruction
In order to use today's common measures of obstruction, it is important to understand basic bladder output and urethral resistance relations. Over the past 35 years several concepts regarding urethral resistance, bladder contractility, and obstruction have been introduced. In 1997, the International Continence Society (ICS) introduced the provisional ICS nomogram, which is now recommended for the diagnosis of obstruction in older men with LUTS suggestive of benign prostatic obstruction (BPO).16 A brief review of the history of pressure-flow analysis follows in an effort to explain the ICS nomogram and its application.
Attempts to mathematically define urethral resistance date back to the early 1960s.17 Early equations calculating urethral resistance, such as R = Pves/Q (where R = resistance, Pves = vesical pressure, and Q = flow rate), followed standard hydro-dynamic formulae calculating outlet resistance. Unfortunately, these concepts failed to consider that the urethra has an active and distensible nature and is not a rigid tube. They also failed to consider the importance of bladder volume. Rigid tube hydrodynamics were abandoned in favor of more dynamic ways to analyze micturition.
In 1972, Griffiths introduced Bladder Output Relation (BOR), which depicts the interrelation between bladder pressure and uroflow at a given volume.18,19 According to the BOR, for any given bladder there is a specific bladder output relation and the higher the bladder pressure, the lower the flow and vice versa. The BOR essentially measures the function of the bladder independent of the function of the urethra.
Griffiths further defined a method to evaluate urethral resistance independent of bladder function: the urethral resistance relation (URR).18 According to this relation, as bladder pressure rises, the flow rate will be zero until the intrinsic bladder pressure equals the intrinsic urethral pressure. At this point flow will start and the flow rate will rise rapidly with further increases in the intrinsic bladder pressure. If pairs of simultaneously measured values of detrusor pressure and flow rate are plotted against one another throughout the course of a micturition event, a curve is obtained that shows the resistance to flow independent of detrusor function, representing the urethral resistance relation. A change in one of these relations during micturition would not affect the curve representing the other relation but would result in the point of intersection to move along that curve.
In 1979, Abrams and Griffiths defined a simple nomogram for the diagnosis of obstruction in males.12 The researchers collected pressure-flow data on 117 males older than age 55 years, who were evaluated for possible prostatic obstruction. By comparing pressure-flow data between these patients and plotting the Qmax on the X axis and the detrusor pressure (Pdet) at maximum flow (Pdet @ Qmax) rate on the Y axis, they created 3 zones representing obstructed, unobstructed, and equivocal micturition. The zone boundaries were created by a combination of empiric observations and theoretical considerations.13 Conceptually, the Abrams-Griffiths nomogram does not permit a diagnosis of impaired detrusor contractility with or without coexisting BOO.
The passive urethral resistance relation (PURR) developed by Schafer20,21 in 1983 constitutes a simplified model of Griffith's URR. The PURR curve describes the relationship between pressure and flow during the period of lowest urethral resistance (ie, during complete relaxation), and therefore defines the lowest urethral resistance during a single voiding event. The importance of a minimum opening pressure in describing a collapsible tube is considered. Outlet function is characterized by 2 simple parameters: the minimum opening pressure, reflecting collapsibility of the tube, and the cross-sectional area of the flow-rate controlling zone, reflecting extensibility.22 Therefore, the PURR curve is a method of assessing the presence or absence of BOO independent of inherent detrusor strength.
The PURR was the first attempt to quantify relevant features of the voiding cycle describing the interplay of detrusor capability and bladder outlet resistance. Schafer subsequently modified the PURR by using a straight line instead of a parabolic curve.23 Schafer divided this linear PURR (LinPURR) curve into 7 zones labeled 0 to VI corresponding to increasing grades of obstruction: grades 0 and 1, no obstruction; grade 2, equivocal or mild obstruction; grades 3 to 6, increasing severity of obstruction. The boundary between grades 2 and 3 corresponds to the boundary between equivocal and obstructed in the Abrams-Griffiths nomogram.
The linear PURR also allows for the assessment of detrusor contractility independent of obstruction (strong, normal, weak, and very weak). Finally in 1989, Griffiths and associates developed a single urethral resistance parameter, URA.24 Using data from a mixed group of patients, they determined that obstruction is represented by URA values greater than 29 cm H2 O.
In the past 10 years, work has been done to simplify the diagnosis of BOO in men and to create a standardized method for diagnosis, based on the work of different authors described above. Lim and Abrams showed that patients were identically classified by the Abrams-Griffiths and Schafer nomograms and there was only a 6% discrepancy between these and the URA nomogram described by Griffiths.25 They also described the Abrams-Griffiths number derived from the slope of the dividing obstructed and equivocal groups on the Abrams-Griffiths nomogram and the same line dividing the obstructed (II) and slightly obstructed (III) on the Schafer nomogram. The Abrams-Griffiths number was later renamed the bladder outlet obstruction index (BOOI) and is represented by the equation: BOOI = Pdet @ Qmax − 2 Qmax.
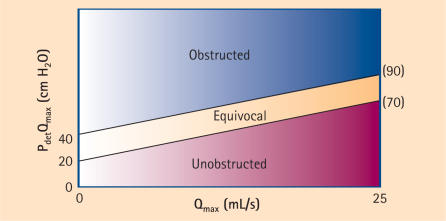
Based on these findings, the provisional ICS nomogram was subsequently published.16 Using this nomogram, men can be divided into obstructed, equivocal, and unobstructed according to their BOOI: BOOI > 40 = obstructed; BOOI 20−40 = equivocal; and BOOI < 20 = unobstructed (Figure 1). For purposes of standardization, this nomogram is now recommended for use in older men with LUTS suggestive of BPO.
Figure 1.
The ICS nomogram. Patients are divided into 3 classes: unobstructed, equivocal, and obstructed, based on the Bladder Outlet Obstruction Index (BOOI). Modified from Abrams.26 ICS, International Continence Society; Pdet detrusor pressure; Qmax, maximum flow rate.
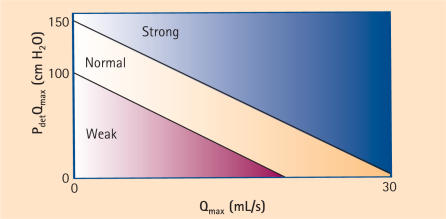
Furthermore, an index for bladder contractility can be calculated from the contractility groups derived from the Schaefer nomogram. The bladder contractility index (BCI) is represented by the following formula: BCI = Pdet Qmax + 5 Qmax. Using this formula, contractility can be divided into strong > 150, normal 100–150, and weak < 100.26 This is represented by the bladder contractility nomogram (Figure 2).
Figure 2.
Bladder contractility nomogram. Patients are divided into 3 classes: strong, normal, and weak contractility according to the Bladder Contractility Index (BCI). Modified from Abrams.26 Pdet, detrusor pressure; Qmax, maximum flow rate.
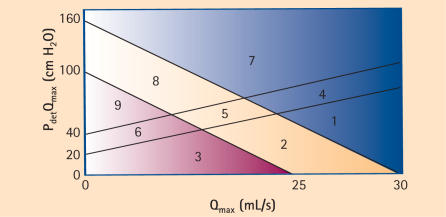
Both the BOOI and BCI can be simply calculated without the use of computer programs or even the nomogram for that matter. In addition, according to Abrams, the two can be combined to categorize men into 1 of 9 groups representing the spectrum of contractility and obstruction (ie, from no obstruction and good contractility to obstruction and weak contractility26) (Figure 3).
Figure 3.
Composite nomogram permitting categorization of patients into 9 zones based on the BOOI and BCI. Modified from Abrams.26 BOOI, Bladder Outlet Obstruction Index; BCI, Bladder Contractility Index; Pdet, detrusor pressure; Qmax, maximum flow rate.
The work of several innovators over the past 35 years has led to a simplified method of diagnosing obstruction and assessing bladder contractility in men. These methods do require urodynamic testing, which is somewhat invasive, but pressure-flow analysis remains the gold standard for the diagnosis of obstruction by which all other methods must be compared.
Noninvasive Measurement of Pressure and Flow
The invasive nature of urodynamic testing has somewhat limited its use, leading to the development of several noninvasive techniques to measure bladder pressure. These techniques involve the measurement of isovolumetric bladder pressure combined with a free flow rate to diagnose obstruction. This is accomplished by occluding urinary flow and measuring the bladder pressure transmitted along a fluid column between the bladder and the occlusion. The pressure generated by the bladder against a closed outlet (isovolumetric pressure) theoretically should differentiate between low flow caused by obstruction (high pressure) and low flow caused by impaired contractility (low pressure). An excellent review on the state of noninvasive measures of pressure was recently written by Blake and Abrams.27
Two techniques have been described. In the first, an external condom catheter is used to interrupt flow distal to the urethral meatus. A pressure transducer is located between the penis and the point of occlusion along the catheter.28 The second method uses a penile compression cuff that can occlude the urethra before initiation of voiding or after voiding has commenced. In this case the cuff is inflated to increase pressure and the pressure transducer is connected to the inflatable cuff.29 Both methods have been shown to reproducibly measure pressure and flow and correlate reasonably well with invasive pressure from studies. However there is better correlation with a minimal voided volume of 150 mL.30–33
There are several downsides to noninvasive methods including leakage from condom and condom compliance, inhibited voiding especially with the cuff technique, and cuff release problems.27 In addition, there is no abdominal pressure monitoring to accurately measure abdominal straining and there is no assessment of the storage phase (CMG). Although clearly there are some flaws in the noninvasive measurement of pressure and flow, these techniques seem promising and when eventually perfected for wide-spread use may offer an additional diagnostic test for assessing men with LUTS.
Bladder Outlet Obstruction in Women
The definitions and nomograms that are used to describe BOO in men do not apply to women. Clearly, men and women have unique micturitional characteristics. What is normal voiding pressure and flow rate for men is not necessarily normal for women. The nomograms in men were devised based on the clinical presentation and response to treatment of men with BPO.
In women there is no condition that is as common as BPH and BPO and therefore developing nomograms by similar methods is difficult. The causes of obstruction in women vary greatly from anatomic (pelvic prolapse, pelvic masses, iatrogenic obstruction after stress incontinence) to functional (dysfunctional voiding, primary bladder neck obstruction) without one pre-dominant diagnosis. Despite this, there has been a great interest over the past decade in defining BOO in women.
Early definitions of obstruction were based on flow rate alone, even though this concept has never been accepted in males. Farrar and colleagues used only flow rates to diagnose obstruction as they believed that low flow in the presence of normal or low detrusor pressures might be an indication of “relative” obstruction. This was defined as a maximum flow rate of < 15 mL/sec with a volume of 200 mL or more.34 Bass and Leach have stated that a peak flow of > 15 mL/sec with a voided volume of > 100 mL, a normal uroflow curve configuration, and no significant postvoid residual usually excludes outlet obstruction.35
Other authors introduced voiding pressure into the definition. Massey and Abrams proposed that 2 or more of the following 4 parameters be included: flow rate < 12 mL/sec, detrusor pressure at peak flow > 50 cm/H2O, urethral resistance (Pdet @ Qmax/Qmax2) > 0.2, or significant residual urine in the presence of high pressure or resistance.36 The proposed pressure and flow criteria are similar to those used in men. As a result only 2.7% of the 5948 females who presented for urodynamic evaluation for a variety of complaints were “obstructed.”
In 1998 Chassagne and colleagues proposed cutoff values for voiding pressure and flow rate.37 They prospectively studied 2 groups of women. Obstructed women (n = 35) were classified based on a diagnosis of clinical obstruction. They were divided into 3 groups independent of urodynamic findings: 1) after incontinence surgery, 2) secondary to cystocele, and 3) other etiologies. The unobstructed or control group consisted of 135 women with stress urinary incontinence and no evidence of clinical obstruction.
The authors used receiver operator characteristic (ROC) curve analysis to determine the optimum cutoff values for Qmax and Pdet @ Qmax. When Qmax and Pdet @ Qmax were used simultaneously to predict obstruction, the best combination was obtained using a Qmax of 15 mL/sec or less and a Pdet @ Qmax of more than 20 cm H2O (sensitivity 74.3%; specificity 91.1%).
This group expanded its analysis in 2000, including 87 clinically obstructed women and 124 controls, and modified their recommendations for obstruction to include a Qmax < 11 mL/sec and a PdetQmax > 21 cm H2O (sensitivity 91.5%; specificity 73.6%).38 Most recently, the group used a similar analysis to compare 169 clinically obstructed women to 20 asymptomatic normal controls, citing the fact that previous controls (women with stress incontinence) might not reflect a true control and in fact may have a lower than normal outlet resistance.39 In this study, the authors calculated that the optimal values to use were Qmax < 12 and PdetQmax > 25. They also found that each individual parameter, if abnormal, was suggestive of obstruction, even if the other parameter was normal. In all studies there was a large overlap in values for Qmax and PdetQmax for individual patients who were obstructed or unobstructed.
In 1999, we reported the use of simultaneous fluoroscopic imaging of the bladder outlet during voiding to help make the diagnosis of obstruction.40 We defined bladder outlet obstruction in women (using videourodynamics) as radiographic evidence of obstruction between the bladder neck and distal urethra in the presence of a sustained detrusor contraction, without the application of strict pressure-flow criteria. We have found videourodynamics to be an easy and practical way to diagnose bladder outlet obstruction in women. Equally important, it also localizes the site.
Using these criteria in 261 consecutive women with nonneurogenic voiding dysfunction we found 29% to be obstructed.40 There was a significant difference in both maximum flow (Qmax) and detrusor pressure at maximum flow (Pdet @ Qmax), but the parameters are not what we would expect to see in men. As was demonstrated in the ROC analyses, there also was significant overlap in voiding parameters among obstructed and unobstructed patients (Table 1).
Table 1.
Comparison of Urodynamic Parameters in Obstructed and Unobstructed Patients Using Videourodynamic Criteria*
| Urodynamic Parameter | Obstructed Patients (n = 76) | Unobstructed Patients (n = 185) | P |
| Qmax mL/s | 9.0 ± 6.2 | 20.1 ± 10.0 | < .001 |
| Pdet @ Qmax cm H20 | 42.8 ± 22.8 | 22.1 ± 11.3 | < .001 |
| Postvoid residual (mL) | 157 ± 183 | 33 ± 91 | < .001 |
| Bladder capacity (mL) | 381 ± 170 | 347 ± 147 | .11 |
| Detrusor instability | 45% | 41% | .62 |
Qmax, maximum flow rate; Pdet @ Qmax, detrusor pressure at maximim flow rate.
Defined by Nitti, et al.40
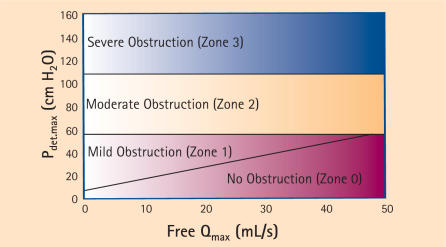
In 2000, Blaivas and Groutz created a nomogram using some of the principles cited in the 2 previously mentioned studies.41 They defined BOO as a free Qmax < 12 mL/sec combined with a Pdet Qmax of > 20 cm H2O in pressure-flow study, or obvious radiographic obstruction in the presence of a sustained detrusor contraction of > 20 cm H2O, or urinary retention or the inability to void with a transurethral catheter in place despite a sustained detrusor contraction of > 20 cm H2O.
Citing the difficulty in performing uroflowmetry in women with a catheter in place, and the fact that there was a significantly higher flow rate in the same woman without a catheter, they chose to use a noninvasive flow rate in their nomogram. Also, because they found no statistical difference in Pdet Qmax in obstructed versus unobstructed patients, they chose Pdet.max (which enables analysis in patients with urinary retention) as the pressure parameter. Using cluster analysis to classify patients with low and moderate grade obstruction, they formulated the 4-zone nomogram shown in Figure 4.
Figure 4.
The Blaivas-Groutz nomogram for female obstruction. Modified from Blaivas and Groutz.41 Pdet, detrusor pressure; Qmax, maximum flow rate.
Although pressure-flow analysis for BOO in women is not yet as standardized as it is in men, the concept of relatively high pressure and relatively low flow when compared to normals as a measure of obstruction prevails. It has been shown that there is reasonable agreement among the 3 different methods.42 Future studies will help to standardize the diagnosis of obstruction in women.
Conclusions
Voiding pressure flow studies remain the gold standard for the diagnosis of BOO. In fact, obstruction itself is defined based on the pressure-flow relation. Standardization of the pressure-flow relation and the characterization of obstruction is better defined in men due to the prevalence of obstruction and accepted treatments to relieve it. Recent work on obstruction in women is aimed at clarifying and standardizing its definition. The ability to consistently diagnose obstruction in a noninvasive manner will represent a significant advance in the evaluation of lower urinary tract dysfunction and LUTS. Non-invasive methods of determining the pressure-flow relation, such as determination of isovolumetric bladder pressure with noninvasive flow rate, hold promise. In addition, other non-invasive parameters such as the measurement of bladder wall thickness and bladder weight may also contribute to the diagnosis of BOO.
Main Points.
Urodynamics with pressure flow studies remains the gold standard for diagnosing bladder outlet obstruction (BOO) and other voiding and storage abnormalities responsible for lower urinary tract symptoms (LUTS) and voiding dysfunction. Urodynamic studies are most useful when their results will affect treatment and therefore should be used judiciously.
Simultaneously measuring detrusor pressure and urinary flow rate during voluntary voiding is the best way currently available to access 2 critical parameters of bladder and outlet function: detrusor contractility (normal vs impaired) and outlet resistance (obstructed vs unobstructed).
Noninvasive techniques that measure bladder pressure involve the measurement of isovolumetric bladder pressure combined with a free flow rate to diagnose obstruction. Although there are downsides to noninvasive techniques, including the lack of abdominal pressure monitoring and assessment of the storage phase, they hold promise and may offer an additional diagnostic test for the assessment of men with LUTS.
Definitions and nomograms used to describe BOO in men do not apply to women, and there is great interest in defining BOO in women. The causes of obstruction in women can vary greatly from anatomic (pelvic prolapse, pelvic masses) to functional (dysfunctional voiding, primary bladder neck obstruction) without one predominant diagnosis.
Although pressure-flow analysis for BOO in women is not yet as standardized as it is in men, the concept of relatively high pressure and relatively low flow when compared to normals as a measure of obstruction prevails. Future studies will help standardize the diagnosis of obstruction in women.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Urology. 2003;61:38–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 2.Abrams P. New words for old: lower urinary tract symptoms for “prostatism.”. BMJ. 1994;308:929–930. doi: 10.1136/bmj.308.6934.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nitti VW, Kim Y, Combs AJ. Correlation of the AUA symptom index with urodynamics in patients with suspected benign prostatic hyperplasia. Neurourol Urodyn. 1994;13:521–527. doi: 10.1002/nau.1930130504. [DOI] [PubMed] [Google Scholar]
- 4.Yalla SV, Sullivan MP, Lecamwasam HS. Correlation of American Urological Association symptoms index with obstructive and nonobstructive prostatism. J Urol. 1995;153:674–679. [PubMed] [Google Scholar]
- 5.Sirls LT, Kirkemo AK, Jay J. Lack of correlation of the American Urological Association symptom 7 index with urodynamic bladder outlet obstruction. Neurourol Urodyn. 1996;15:447–457. doi: 10.1002/(SICI)1520-6777(1996)15:5<447::AID-NAU2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Van Ventrooij GEPM, Boon TA. The value of symptom score, quality of life score, maximal urinary flow rate, residual volume and prostate size for the diagnosis of obstructive benign prostatic hyperplasia: a urodynamic analysis. J Urol. 1996;155:2014–2018. doi: 10.1097/00005392-199606000-00057. [DOI] [PubMed] [Google Scholar]
- 7.Blaivas JG. Multichannel urodynamic studies in men with benign prostatic hyperplasia: indications and interpretation. Urol Clin North Am. 1990;17:543–552. [PubMed] [Google Scholar]
- 8.Abrams P, Bruskewitz R, De La Rosette J, et al. The diagnosis of bladder outlet obstruction: urodynamics. In: Cockett ATK, Khoury S, Aso Y, et al., editors. Proceedings, the 3rd International Consultation on BPH. World Health Organization; 1995. pp. 299–367. [Google Scholar]
- 9.Chancellor MB, Blaivas JG, Kaplan SA, Axelrod S. Bladder outlet obstruction versus impaired detrusor contractility: the role of uroflow. J Urol. 1991;145:810–812. doi: 10.1016/s0022-5347(17)38458-6. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenberg TC, Andersen JT, Klarskov P, et al. High flow infravesical obstruction in men: symptomatology, urodynamics and the results of surgery. J Urol. 1982;127:943–945. doi: 10.1016/s0022-5347(17)54140-3. [DOI] [PubMed] [Google Scholar]
- 11.George N, Slade N. Hesitance and poor stream in men without bladder outflow obstruction — the anxious bladder. Br J Urol. 1979;51:506–509. doi: 10.1111/j.1464-410x.1979.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 12.Abrams PH, Griffiths D. The assessment of pro-static obstruction from urodynamic measurements and from residual urine. Br J Urol. 1979;51:129–134. doi: 10.1111/j.1464-410x.1979.tb02846.x. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths DJ. Pressure-flow studies of micturition. Urol Clin North Am. 1996;23:279–297. doi: 10.1016/s0094-0143(05)70311-6. [DOI] [PubMed] [Google Scholar]
- 14.McGuire EJ. The role of urodynamic investigation in the assessment of benign prostatic hyperplasia. J Urol. 1992;148:1133–1136. doi: 10.1016/s0022-5347(17)36841-6. [DOI] [PubMed] [Google Scholar]
- 15.McGuire EM, Woodside JR, Borden TA. Prognostic value of urodynamic testing in myelodysplasic children. J Urol. 1981;126:205–209. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths D, Hofner K, van Mastrigt R, et al. Standardisation of terminology in lower urinary tract function: pressure flow studies of voiding, urethral resistance and urethral obstruction. Neurourol Urodyn. 1997;6:1–18. doi: 10.1002/(sici)1520-6777(1997)16:1<1::aid-nau1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Gleason DM, Lattimer J. The pressure-flow study: a method for measuring bladder neck resistance. J Urol. 1962;87:844–852. doi: 10.1016/S0022-5347(17)65057-2. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths DJ. The mechanics of the urethra and of micturition. Br J Urol. 1973;45:497–507. doi: 10.1111/j.1464-410x.1973.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths DJ. Basics of pressure-flow studies. World J Urol. 1995;13:30–33. doi: 10.1007/BF00182663. [DOI] [PubMed] [Google Scholar]
- 20.Schafer W. The contribution of the bladder outlet to the relation between pressure and flow rate during micturition. In: Hinman F Jr, Boyarsky S, editors. Benign Prostatic Hypertrophy. New York, NY: Springer Verlag; 1983. pp. 470–496. [Google Scholar]
- 21.Schafer W. Urethral resistance? Urodynamic concepts of physiological and pathological bladder outlet function during voiding. Neurourol Urodyn. 1985;4:161–201. [Google Scholar]
- 22.Schafer W. Urodynamics of micturition. Curr Opin Urol. 1992;2:252–256. [Google Scholar]
- 23.Schafer W. Principles and clinical application of advanced urodynamic analysis of voiding function. Urol Clin North Am. 1990;17:553–566. [PubMed] [Google Scholar]
- 24.Griffiths DJ, van Mastrigt R, Bosch R. Quantification of urethral resistance and bladder function during voiding, with special reference to the effects of prostate size reduction in urethral obstruction due to benign prostatic hyperplasia. Neurourol Urodyn. 1989;8:17–27. [Google Scholar]
- 25.Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol. 1995;13:34–39. doi: 10.1007/BF00182664. [DOI] [PubMed] [Google Scholar]
- 26.Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int. 1999;84:14–15. doi: 10.1046/j.1464-410x.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 27.Blake C, Abrams P. Non invasive techniques for the measurement of isovolumetric bladder pressure. J Urol. 2004;171:12–19. doi: 10.1097/01.ju.0000102685.44036.b9. [DOI] [PubMed] [Google Scholar]
- 28.Schafer W, Kirschner-Hermans R, Jakse G. Non invasive pressure/flow measurement for precise grading of bladder outflow obstruction. J Urol. 1994;151(suppl):323A. [abstract] [Google Scholar]
- 29.McRae LP, Bottaccini MR, Gleason DM. Non invasive quantitative method for measuring isovolumetric bladder pressure and urethral resistance in the male: I. experimental validation of the theory. Neurourol Urodyn. 1995;14:101–114. doi: 10.1002/nau.1930140202. [DOI] [PubMed] [Google Scholar]
- 30.Pel JJ, Bosch JL, Lycklama a Nijeholt AA, van Mastrigt R. Development of a non-invasive strategy to classify bladder outlet obstruction in male patients with LUTS. Neurourol Urodyn. 2002;21:117–125. doi: 10.1002/nau.10046. [DOI] [PubMed] [Google Scholar]
- 31.Drinnan MJ, Pickard RS, Ramsden PD, Griffiths CJ. Assessment of prostatic obstruction: a cuff may be enough. Neurourol Urodyn. 2003;22:40–44. doi: 10.1002/nau.10059. [DOI] [PubMed] [Google Scholar]
- 32.Drinnan MJ, Robson W, Reddy M, et al. Transmission of penile cuff pressure to the penile urethra. J Urol. 2001;166:2545–2549. [PubMed] [Google Scholar]
- 33.McIntosh S, Pickard RS, Drinnan M, et al. Non invasive measurement of bladder pressure: minimum voided volume and test/retest reproducibility. Paper presented at: Annual Meeting of the International Continence Society; August 27–29, 2002; Heidelberg, Germany. [Google Scholar]
- 34.Farrar DJ, Osborne JL, Stephenson TL, et al. A urodynamic view of bladder outflow obstruction in the female: factors influencing the results of treatment. Br J Urol. 1976;47:815–822. doi: 10.1111/j.1464-410x.1975.tb04062.x. [DOI] [PubMed] [Google Scholar]
- 35.Bass JS, Leach GE. Bladder outlet obstruction in women. Problems in Urology. 1991;5:141–154. [Google Scholar]
- 36.Massey JA, Abrams PA. Obstructed voiding in the female. Br J Urol. 1988;1:36–39. doi: 10.1111/j.1464-410x.1988.tb09158.x. [DOI] [PubMed] [Google Scholar]
- 37.Chassagne S, Bernier PA, Haab F, et al. Proposed cutoff values to define bladder outlet obstruction in women. Urology. 1998;51:408–411. doi: 10.1016/s0090-4295(97)00634-1. [DOI] [PubMed] [Google Scholar]
- 38.Lemack GE, Zimmern PE. Pressure flow analysis may aid in identifying women with outflow obstruction. J Urol. 2000;163:1823–1828. [PubMed] [Google Scholar]
- 39.Defreitas GA, Zimmern PE, Lemack GE, Shariat SF. Refining diagnosis of anatomic female bladder outlet obstruction: comparison of pressure-flow study parameters in clinically obstructed women with those of normal controls. Urology. 2004;64:675–679. doi: 10.1016/j.urology.2004.04.089. [DOI] [PubMed] [Google Scholar]
- 40.Nitti VW, Tu LM, Gitlin J. Diagnosing bladder outlet obstruction in women. J Urol. 1999;161:1535–1540. [PubMed] [Google Scholar]
- 41.Blaivas JG, Groutz A. Bladder outlet obstruction nomogram for women with lower urinary tract symptomatology. Neurourol Urodyn. 2000;19:553–564. doi: 10.1002/1520-6777(2000)19:5<553::aid-nau2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Fleischmann N, Hartanto V, Rosenblum N, et al. Comparison of diagnostic criteria for female bladder outlet obstruction. J Urol. 2004;171(suppl):450. doi: 10.1016/j.juro.2006.07.031. [abstract] [DOI] [PubMed] [Google Scholar]