Abstract
The early release of cytokines by cells involved in innate immunity is an important host response to intracellular pathogens. Gamma interferon (IFN-γ) is an important cytokine produced during the early stages of an infection by macrophages, natural killer (NK) cells, and other cell types, and it is also a central cytokine mediator for the induction of cellular or Th1 immunity. To better understand innate and adaptive immune responses after infection with Porcine reproductive and respiratory syndrome virus (PRRSV), we investigated serum IFN-γ concentrations and the duration of viremia. For 2 strains of atypical PRRSV, IFN-γ was detectable in swine serum soon after infection and lasted for approximately 3 wk. Serum concentrations of IFN-γ peaked at about 10 d after inoculation and returned to approximately baseline levels by day 22. However, individual pigs manifested short, sporadic increases in the serum concentration of IFN-γ from 18 to 50 d after inoculation. Prior vaccination blocked the serum IFN-γ response associated with homologous virus challenge and altered the kinetics of the response after heterologous challenge. Two other respiratory viruses of pigs, Porcine respiratory coronavirus and Swine influenza virus, do not appear to induce serum IFN-γ. The early production of IFN-γ in PRRSV-infected pigs might result from activation of NK cells, a response that is more characteristic of immune pathways stimulated by intracellular bacterial and protozoan infections.
Résumé
La réponse hâtive en cytokines par les cellules impliquées dans l’immunité innée joue un rôle important dans la réponse envers les agents pathogènes intracellulaires. L’interféron gamma (IFN-γ) est une cytokine importante produite dans les premiers stages d’une infection par les macrophages, par les cellules tueuses naturelles (NK), ainsi que par d’autres types cellulaires, et elle est également une cytokine centrale médiatrice pour l’induction d’une réponse cellulaire ou immunité Th1. Afin de mieux comprendre les réponses immunitaires innées et acquises suite à l’infection par le virus du syndrome reproducteur et respiratoire porcin (PRRSV), nous avons étudié les concentrations sériques d’IFN-γ et la durée de la virémie. Pour deux souches atypiques de PRRSV, la présence d’IFN-γ était détectable dans le sérum de porc tôt après l’infection et dura environ 3 sem. Les concentrations sériques d’IFN-γ atteignirent leur maximum environ 10 j après l’inoculation et revinrent aux alentours du niveau de base au jour 22. Toutefois, quelques animaux présentèrent des augmentations de concentration sérique d’IFN-γ sporadiques de courte durée du jour 18 au jour 50 après l’inoculation. Une vaccination préalable empêcha la réponse sérique en IFN-γ associée à un test de provocation avec la souche homologue et modifia la cinétique de la réponse à un test de provocation avec une souche hétérologue. Deux autres virus respiratoires porcins, le virus de l’influenza porcin et le coronavirus respiratoire porcin, ne semblent pas induire la production d’IFN-γ. La production hâtive d’IFN-γ chez des porcs infectés par le PRRSV pourrait résulter d’une activation des cellules NK, une réponse qui est plutôt caractéristique des mécanismes immunitaires stimulés par les infections par les bactéries intracellulaires et les protozoaires.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is present worldwide and is the most economically important infectious disease for swine production. The etiologic agent is a single-stranded, positive-sense RNA virus belonging to the family Arteriviridae (1,2). The disease results in mummified fetuses, stillbirths, and weak piglets born alive or an interstitial pneumonia in nursery-age and growing pigs that is characterized by an influx of a mixed population of mononuclear cells. Markedly enlarged, hyperplastic lymph nodes are also characteristic of infection with Porcine reproductive and respiratory syndrome virus (PRRSV).
The respiratory disease is seen with PRRSV strains of the North American genotype (3). Infection is persistent, lasting 6 to 7 wk before viral clearance from the lungs; virus titers peak 7 to 9 d after inoculation (4,5). The permissive cell type for PRRSV replication is the alveolar macrophage, particularly those of higher density, and tissue histiocytes (5–7). Alveolar macrophages infected in vitro with PRRSV are lysed in 48 h. However, even more numerous after PRRSV infection are uninfected bystander cells in lungs, lymph nodes, and other tissues that undergo apoptosis (8–11). In bronchoalveolar lavage (BAL) fluids from PRRSV-infected pigs, only about 1% to 2% of the cells replicate virus (5,12). Despite virus replication in a subpopulation of macrophages, the number of pulmonary macrophages increases approximately 5-fold for 2 mo after PRRSV infection, notably by infiltration of circulating monocytes (4).
The porcine immune response to PRRSV is unconventional. Nonspecific innate immunity is lacking in terms of interference with virus replication by type I interferon production (13). Natural killer (NK) cell activity after infection has not been studied adequately. Both the cell-mediated immune (CMI) response and the induction of neutralizing antibodies to PRRSV appear to be weak or impaired (14–16). Apparently, cellular immunity to PRRSV infection is delayed in onset, and the number of virus-specific T-cells secreting gamma interferon (IFN-γ), a key cytokine for the induction of CMI or Th1 immunity, increases slowly (15). To better understand innate and adaptive immune responses to PRRSV infection, we investigated serum IFN-γ levels and the duration of viremia after the infection of weaned pigs with 2 strains of atypical PRRSV.
Materials and methods
Viruses and cell cultures
Two strains of PRRSV were used in this study: strain JA-142 was isolated at the US Department of Agriculture National Animal Disease Center (NADC), Ames, Iowa, USA, and strain SDSU-73 was kindly provided by Dr. Mike Roof, Boehringer Ingelheim Vetmedica, Ames, Iowa. Both strains had been isolated from severe outbreaks of PRRS that have been referred to as “acute” or “atypical” (17,18). The viruses were propagated in MARC-145 cells (19). Stock preparations of both strains for pig inoculation were passaged less than 5 times in cell culture.
The pigs were also inoculated with the Ind/89 isolate (20) of Porcine respiratory coronavirus (PRCV), which was isolated at the NADC from swine nasal swab specimens in swine testicular (ST) cells and then passed 2 additional times in ST cells to increase the virus titer. In addition, the pigs were inoculated with Swine influenza virus (SIV), subtype H3N2, prepared from lung samples from a farm with severe respiratory disease that were kindly provided by Dr. Pat Halbur, State Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa. To maintain virulence, the SIV was passaged only in pigs as lung homogenates and lung lavage fluids. The pigs were inoculated with the 7th pig passage. The titer of the SIV inoculum was determined on Madin–Darby canine kidney cells.
Experimental design
Three animal experiments were conducted, under the guidelines of the Institutional Animal Care and Use Committee. In experiment I, 19 PRRSV-seronegative, outbred pigs were assigned after weaning to 3 groups housed in 3 separate isolation rooms at the NADC. At 5 wk of age, the pigs were first bled and then inoculated intranasally with 2 × 104 tissue culture infective dose 50 (TCID50) of either PRRSV strain SDSU-73 (n = 7) or JA-142 (n = 6) or, as sham inoculation (n = 6), with 2 mL of conditioned medium from noninoculated MARC-145 cells. For the next 8 wk, the pigs were observed each morning and evening for clinical signs. At different intervals after inoculation, serum samples were assayed for infectious virus in MARC-145 cells and for serum IFN-γ concentration with the use of a swine enzyme-linked immunosorbent assay (ELISA) (BioSource International, Camarillo, California, USA).
In experiment II, 30 pigs were used to determine the effects of prior PRRSV vaccination on the induction of IFN-γ as measured in serum. The pigs were purchased from a high health herd and delivered to the NADC when weaned at 2 wk of age. The pigs were negative for PRRSV antibodies. The pigs were randomly assigned to 6 groups (5 pigs per group), kept in separate isolation rooms, and allowed to adapt to their new environment for another week. At 3 wk of age, 3 of the groups were vaccinated once intramuscularly (IM) with modified live PRRS ATP vaccine (Boehringer Ingelheim Vetmedica, St. Joseph, Missouri, USA). At 11 wk of age, 1 nonvaccinated and 1 vaccinated group were challenged intranasally with 2 × 104 TCID50 of PRRSV strain JA-142, the parental and homologous strain of the modified-live-virus vaccine. Likewise, 1 nonvaccinated group and 1 vaccinated group were challenged intranasally with 2 × 104 TCID50 of PRRSV strain SDSU-73 (heterologous challenge). As controls, the other 2 groups of nonvaccinated and vaccinated pigs were left unchallenged. For all groups, serum samples were collected for IFN-γ measurement before challenge inoculation and on days 5 and 10 after challenge.
In experiment III, to measure serum IFN-γ induced by infection with either PRCV or SIV, 2 groups of 4 pigs each were housed in 2 isolation rooms. The pigs were negative for antibodies to PRCV and SIV subtype H3N2. At 6 wk of age, both groups were anesthetized for deep intranasal inoculation with a syringe adapted with a tight-fitting nasal tip. For anesthesia, we used an IM injection of a mixture of xylazine (22 mg/mL), zolazepam (33 mg/mL; Fort Dodge Animal Health, Fort Dodge, Iowa, USA), and ketamine (44 mg/mL), at a dose of 1 mL/5.5 kg of body weight. Both viruses were given at a dose of 1.5 mL per nostril. The titer of the PRCV inoculum was 6 × 107 plaque-forming units per milliliter and the titer of the SIV subtype H3N2 in lung lavage fluids 4 × 105 TCID50/mL. Blood for serum preparation was collected before inoculation and at the same intervals thereafter as in experiment I but only for 14 d. The pigs were observed twice daily for clinical signs.
Detection of viremia
The PRRSV titers in serum were determined in MARC-145 cells. We prepared a 10-fold dilution series of serum in minimum essential medium (MEM; JRH Biosciences, Lenexa, Kansas, USA) containing 5% fetal bovine serum, 25 μg/mL of gentamicin (Phoenix Pharmaceutical, St. Joseph, Missouri, USA), and 1.25 μg/mL of amphotericin B (Bristol-Myers Squibb, Princeton, New Jersey, USA). The medium was removed from MARC-145 cells that had grown for 3 d in 96-well plates, and 50 μL of undiluted or diluted serum was added to each well. This was repeated for each sample in quadruplicate. After incubation of the plates for 1 h at 37°C in a 5% CO2 incubator, 150 μL of MEM containing 5% fetal bovine serum, gentamicin, and amphotericin B was added to each well. The plates were incubated at 37°C with 5% CO2 and observed for cytopathic effects for 7 d after inoculation. Titers were calculated by the Karber 50% endpoint method.
Assay of IFN-γ
Using the swine IFN-γ ELISA kit, we assayed 50-μL serum samples in duplicate, following the manufacturer’s protocol. The plates were read at 450 nm on a SPECTRAmax 190 Microplate Spectrophotometer (Molecular Devices, Sunnyvale, California, USA). For each assay, a standard curve was generated with the manufacturer’s IFN-γ standards to determine the IFN-γ concentration in the individual samples. The optical density data were converted to IFN-γ concentrations by means of SOFTmax PRO version 3.1 software (Molecular Devices).
Statistical analysis
Virus titers in serum were analyzed by a 2-factor, mixed-model, repeated-measures analysis of variance (ANOVA). For IFN-γ concentrations in the serum of experiment II pigs, 3 single-factor ANOVAs were performed to compare the 6 treatment groups at each day after challenge. For IFN-γ concentrations in the serum of experiment III pigs, 4 one-way ANOVAs were performed to compare the 2 treatments at each day after infection. If a significant F-test value for a treatment group was obtained from an ANOVA, Duncan’s multiple range test at a P-value of 0.05 was used as the multiple comparison test to compare group means.
Results
Clinical signs and serum IFN-γ levels after inoculation
The pigs inoculated with PRRSV strains SDSU-73 and JA-142 showed mild clinical signs for more than 1 wk after inoculation. The main clinical sign for each group was inappetence; a few pigs in each group had rough hair coats, and some had intermittent sneezing and coughing. The sham-inoculated control pigs were healthy throughout the 57-d study period.
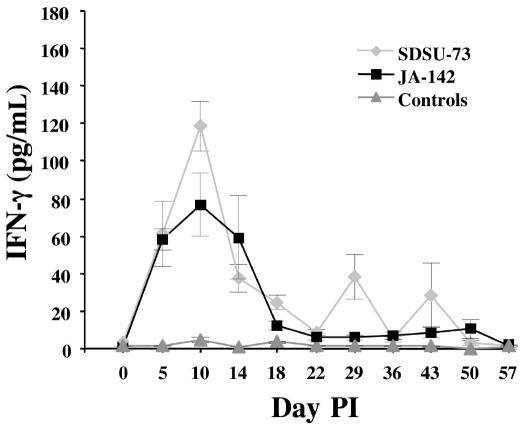
Figure 1 shows the serum IFN-γ concentrations after PRRSV and sham inoculation. By day 5, all 6 pigs inoculated with strain JA-142 had increasing concentrations of IFN-γ in their serum (range 17.5 to 82 pg/mL). At this point the concentrations were also increasing in the pigs inoculated with SDSU-73, but the range was wider: from ~5 to 214 pg/mL (these extremes in 1 pig each). The group means peaked on day 10 in both groups, at 119 ± 50 (standard error) pg/mL and 76 ± 59 pg/mL for the SDSU-73-inoculated pigs and the JA-142-inoculated pigs, respectively. After day 10 the serum concentration of IFN-γ in both infected groups of pigs began to decline. Two of the sham-inoculated pigs had mildly increased concentrations of serum IFN-γ, peaking on day 10 at approximately 10.2 and 16.4 pg/mL, respectively.
Figure 1.
Serum concentrations of gamma interferon (IFN-γ) in pigs post inoculation (PI) with Porcine reproductive and respiratory syndrome virus (PRRSV), strain SDSU-73 (n = 7) or strain JA-142 (n = 6), or after sham inoculation (n = 6). For all 4 figures, all serum samples were analyzed in duplicate for swine IFN-γ with a commercial enzyme-linked immunosorbent assay kit, and the values are means ± the standard error for each group.
About 3 wk after inoculation, the serum concentrations of IFN-γ in both groups of PRRSV-inoculated pigs had returned to values that approached the levels in the sham-inoculated pigs; however, the concentrations were consistently slightly elevated in the PRRSV-inoculated pigs compared with the control pigs until day 57 (Figure 1). This was due in part to transient bursts of serum IFN-γ activity in individual PRRSV-inoculated pigs at either single sample points on or after day 18 or at 2 points separated by days with low IFN-γ concentrations: 5 of the 7 SDSU-73-inoculated pigs and 4 of the 6 JA-142-inoculated pigs showed spikes between days 18 and 50. The single spikes were seen only in the PRRSV-inoculated pigs and not in the control pigs.
Viremia
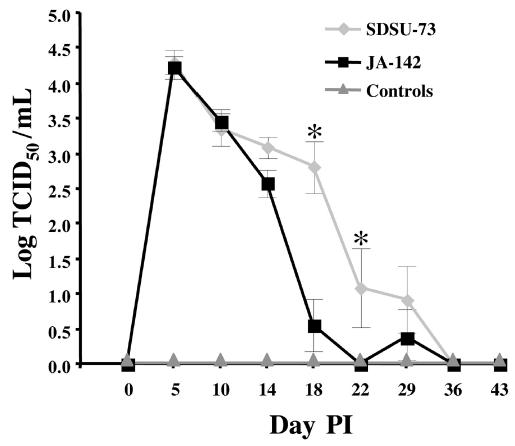
The geometric mean virus titers in the serum were similar in the 2 groups of PRRSV-inoculated pigs through day 14 (Figure 2). On day 18 all of the SDSU-73-inoculated pigs were viremic, but only 3 of these 7 pigs were still viremic by days 22 and 29, and 2 of the pigs with recoverable virus on day 29 were not viremic on day 22. Among the 6 JA-142-inoculated pigs, PRRSV was recovered from the serum of only 2 on day 18, and a different pig had recoverable virus in its serum on day 29; virus was not detected in the serum of any pig on day 22. Hence, virus was recovered from pigs inoculated with both strains of PRRSV through day 29, but on and after day 18 virus recovery was more frequent in those inoculated with strain SDSU-73.
Figure 2.
Serum titers of PRRSV, expressed as the logarithm of the median tissue culture infective dose (TCID50), in the same 3 groups of pigs. The asterisks indicate that the group mean titers were significantly different (P ≤ 0.05) only on days 18 and 22 PI.
Peak viremia occurred on day 5 (Figure 2). By day 18 the serum titer for strain JA-142 had been reduced by almost 4 orders of magnitude, whereas by day 29 more than 3 logs of strain SDSU-73 had been cleared from the serum.
Serum IFN-γ concentrations in vaccinated and challenged pigs
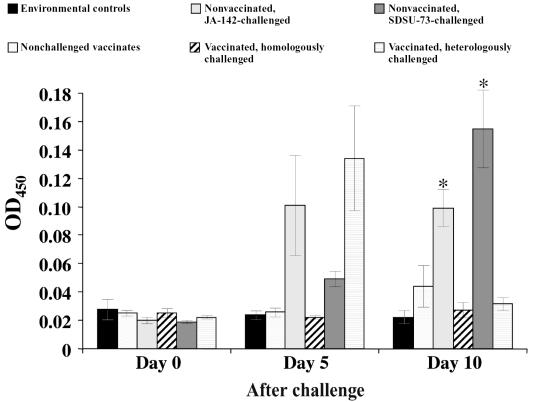
Experiment II was carried out to determine if prior vaccination with modified live PRRSV vaccine would block or enhance the IFN-γ response after homologous and heterologous PRRSV challenge. Pigs in 3 groups (5 pigs per group) were vaccinated 8 wk before challenge, and 3 groups of pigs (5 pigs per group) remained nonvaccinated before challenge. The average serum IFN-γ concentration in each group of pigs before and at 5 and 10 d after challenge are illustrated in Figure 3. As consistently observed, the serum IFN-γ concentration in the nonvaccinated pigs challenged with either strain of PRRSV was elevated by day 5, and for those challenged with strain SDSU-73 the IFN-γ concentration continued to increase through day 10. For the vaccinated pigs challenged with the homologous virus (JA-142), the serum IFN-γ concentration remained at background levels on both days 5 and 10. For the vaccinated pigs challenged with heterologous strain SDSU-73, the serum concentration of IFN-γ spiked on day 5 and then returned to background levels on day 10. The differences between the vaccinated and nonvaccinated groups challenged with either the homologous strain or the heterologous strain of PRRSV were statistically significant (P ≤ 0.05) on day 10 but not on day 5. The nonvaccinated, nonchallenged pigs and the vaccinated, nonchallenged pigs showed background IFN-γ levels at each of the 3 sampling times.
Figure 3.
Serum IFN-γ concentrations before and on days 5 and 10 after homologous and heterologous challenge of vaccinated and nonvaccinated pigs. On day 10 the asterisks indicate significant differences (P ≤ 0.05) between vaccinated and nonvaccinated groups whether challenged with PRRSV strain JA-142 or strain SDSU-73. The differences between the environmental control pigs and the 3 groups of vaccinated pigs were not significant on day 10. OD — optical density.
Serum IFN-γ concentrations after PRCV or SIV inoculation
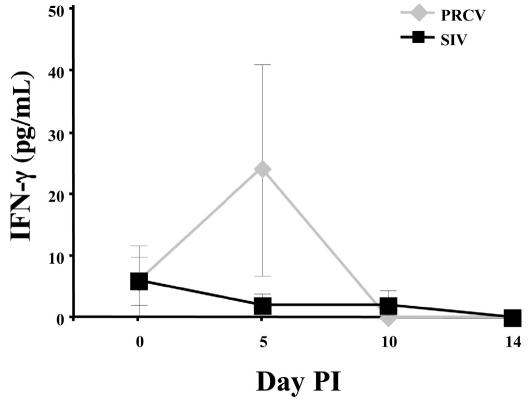
To determine if other swine respiratory viruses cause elevation of the serum IFN-γ concentration, we inoculated 2 groups of pigs (4 pigs per group) with either PRCV or SIV. In contrast to PRRSV, which infects alveolar macrophages, PRCV and SIV primarily infect epithelial cells in the respiratory tract. Figure 4 shows the serum IFN-γ concentrations for 14 d after inoculation with PRCV or SIV. In 1 of the 4 PRCV-infected pigs, the concentration spiked on day 5 (to 75.9 pg/mL) and then returned to low levels on days 10 and 14. In the other 3 pigs, the concentration remained at the day 0 level or was negative on day 5. For SIV, the concentration was low on days 5, 10, and 14 in all the pigs. The group means at day 5 for the PRCV-inoculated pigs and the SIV-inoculated pigs were not significantly different.
Figure 4.
Serum IFN-γ concentrations after inoculation with Porcine respiratory coronavirus (PRCV) or Swine influenza virus (SIV).
Discussion
Our results indicate that IFN-γ is detectable in serum soon after PRRSV infection of pigs. This result contrasts with the reported weak and slow onset of cellular immunity to PRRSV, which is based on an ELISPOT assay that enumerates virus-specific T-cell responses for IFN-γ biosynthesis and secretion (14–16). This apparent contradiction is due to different measurements of early and later components of immunity. The 2 components of antiviral immunity, 1 for rapid, nonspecific host defence and the other for virus-specific immunity, are interrelated (21,22). The IFN-γ ELISPOT assay measures adaptive T-cell responses to specific viral antigens. The early production of IFN-γ as measured in serum might result from innate immune responses, most likely from stimulated NK cells (23,24).
The cytokine cascade immediately after the primary infection of macrophages with PRRSV until the detection of serum IFN-γ is not known. Cells credited with IFN-γ secretion are T-cells and NK cells (25,26). The secretion of IFN-γ by CD4 lymphocytes is measured as an initial step in virus-specific, adaptive immunity, whereas NK cells are a component of innate viral immunity, and, on the basis of our findings of high levels of IFN-γ in the serum after PRRSV inoculation, are a good candidate to account for the early clearance of PRRSV viremia and reduction of virus load in the lungs. Furthermore, susceptible macrophages and recruited monocytes in the lung probably become activated by IFN-γ and might become resistant to infection in response to the IFN-γ release, because the peak virus titer in BAL fluids and the high levels of IFN-γ transcriptional activity in BAL fluid cells both occur approximately 9 d after infection (27). Activation and resistance of bystander macrophages, thus, might account for only a small subpopulation of BAL cells being permissive for virus replication.
Polyclonal activation of lymphocytes is another possible source of the serum IFN-γ observed in the current study. Recognized as a factor contributing to the pathogenesis of disease caused by selected pathogens, polyclonal lymphocyte activation is thought to obliterate specific protective immune responses of a host by reducing the availability of lymphocyte clones capable of responding to antigens (28,29). Polyclonal lymphocyte activation can also represent an obstacle to optimal vaccine design. In normal pigs, polyclonal mitogenic activation of lymphocytes will result in IFN-γ production by lymphocytes (30). The possibility of polyclonal activation of lymphocytes in piglets infected with PRRSV has been suggested by the presence of B-cell hyperplasia, immune complexes, and auto-antibodies in PRRSV-inoculated piglets raised in gnotobiotic isolators (31,32). If this also occurs in conventionally raised pigs, then polyclonal activation of lymphocytes may indeed represent a virulence mechanism for PRRS. This would be consistent with a theory of PRRSV-induced, long-lasting polyclonal activation, resulting in the onset of autoimmunity, production of IFN-γ, and prevention of or delay in onset of a protective immune response.
The PRRSV strains that we used induce different serum levels of IFN-γ. Strain SDSU-73 stimulated higher levels of IFN-γ, on average, than did strain JA-142. Peak viremia on days 5 and 10 after inoculation was the same for both strains, but the higher IFN-γ concentration induced by SDSU-73 corresponded with a slower clearance of this strain from the serum on days 14 through 29. Thus, for these 2 PRRSV strains, higher serum levels of IFN-γ on day 10 correlated positively with a slightly longer duration of viremia. This correlation needs to be investigated further with additional PRRSV strains.
Peak IFN-γ values of 119 ± 50 pg/mL after SDSU-73 inoculation and 76 ± 59 pg/mL after JA-142 inoculation represent significant in vivo levels of IFN-γ release. The systemic release of IFN-γ was detectable in all the PRRSV-inoculated pigs and lasted for about 3 wk. Although each animal responded to PRRSV with IFN-γ activity, the levels from pig to pig were highly variable, as shown by the large standard error of the means (Figure 1). After the first 3 wk of peak activity the IFN-γ concentration did not return to background levels in either the SDSU-73-inoculated pigs or the JA-142-inoculated pigs, remaining elevated through day 50. Transient bursts of IFN-γ activity were observed in pigs of both groups, at a single time point for each pig and at different times for different pigs. The residual serum IFN-γ and transient bursts of activity were absent in the control pigs and, thus, may reflect a cytokine response to further rounds of undetected virus replication.
Prior vaccination appeared to block the serum IFN-γ response, at least the response to challenge with the homologous parental, wild-type PRRSV. The serum IFN-γ release in response to JA-142 challenge was blocked in vaccinated pigs at both days 5 and 10 after challenge (Figure 3). Moreover, there was a significant reduction in serum IFN-γ concentration at 10 d after heterologous SDSU-73 challenge. However, at 5 d after SDSU-73 challenge, the serum IFN-γ response was transient and significantly exaggerated and variable (large standard errors) from pig to pig. Most likely this resulted from the lesser degree of protective immunity provided by the vaccine and the greater replication of the heterologous virus after challenge. At 8 wk after vaccination, when adaptive immunity is engaged (14), it is unknown whether the exaggerated serum response 5 d after SDSU-73 challenge resulted from adaptive immunity and virus-specific IFN-γ release from CD4 lymphocytes or from innate immunity that occurs after primary PRRSV infection. Also, for the homologous JA-142 challenge, it is possible that serum IFN-γ release was not completely blocked but, indeed, accelerated and was present at intervals before 5 d.
Apparently PRCV and SIV, which infect respiratory tract epithelial cells, do not induce an early serum IFN-γ response. For SIV, only background levels of IFN-γ were detected during the first 14 d after inoculation. For PRCV, 1 of 4 infected pigs released measurable serum IFN-γ after inoculation, but only on day 5. Further studies are needed with PRCV to ascertain whether there is a consistent and reproducible early IFN-γ response.
Our working hypothesis is that porcine NK cells are important for early control of PRRSV infection. The virus load in the blood and lungs peaks at 5 to 10 d, and then there is a 1000- to 10 000-fold reduction of infectious virus (Figure 2) before the induction of virus-specific neutralizing antibodies and cellular immunity (33). Moreover, type I interferon induction is absent and not a factor in controlling early virus replication. Thus, early production of IFN-γ probably results from activation of NK cells, which is more characteristic of immune pathways stimulated by intracellular bacterial and protozoan infections (34). Early systemic IFN-γ further activates additional NK cells via a positive feedback loop for rapid clearance of PRRSV from serum and may facilitate but may not necessarily be essential for Th1 immunity.
Acknowledgments
The authors are grateful to David Michael, Andrew Gibson, and Gary Buck for technical assistance and for assistance with animal care.
Footnotes
Disclaimer: Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
References
- 1.Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629–633. [PubMed] [Google Scholar]
- 2.Meulenberg JJ, Hulst MM, De Meijer EJ, et al. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS) is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benfield DA, Collins JE, Dee SA, et al. Porcine reproductive and respiratory syndrome. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. Ames, Iowa: Iowa State University Press, 1999:201–232.
- 4.Labarque GG, Nauwynck HJ, Van Reeth K, Penseart MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000;81:1327–1334. doi: 10.1099/0022-1317-81-5-1327. [DOI] [PubMed] [Google Scholar]
- 5.Mengeling WL, Lager KM, Vorwald AC. Diagnosis of porcine reproductive and respiratory syndrome. J Vet Diagn Invest. 1995;7:3–16. doi: 10.1177/104063879500700102. [DOI] [PubMed] [Google Scholar]
- 6.Duan X, Nauwynck HJ, Penseart MB. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV) Arch Virol. 1997;142:2483–2497. doi: 10.1007/s007050050256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molitor TW, Bautista EM, Choi CS. Immunity to PRRSV: doubleedged sword. Vet Microbiol. 1997;55:265–276. doi: 10.1016/S0378-1135(96)01327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi C, Chae C. Expression of tumor necrosis factor-α is associated with apoptosis in lungs of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Res Vet Sci. 2002;72:45–49. doi: 10.1053/rvsc.2001.0519. [DOI] [PubMed] [Google Scholar]
- 9.Labarque GG, Nauwynck HJ, Van Reeth K, Penseart MB. Apoptosis in the lungs of pigs during an infection with a European strain of porcine reproductive and respiratory syndrome virus. Adv Exp Med Biol. 2001;494:691–697. doi: 10.1007/978-1-4615-1325-4_102. [DOI] [PubMed] [Google Scholar]
- 10.Sirinarumitr T, Zhang Y, Kluge JP, et al. A pneumovirulent United States isolate of porcine reproductive and respiratory syndrome virus induces apoptosis in bystander cells both in vitro and in vivo. J Gen Virol. 1998;79:2989–2995. doi: 10.1099/0022-1317-79-12-2989. [DOI] [PubMed] [Google Scholar]
- 11.Sur JH, Doster AR, Osorio FA. Apoptosis induced in vivo during acute infection by porcine reproductive and respiratory syndrome virus. Vet Pathol. 1998;35:506–514. doi: 10.1177/030098589803500605. [DOI] [PubMed] [Google Scholar]
- 12.Duan X, Nauwynck HJ, Penseart MB. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV) Vet Microbiol. 1997;56:9–19. doi: 10.1016/S0378-1135(96)01347-8. [DOI] [PubMed] [Google Scholar]
- 13.Murtaugh MP, Xiao Z, Zuckermann F. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15:533–547. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 14.Batista L, Pijoan C, Dee S, et al. Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can J Vet Res. 2004;68:267–273. [PMC free article] [PubMed] [Google Scholar]
- 15.Meier WA, Galeota J, Osorio FA, et al. Gradual development of the interferon-γ response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 16.Xiao Z, Batista L, Dee S, et al. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J Virol. 2004;78:5923–5933. doi: 10.1128/JVI.78.11.5923-5933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman J, Epperson W, Wills RW, McKean JD. Results of the recent survey of the membership of the AASP for outbreaks of sow abortion and mortality. J Swine Health Prod. 1997;5:74–75. [Google Scholar]
- 18.Mengeling WL, Lager KM, Vorwald AC. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am J Vet Res. 1998;59:1540–1544. [PubMed] [Google Scholar]
- 19.Kim HS, Kwang J, Yoon IJ, et al. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 20.Wesley RD, Woods RD, Hill HT, Biwer JD. Evidence for a porcine respiratory coronavirus, antigenically similar to transmissible gastroenteritis virus, in the United States. J Vet Diagn Invest. 1990;2:312–317. doi: 10.1177/104063879000200411. [DOI] [PubMed] [Google Scholar]
- 21.Levy DE, Marie I, Prakash A. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr Opin Immunol. 2003;15:52–58. doi: 10.1016/s0952-7915(02)00011-0. [DOI] [PubMed] [Google Scholar]
- 22.Royaee AR, Husmann RJ, Dawson HD, et al. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet Immunol Immunopathol. 2004;102:199–216. doi: 10.1016/j.vetimm.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharton TM, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unanue ER. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol Rev. 1997;158:11–25. doi: 10.1111/j.1600-065x.1997.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 25.Unanue ER. Innate immunity in bacterial infections. In: Kaufmann SHE, Sher A, Ahmed R, eds. Immunology of Infectious Diseases. Washington, DC: ASM Press, 2002:93–103.
- 26.Carter QL, Curiel RE. Interleukin-12 (IL-12) ameliorates the effects of porcine respiratory and reproductive syndrome virus (PRRSV) infection. Vet Immunol Immunopathol. 2005;107:105–118. doi: 10.1016/j.vetimm.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Suradhat S, Thanawongnuwech R. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84:2755–2760. doi: 10.1099/vir.0.19230-0. [DOI] [PubMed] [Google Scholar]
- 28.Hovav A, Davidovitch L, Nussbaum G, et al. Mitogenicity of the recombinant mycobacterial 27-kilodalton lipoprotein is not connected to its antiprotective effect. Infect Immun. 2004;72:3383–3390. doi: 10.1128/IAI.72.6.3383-3390.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reina-San-Martin B, Cosson A, Minoprio P. Lymphocyte polyclonal activation: a pitfall for vaccine design against infectious agents. Parasitol Today. 2000;16:62–67. doi: 10.1016/s0169-4758(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 30.Darwich L, Salasch M, Plana-Duran J, et al. Cytokine profiles of peripheral blood mononuclear cells from pigs with post-weaning multisystemic wasting syndrome in response to mitogen, superantigen or recall viral antigens. J Gen Virol. 2003;84:3453–3457. doi: 10.1099/vir.0.19364-0. [DOI] [PubMed] [Google Scholar]
- 31.Lamontagne L, Page C, Larochelle R, et al. Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol Immunopathol. 2001;82:165–181. doi: 10.1016/s0165-2427(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 32.Lemke CD, Haynes JS, Spaete R, et al. Lymphoid hyperplasia resulting in immune dysregulation is caused by porcine reproductive and respiratory syndrome virus infection in neonatal pigs. J Immunol. 2004;172:1916–1925. doi: 10.4049/jimmunol.172.3.1916. [DOI] [PubMed] [Google Scholar]
- 33.Murtaugh MP. PRRS immunology: What are we missing? Proc Annu Meet Am Assoc Swine Vet. 2004:359–367. [Google Scholar]
- 34.Biron CA, Dalod M, Salazar-Mather TP. Innate immunity and viral infections. In: Kaufmann SHE, Sher A, Ahmed R, eds. Immunology of Infectious Diseases. Washington, DC: ASM Press, 2002:139–160.