Abstract
Glandular prostate epithelial cells of the peripheral zone are unique among normal cells in their dependence on glycolysis for energy production, due to a zinc-mediated enzymatic block in the citric acid cycle. Lonidamine (LND), a derivative of indazole-3-carboxylic acid, is thought to disrupt energy metabolism by interfering with glycolysis and to cause cell apoptosis. We evaluated the efficacy of oral LND treatment in subjects with symptomatic benign prostatic hyperplasia (BPH). The following reports the findings of an open-label study of orally administered LND. Thirty subjects with symptomatic BPH received oral LND (150 mg/day) once daily for 28 days. Subjects were assessed at baseline, at active-therapy assessment visits (days 14, 28), and 1, 2, 3, and 6 months post-therapy, for prostate volume (PV) by transrectal ultrasound (TRUS), maximum flow rate (Qmax) on uroflowmetry, postvoid residual urine volume (PVR), International Prostate Symptom Score (IPSS), prostate-specific antigen (PSA) levels, serum chemistry, and adverse events.
Key words: Lonidamine, Benign prostatic hyperplasia, Prostate volume, Prostate-specific antigen, International Prostate Symptom Score, Uroflowmetry
Benign prostatic hyperplasia (BPH) is recognized as a chronic progressive disease associated with considerable morbidity, such as hematuria, urinary infections, the risk of acute urinary retention (AUR), and the need for surgery. Due to increased life expectancy, the proportion of men at risk for BPH and its morbidity is increasing. Effective management of this disease is essential to improve patient well-being and to reduce the economic burden for the community. Treatment endpoints are reduction in prostate volume (PV), decrease in International Prostate Symptom Score (IPSS), and improvement in the urine flow rate. Through these changes, therapy should ultimately affect the natural course of the disease, reducing the lifetime risk of AUR- and BPH-related surgery.1–3
The typical onset of action of α-blockers is within 2 weeks, relaxing the adrenergic receptors in the stroma and the smooth muscle of the prostate and bladder neck. The expected effect is a 15% to 20% reduction in IPSS and a 10% to 15% improvement in flow rate. However, α-blockers have a discontinuation rate of 5% to 10% due to well-known side effects such as orthostatic hypotension, dizziness, ejaculatory disorders, or refractoriness to the drug.4 The α-blockers delayed time to progression of AUR and the need for invasive therapy but did not reduce the risk of these events.5
5-α reductase inhibitors (5ARIs) inhibit the production of dihydrotestosterone, causing a PV reduction by 16%, an increase in the urinary flow rate by 2.2 mL/s, and a reduction in IPSS by 5.0 points. Unlike α-blockers, 5ARIs do have an impact on the natural course of the disease, reducing the incidence of AUR- and BPH-related surgery by 67% and 64%, respectively. Unfortunately, the time required to obtain symptom relief using ARIs is at least 3 to 6 months. Moreover, more than 10% of patients present with reduced libido, impotence, ejaculatory disorders, and gynecomastia.6,7
We explored a novel therapeutic approach to BPH by exploiting the unique metabolic environment of the human prostate. Glandular prostate epithelial cells of the peripheral zone are unique among normal cells in their dependence on glycolysis for energy production.8 This dependence is the result of a zinc-mediated enzymatic block in the citric acid cycle that mediates an essential function of the prostate gland—the secretion of extraordinarily high levels of citrate and zinc.9
Lonidamine (LND), a derivative of indazole-3-carboxylic acid, is thought to disrupt energy metabolism by interfering with glycolysis. It inhibits ADP- and uncoupler-stimulated respiration on various NAD- and FAD-linked substrates,10 inhibits oxygen consumption and hexokinase activity in Ehrlich ascites tumor cells,11,12 and selectively induces apoptosis in a citrate-producing prostate cell line.13
Currently approved in Italy for oncologic indications, LND has been studied extensively in humans for more than 20 years, and generally shows only mild, temporary side effects.14 Based on LND's clinical safety history, its potential novel mechanism of action, and preclinical data, we investigated the effects of once-daily oral doses of LND on PV, PSA level, urine flow, residual volume, and IPSS in men with symptomatic BPH.
Materials and Methods
Study Design
Protocol TH-CR-201 was originally designed as an open-label, dose-comparison study of orally administered LND for the treatment of symptomatic BPH. A total of 60 subjects with BPH, 30 per dose group, were enrolled to receive treatment with oral LND. Group A, reported herein, received LND 150 mg PO qd, 7 days per week for 4 weeks. Group B was to receive LND 150 mg PO tid, 5 days per week for 4 weeks.
Group A subjects were assessed at baseline, at active-therapy assessment visits (days 14 and 28), and during a post-therapy follow-up visit for PV by transrectal ultrasound of the prostate (TRUS) (ultrasound, multiplanar 7.5 Mhz transrectal probe; B-K Medical, Herlev, Denmark), uroflowmetry (Urodyn-1000®; Dantec Corp., Skovlunde, Denmark), IPSS, serum PSA level, adverse events, serum chemistry, and hormonal profile. Subjects returned for follow-up evaluations at 1, 2, 3, and 6 months postdosing. Because of the magnitude of response in Group A, the study was closed and no Group B patients were enrolled.
Patients
Between March and August of 2004, 30 patients were recruited for the study at Bari University Hospital. Men between the ages of 50 and 80 years were eligible for inclusion if they had experienced lower urinary tract symptoms (LUTS) for at least 3 months, had a total prostate volume (TPV) > 30 cc as measured by TRUS, a Qmax < 15 mL/s as measured by uroflowmetry, an IPSS > 13, a PSA level > 1.0 ng/mL, and were able to comply with the prescribed treatment protocol and evaluations. In addition to TPV, transition zone volume (TZV) was also recorded but not used as an inclusion criterion. All patients with a PSA level > 4 ng/mL had a sextant ultrasound-guided prostate biopsy to rule out areas of prostate cancer.
Subjects were excluded if they had any of the following:
prior BPH therapy, other than α-blockers;
prior prostate surgery, except for biopsies;
current or past evidence of malignant disease of the prostate;
active cardiac, renal, or hepatic disease, evidenced by creatinine >1.8 mg/dL, alanine aminotransferase (ALT) or aspartate aminotransferase (AST) > 2.5 times the normal upper limit, a history of myocardial infarction, congestive heart failure, or unstable cardiac arrhythmias within 6 months prior to study entry;
uncontrolled diabetes mellitus (fasting blood glucose > 200 mg/dL);
participation in an investigational drug study within the past 30 days; or
concomitant diseases or conditions that could interfere with the conduct of the study or pose an unacceptable risk to the subject.
α-Blocker therapy was not allowed during or 14 days prior to the study. All subjects gave informed consent according to our institutional guidelines.
Assessment
Pretreatment evaluation included the following:
a record of all medications taken 2 weeks prior to study entry;
a serum chemistry panel including sodium, potassium, chloride, blood urea nitrogen, creatinine, AST, and ALT;
a hormonal profile (follicle-stimulating hormone [FSH], luteinizing hormone [LH], prolactin [PRL], testosterone, free testosterone);
TRUS measurement of TPV and TZV;
PSA levels;
uroflowmetry; and
IPSS.
On study days 14 and 28, patients were evaluated for 1) PV by TRUS, 2) PSA levels, 3) uroflowmetry, 4) adverse events, and 5) concomitant medication assessments.
On day 28, patients also underwent a physical examination including digital rectal examination, temperature, vital signs, evaluation of serum chemistry panel, hormonal profile, and IPSS. On day 56, patients were also evaluated for PV and uroflowmetry. On day 84 and day 112, patients were evaluated by physical exam and uroflowmetry. At the last follow-up visit (day 200), patients were evaluated for PV, PSA levels, uroflowmetry, IPSS, adverse events, and concomitant medication assessments.
Statistical Analysis
The primary efficacy endpoints were the changes from baseline to day 28 in PV, Qmax, IPSS, and PSA levels. Efficacy was analyzed by paired t-tests of the percentage change in ultrasound PV and PSA levels, change in Qmax on uroflowmetry, and IPSS from baseline. Nonparametric tests were also performed to examine the robustness of the results. All tests were 2-sided and no adjustments were made for multiple tests. Missing data at day 14 and at day 28 were replaced using the last observation carried forward procedure.
Results
After signing informed consent, 30 patients were enrolled and given LND therapy between March and August 2004. The patient characteristics are outlined in Table 1. During the study, 4 dropouts were registered. One patient with a large prostate (over 100 cc) experienced gross hematuria apparently not related to drug treatment, but for safety reasons the LND treatment was stopped. One patient was removed at day 84 because of noncompliance with the protocol. Two patients were dropped out at the last visit (day 200), 1 due to voluntary withdrawal and 1 because of loss to follow-up.
Table 1.
Baseline Patient Characteristics
| Parameters | Mean (Range) |
|---|---|
| Age (y) | 63 (49–75) |
| Previous use of α-blockers (patients) | 14 (47%) |
| Prostate volume (cc) | 55.4 (30–121) |
| Qmax (mL/s) | 9.4 (4.3–14) |
| Postvoid volume (cc) | 82.1 (0–300) |
| Serum PSA level (ng/mL) | 3.6 (1.1–8.2) |
| IPSS | 19.5 (14–29) |
Qmax, maximum urinary flow rate; PSA, prostate- specific antigen; IPSS, International Prostate Symptom Score.
In all subjects tested, the once-daily regimen of oral LND was well tolerated, with no significant therapy-related side effects. No occurrences of myalgia, testicular pain, sexual dysfunction, or cardiovascular events were reported. There were no significant changes in serum chemistry except for 1 patient who had a transient 3-fold elevation of ALT. A slight but significant increase in the mean serum FSH and LH and decrease in free testosterone were observed, but no significant change in PRL and total testosterone was seen.
Prostate Volume
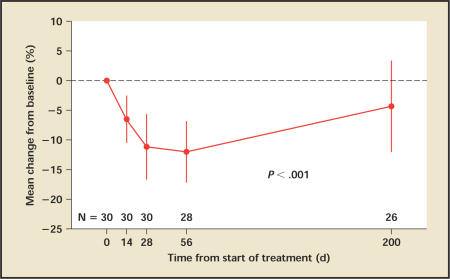
Mean TPV decreased significantly during the course of treatment, remaining stable after 1 month of follow-up and slowly returning close to baseline by 6 months (Figure 1). At the end of the treatment, the mean percentage change from baseline was −11.2%, being −12.0% at day 56, and −4.3% at day 200. Fifteen patients (50%) had more than a 10% reduction in TPV at day 28, and 7 patients (23%) had more than a 20% reduction in TPV; 23% of the patients showed no improvement.
Figure 1.
Prostate volume: Mean percentage change in prostate volume from baseline. Vertical lines denote 95% confidence intervals.
Unlike the TPV values, the change of the TZV was minimal and statistically not significant. TZV was 23.4 cc at baseline, 23.1 cc (−1.3%) at 14 days, and 22.5 cc (−3.8%) at 28 days, 23.7 cc (+1.3%) at day 56, and 23.8 cc (+1.7%) at day 200.
PSA Level
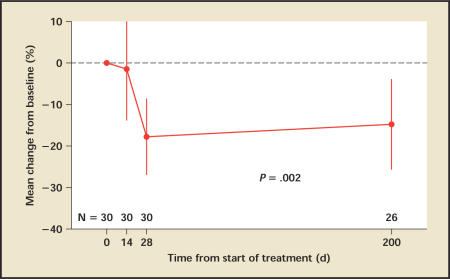
At day 14, serum PSA levels decreased modestly by a mean of 1.5% (95% CI, −14.1% to 11.2%) from a baseline of 3.6 ng/mL (Figure 2). By day 28, however, PSA levels had dropped on average by 17.8% (95% CI, −27.2% to −8.4%; P < .001). At day 200, the serum PSA levels had increased on average to 3.1 ng/mL, but a mean change of −14.8% from baseline remained.
Figure 2.
PSA: Mean percentage change from baseline. Vertical lines denote 95% confidence intervals. PSA, prostate-specific antigen.
IPSS
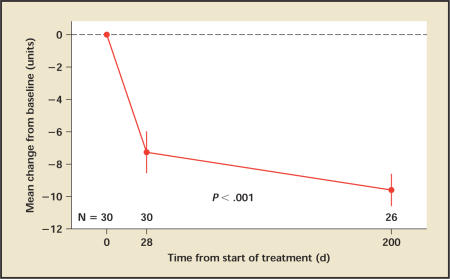
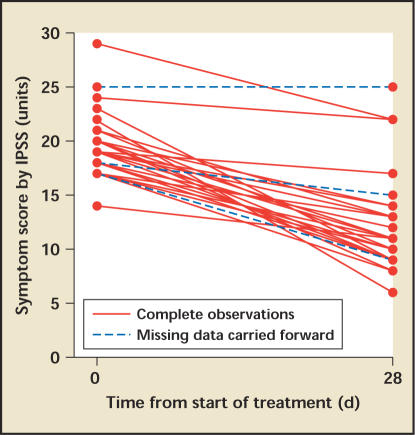
Patients' IPSS decreased by 7.3 points from an average of 19.5 at baseline to 12.2 on day 28 (P < .001), with an additional decrease at day 200 (Figure 3). The decreases were consistent among patients (Figure 4). At day 28 five patients (17%) had a decrease of 0 to 3 points, 8 patients (27%) had a decrease of 4 to 6 points, and 17 patients (47%) had a decrease of 7 or more points. Patients with a higher baseline score were more likely to have a larger decrease.
Figure 3.
IPSS: Mean change from baseline at 28 days and 6 months follow-up. Vertical lines denote 95% confidence intervals. IPSS, International Prostate Symptom Score.
Figure 4.
Individual IPSS at baseline and day 28. IPSS, International Prostate Symptom Score.
Uroflowmetry and PVR
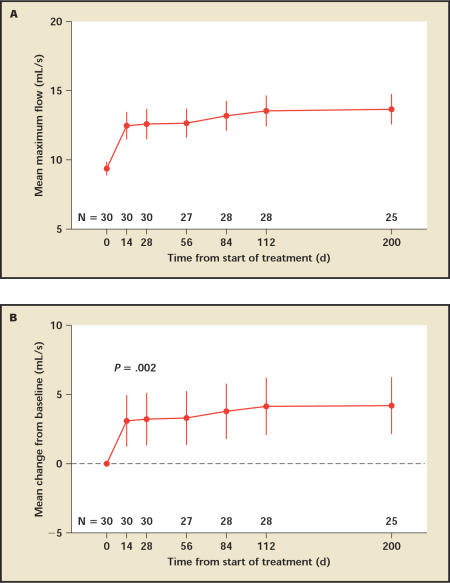
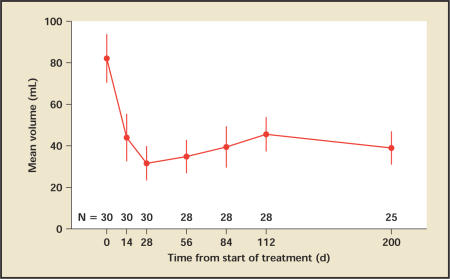
Patients experienced a significant increase in urine flow from a mean of 9.4 mL/s to 12.5 mL/s by day 14, and continued to improve until the end of the follow-up, reaching 13.7 mL/s at day 200 (Figure 5). By day 28, the end of treatment, 12 patients (40%) had more than 3 mL/s flow improvement over baseline and 7 patients (23%) had more than 5 mL/s flow improvement over baseline. Similarly, the postvoid residual volume (PVR) improved significantly after day 14 and continued to improve throughout the treatment. PVR decreased from a mean of 82 cc to 44 cc at day 14, reaching the nadir of 32 cc at day 28, and slowly increasing to reach 39 cc at 6 months (the next follow-up visit) (Figure 6).
Figure 5.
Qmax by uroflowmetry. A. Mean maximum flow rate with standard error. B. Mean change in maximum flow rate from baseline. Vertical lines denote 95% confidence intervals.
Figure 6.
Mean postvoid residual urine volume with standard error at each visit. There is a statistically significant improvement from baseline at all visits.
Discussion
This is the first study on the use of lonidamine in the treatment of symptomatic BPH. Based on the extensive literature available on the safety of LND in an oncology setting, it was decided to initiate an open-label, phase II trial. This is a “proof of concept” study in which, however, a placebo arm was not used. The drawback in this design is the placebo effect that may elicit misleading results, if not properly corrected for, especially considering the small number of patients and the absence of a blind run-in period.
Patients were selected on the basis of the risk of disease progression and of developing complications. In fact, before study entry, 50% of the patients were severely symptomatic and were taking α-blockers. All study endpoints were achieved for the 150 mg qd dose arm and the higher dose arm (150 mg tid) was never begun.
The dose level was selected based on the extensive clinical literature published on LND. We reduced the typical dose of 150 mg PO tid to a once-daily regimen in order to determine whether the already mild side effects could be further mitigated while maintaining the therapeutic activity, as well as to help foster patient compliance.
The efficacy results suggest that low doses of oral LND induce a rapid and substantial response in patients with symptomatic BPH. Despite the short duration of the treatment, patients achieved a significant reduction in PV, increase in Qmax, decrease in residual urine volume, and reduction in IPSS by day 14. Prostate volume and BPH-related symptoms continued to improve throughout the 28 days of LND treatment, reaching the maximum response. During the early post-treatment period (56 days), all the efficacy parameters remained stable, whereas at the 3 and 6 month follow-up visits there was a gradual return to baseline parameters for PV and PSA levels. The correlation and the magnitude of response of all parameters taken as study endpoints reduce the risk of a misleading placebo effect.
BPH causes a wide variability in symptom onset and severity and, therefore, the quest for medical advice is variable, ranging from patients with mild irritant voiding symptoms to those with an impending AUR. The fast-acting effect of LND on PV, Qmax, and IPSS will be particularly useful to reverse an incipient AUR or increase in the success rate of catheter removal after AUR in patients with large PV.
Interestingly, serum PSA levels decreased moderately at day 14 and then steeply at day 28, probably related to prostate cell apoptosis through the proposed mechanism of action-disruption of glycolysis and cellular apoptosis cascade activation. Also of interest, 6-month serum PSA values remained lower than at baseline. The magnitude and rapidity of this response to a compound that acts on PV is unique in the medical treatment of BPH. As part of the LND clinical development, a phase II study in the United States and a phase III study in Europe have been designed and will soon be open to enrollment.
Conclusion
One month of oral LND administration induces a rapid and significant improvement of BPH symptoms and a significant decrease in PV and PSA that are sustained for at least 1 month after cessation of treatment, suggesting that LND may be a safe and effective new therapeutic alternative for the treatment of BPH in patients at high risk of disease progression and the development of complications.
Main Points.
The proportion of men at risk for benign prostatic hyperplasia (BPH), a chronic progressive disease associated with considerable morbidity, is increasing over the years.
Treatment endpoints are reduction in prostate volume (PV), decrease in International Prostate Symptom Score (IPSS), and improvement in the urine flow rate. These endpoints could reduce the lifetime risk of surgery for BPH and acute urinary retention (AUR).
Standard therapeutic options for BPH include α-blockers and 5α-reductase inhibitors (5ARIs). Though effective in reducing IPSS and improving flow rate, α-blockers have a discontinuation rate of 5% to 10% due to side effects. Symptom relief using 5ARIs takes at least 3 to 6 months, and more than 10% of patients present with side effects.
Currently approved in Europe for oncological indications, lonidamine (LND) has been studied extensively in humans for more than 20 years, and generally shows only mild, temporary side effects. In rat models, a single oral dose of LND has been shown to safely, rapidly, and reproducibly reduce the size of the prostate by up to 24%.
Based on LND's clinical safety history and preclinical data, the first study on the use of LND in the treatment of symptomatic BPH took the form a single-arm, open-label phase II study.
Patients were selected on the basis of risk of disease progression and of developing complications. All study endpoints were achieved for the 150 mg qd dose arm and the higher dose arm (150 mg tid) was never begun.
The efficacy results suggest that low doses of oral LND induce a rapid and substantial response in patients with symptomatic BPH. Despite the treatment's short duration, patients achieved a significant reduction in PV, increase in maximum flow rate (Qmax), decrease in residual urine volume, and reduction in IPSS by day 14. Prostate volume and BPH-related symptoms continued to improve throughout the 28 days of LND treatment, reaching the maximum response.
The fast-acting effect of LND on PV, Qmax, and IPSS might be particularly useful to reverse an incipient AUR or increase the success rate of catheter removal after AUR in patients with large PV.
References
- 1.Lepor H, Lowe F. Campbell's Urology. Vol 8. Philadelphia, PA: WB Saunders Company; 2002. Evaluation and non-surgical management of BPH; pp. 1337–1378. [Google Scholar]
- 2.Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol. 2004;171:1029–1035. doi: 10.1097/01.ju.0000097026.43866.cc. [DOI] [PubMed] [Google Scholar]
- 3.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 4.Djavan B, Marberger M. A meta-analysis on the efficacy and tolerability of alpha1-adrenoceptor antagonists in patients with lower urinary tract symptoms suggestive of benign prostatic obstruction. Eur Urol. 1999;36:1–13. doi: 10.1159/000019919. [DOI] [PubMed] [Google Scholar]
- 5.McConnell J, Roehrborn C, Bautista O, et al. The long-term effects of doxazosin, finasteride, and the combination on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2385–2396. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn CG, Bruskewitz R, Nickel JC, et al. Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. J Urol. 2004;171:1194–1198. doi: 10.1097/01.ju.0000112918.74410.94. [DOI] [PubMed] [Google Scholar]
- 7.Roehrborn C, Marks L, Fenter T, et al. Efficacy and safety of dutasteride in the four-year treatment of men with benign prostatic hyperplasia. Urology. 2004;63:709–715. doi: 10.1016/j.urology.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Guzman Barron ES, Huggins C. The metabolism of isolated prostatic tissue. J Urol. 1994;51:630–634. [Google Scholar]
- 9.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granger DL, Lehninger AL. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floridi A, Lehninger AL. Action of the antitumor and antispermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Arch Biochem Biophys. 1983;226:73–83. doi: 10.1016/0003-9861(83)90272-2. [DOI] [PubMed] [Google Scholar]
- 12.Floridi A, Paggi MG, D'Atri S, et al. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res. 1981;41:4661–4666. [PubMed] [Google Scholar]
- 13.Data on file. Threshold Pharmaceuticals, Inc.
- 14.Robustelli della Cuna G, Pedrazzoli P. Toxicity and clinical tolerance of lonidamine. Semin Oncol. 1991;18:18–22. [PubMed] [Google Scholar]