Abstract
Evidence indicates that GABA is an inhibitory neurotransmitter responsible for restricting luteinizing hormone-releasing hormone (LHRH) release before the onset of puberty. LHRH neurons in the hypothalamus of female rhesus monkeys are already active during the neonatal period, but subsequently enter a dormant state in the juvenile/prepubertal period because of an elevated level of GABA in the stalk-median eminence (S-ME). The developmental reduction in tonic GABA inhibition results in an increase in LHRH release in the S-ME, triggering puberty. The reduction in GABA also appears to allow an increase in glutamate release in the S-ME and this glutamate seems to further contribute to the pubertal increase in LHRH release. These observations conducted in non-human primates, as a model for humans, provide some insights into future studies of the importance of GABAergic mechanisms in the relation between onset of puberty and neurodevelopmental disorders including autism.
Introduction
There are several neurological and psychiatric diseases associated with puberty and changes in GABAergic function may, at least in part, be responsible for underlying etiology. First, recent discoveries by several laboratories indicate that an abnormality of the GABAergic neuronal system may be a cause of autism or responsible for some symptoms of autism (Prosser et al., 1997; Hussman, 2001; Menold et al., 2001; Cohen et al., 2002; ; Nurmi et al., 2003; Muhle et al., 2004). Moreover, in some patients, a worsening of autistic behavior is observed in association with puberty (Gillberg, 1984; Mouridsen et al., 1999). Second, it has been well documented that the onset of schizophrenia occurs between late puberty and early young adulthood (Lewis, 1997, Lewis et al., 2005). A significant decrease in the GABA transporter, GAT-1, immuno-reactivity on axonal terminals of a subset of GABA neurons that innervate pyramidal cells in the frontal cortex is observed in patients with schizophrenia when compared to normal human subjects (Woo et al., 1998). Third, the new onset of epileptic seizures tends to occur early in life and during the adolescent period (Robertson et al., 1990; Appleton and Gibbs, 1998). Precocious puberty is also often associated with epilepsy in children (Lennox and Lennox, 1960; Elian, 1970; Mouridsen et al., 1999; Shenoy and Raja, 2004). Furthermore, treatment with sodium valproic acid, a GABA agonist, delays the timing of puberty in children with seizure disorders (Lundberg et al., 1986; Cook et al, 1992) and in genetically epilepsy-prone mice (Snyder and Badura, 1995). It is possible that the pubertal increase in gonadal steroids may sensitize neurocircuits involved in epileptic seizures, but it is also possible that there is a common mechanism of developmental deficiency, i.e., weakened GABA inhibition in the LHRH neuronal system resulting in precocious puberty and weakened GABAergic inhibition in the brain at the pubertal age resulting in epilepsy (Olsen and Avoli, 1997). Bourguignon and colleagues treated an 11-month old child who exhibited severe epileptic seizures and precocious puberty with loreclezole and vigabatrin, GABA agonists. At an earlier stage traditional treatment for epilepsy with phenobarbital was not effective in this patient. However, treatment with loreclezole followed by vigabatrin not only regressed all signs of precocious puberty, but also settled seizure attacks (Bourguignon et al., 1997).
This laboratory has been studying the mechanism of the onset of puberty in the rhesus monkey, as a model for humans. Specifically, results from a series of experiments suggest that the GABAergic neuronal system is, in part, responsible for the timing of puberty in primates (Terasawa, 1995; Terasawa, 2000). Puberty is an important developmental stage during which not only reproductive function is attained (Terasawa and Fernandez, 2001), but also the maturation of the prefrontal cortex, responsible for adolescent behaviors, occurs (Gogtay et al., 2004). Recently, a concept has been proposed that the maturation of the hypothalamus, responsible for puberty, may occur independently from the maturation of the cortices, but there may be common mechanisms governing the maturation of reproductive function and behaviors (Sisk and Foster, 2004). Therefore, a series of observations from this laboratory conducted in non-human primates provide some insights into better understanding the role of GABA function in the possible relation between onset of puberty and clinical changes in autism and other neuropsychiatric disorders.
Developmental changes in luteinizing hormone-releasing hormone relese
The decapeptide, luteinizing hormone-releasing hormone (LHRH, also called gonadotropin-releasing hormone or GnRH), is synthesized in the preoptic area and hypothalamus and is released into the pituitary portal circulation in a pulsatile manner at approximately 60 min intervals in mature primates including humans (Hotchkiss and Knobil, 1994). In juveniles the pulse interval of LHRH release is much longer, 90–120 min (Plant, 1994). Acceleration of the pulse frequency accompanied by an increase in the pulse amplitude, hence an increase in total output of LHRH release, triggers the onset of puberty (Watanabe and Terasawa, 1989). It has also been shown that pulsatile administration of LHRH into juvenile monkeys results in precocious puberty (Wildt et al., 1980), and pulsatile administration of LHRH agonist and antagonist analogs has been used for the treatment of precocious and delayed puberty in humans (Crowley et al., 1985).
Although LHRH neurons in the preoptic area and hypothalamus are reasonably mature at birth (Terasawa and Fernandez, 2001) and release the decapeptide in a pulsatile manner shortly after birth, the adult type of secretory pattern with a higher pulse frequency interval (~60 min) is not established until puberty. This is probably due to the immaturity of transsynaptic input regulating LHRH neurons before puberty, either by 1) insufficient excitatory neuronal input to LHRH neurons, or 2) by inhibitory neuronal input to LHRH neurons suppressing activity of LHRH neurons. Electrical stimulation of the medial basal hypothalamus in prepubertal monkeys results in LHRH release of the same magnitude observed in pubertal monkeys (Claypool et al., 1990). However, the immaturity of inhibitory input in control of LHRH release is the predominant mechanism over the immaturity of excitatory input before the onset of puberty, i.e., activity of LHRH neurons during the neonatal period is elevated for the first few months after birth in rhesus monkeys and several months after birth in humans, but is then suppressed by an unknown source of inhibition until shortly before puberty (Plant, 1994), and this inhibition is central in origin, independent from suppression by the ovarian steroid hormone estrogen (Terasawa et al., 1983; Chongthammakun et al., 1993). Thus, we investigated inhibitory neurotransmitters for LHRH release before puberty.
At an early stage we examined the role of beta-endorphin, an inhibitory neuropeptide. However, we excluded it as a candidate for prepubertal inhibition of LHRH release: The release of beta-endorphin increased concomitant with the pubertal increase in LHRH release (Terasawa and Fernandez, 2001). Subsequently, we examined the role of GABA, a dominant inhibitory neurotransmitter in the hypothalamus, (Decavel and van den Pol, 1990) and have found that GABA is an important neurotransmitter responsible for the timing of puberty.
Developmental changes in GABA release
LHRH neurons release the decapeptide into portal circulation located in the median eminence (ME) and pituitary stalk (S), and appear to be controlled by presynaptic input from GABA neurons. Modulation of LHRH neurons by GABA neurons appears to occur at the cell body and dendrites as well as at the neuroterminals. As the first step to assess the role of GABA in puberty, we measured the simultaneous release of LHRH and GABA in the S-ME. The collection of samples from the S-ME located in the base of the hypothalamus in unanesthetized monkeys is a challenging task, but we have been successful in collecting hypothalamic perfusates for the detection of neurochemical substances using a push-pull perfusion method. As described previously (Terasawa, 1994), a double lumen cannula is inserted into the S-ME with aid of x-ray ventriculograms, and artificial CSF is slowly infused to the area, approximately 1 mm3, through the push cannula while perfusates are continuously collected through the pull cannula using two peristaltic pumps calibrated at identical speeds. Using this method, we are able to measure LHRH, GABA, glutamates, beta-endorphin, neuropeptide Y, prostaglandin E2, and catecholamines and their metabolites in various physiological conditions (Terasawa, 1994). This method is also useful in examining the effects of neurotransmitter agonists and antagonists on neurotransmitter/neuromodulator release, such as LHRH and/or GABA, by direct application through the push cannula.
Developmental changes in GABA and LHRH levels in the same samples were assessed using this method. Perfusate samples from the S-ME are collected from prepubertal monkeys at 13–21 months of age (before any sign of puberty is apparent), early pubertal monkeys at 22–28 months of age (after some signs of puberty, before menarche) and midpubertal monkeys at 34–46 months of age (after menarche, but before first ovulation). LHRH and GABA levels were measured by radioimmunoassay and HPLC with electrochemical detection, respectively. In prepubertal monkeys LHRH levels are low, whereas GABA levels measured in the same samples are high. LHRH levels significantly increase in early pubertal and midpubertal monkeys, whereas GABA levels are significantly low in both early and midpubertal monkeys (Fig. 1, Terasawa et al., 1999). These observations are similar to those reported previously (Mitsushima et al., 1994). Although we were not able to obtain data from monkeys between the neonatal period and 12 months of age because they were not weaned, a scatter plot of GABA levels during the ages of 13 to 46 months (Fig. 2) indicates that there is a clear developmental GABA decrease. Interestingly, this profile in female rhesus monkeys resembles to that described for circulating GABA levels in normal children (Dhossche et al., 2002).
Figure 1.
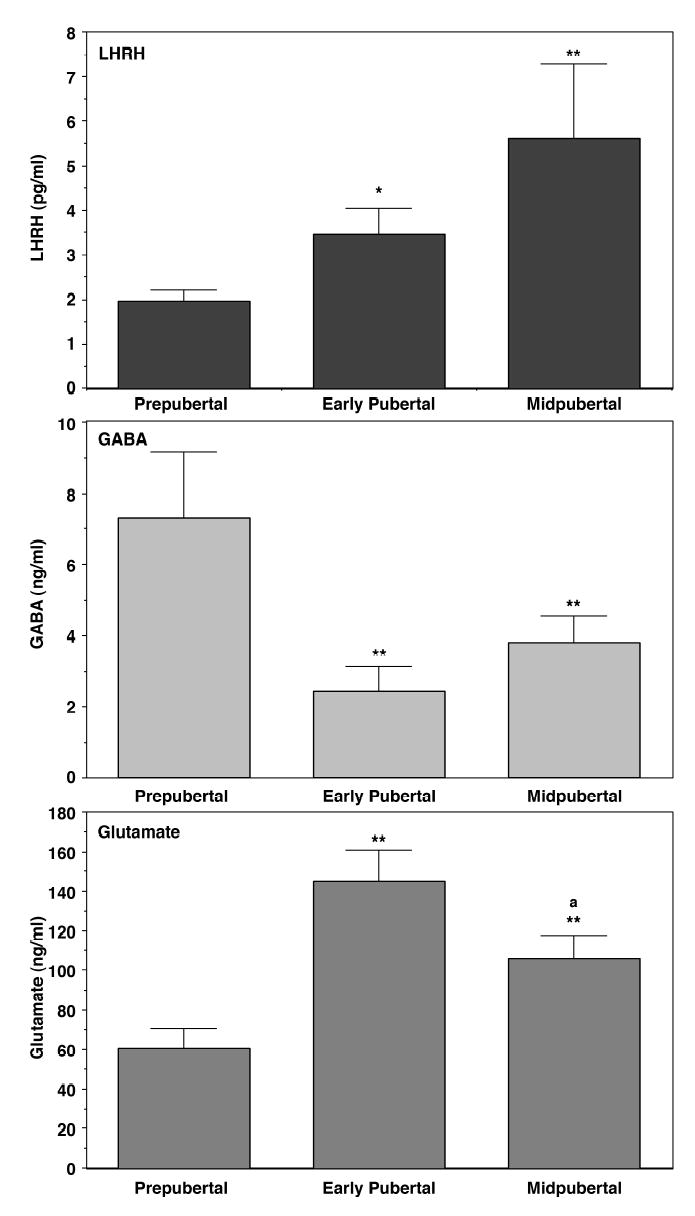
Developmental changes in luteinizing hormone-releasing hormone (LHRH, top), GABA (middle), and glutamate (bottom) levels in the stalk-median eminence of the hypothalamus in female monkeys. Samples were obtained using the push-pull perfusion method. Note that GABA release in the stalk-median eminence decreases, whereas glutamate release increases, when the pubertal increase in LHRH release occurs. The ages of prepubertal, early pubertal, and midpubertal monkeys are 13–20 months (before any sign of puberty), 21–30 months (some signs of puberty, but before menarche), and 34–46 months (after menarche, but before first ovulation), respectively. Ages of menarche and first ovulation in our colony ffemales are ~30 months and ~45 months, respectively. *P<0.05 vs. prepubertal; **p<0.01 vs. prepubertal; aP<0.05 vs. early pubertal monkeys. Modified from Terasawa et al. (1999) with permission.
Figure 2.

A scatter plot of GABA release in the stalk-median eminence of female monkeys at ages of 13–46 months. A gradual decline of GABA levels with age is seen.
Evidence for GABA as an inhibitory neurotransmitter before puberty
A question arises as to whether high levels GABA release in the S-ME before puberty have any physiological significance. To answer this question, two experiments were conducted. First, we examined the effect of the GABAA receptor antagonist, bicuculline, on LHRH release (Mitsushima et al., 1994). Results suggest that bicuculline stimulates LHRH release in prepubertal monkeys by removing endogenous GABA inhibition, whereas exogenous GABA is not effective in suppressing LHRH release until after the onset of puberty, when endogenous GABAergic tone is reduced. The GABAB receptor blocker, saclofen, was not effective in prepubertal monkeys. Second, we examined whether lowering GABA levels in the S-ME by chronic infusion of bicuculline triggers puberty (Keen et al., 1999). The average ages of menarche and first ovulation in female rhesus monkeys in our colony are approximately 30 and 45 months, respectively (Terasawa et al., 1983). Pulsatile infusion of bicuculline into the third ventricle of prepubertal monkeys results in precocious menarche, which occurs 6–8 weeks after the initiation of bicuculline infusion, and in precocious first ovulation, which occurs by 30 months, the age of menarche in control females (Fig. 3, Keen et al., 1999; Richter and Terasawa, 2001). However, since the interval between menarche and first ovulation is not shortened by bicuculline infusion, additional mechanisms, such as the establishment of the stimulatory neuronal system for pulsatile LHRH release, are necessary for the pubertal transition in female primates. The mean ages of menarche and first ovulation in control monkeys receiving saline infusions are not different from the data in colony controls. The results of these two experiments indicate that tonic GABAergic inhibition in the S-ME is, at least in part, responsible for restraining the activity of LHRH neurons before the onset of puberty and reduction in GABAergic inhibition triggers the pubertal increase in LHRH release resulting in the onset of puberty.
Figure 3.
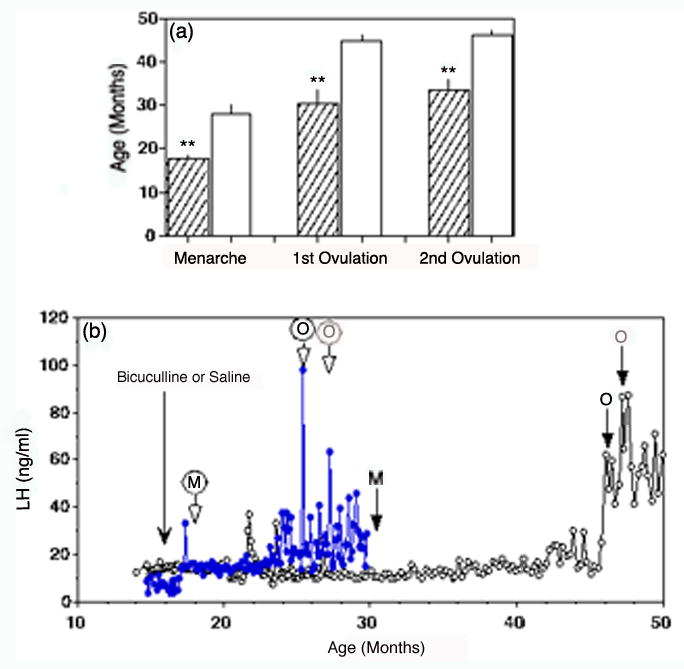
Reducing GABA neurotransmission with the GABAA receptor blocker, bicuculline, advances puberty in female rhesus monkeys. (a) The age of menarche and first ovulation in female rhesus monkeys treated by chronic infusion of bicuculline occur at a significantly earlier age than that in controls. Bicuculline treated group, filled bar; saline-treated control group, open bars. **P<0.01. (b) Representative examples showing LH concentration in a bicuculline treated (filled circles) monkey and a saline-treated control (open circles) monkey. The dramatically earlier onset of puberty is shown by menarche (M) and first and second ovulations (O). Modified from Keen et al., (1999) and Richter and Terasawa (2001) with permission.
Role of glutamic acid decarboxylase in puberty
In presynaptic neurons GABA is synthesized from glutamate by decarboxylation in the presence of glutamic acid decarboxylase (GAD), stored in vesicles, and released by exocytosis upon depolarization in the presence of extracellular Ca2+ (Rando et al., 1981). There are two different proteins, GAD67 and GAD65, derived from respective genes (Martin and Rimvall, 1993; Erlander and Tobin, 1991). To assess the possible involvement of GADs in puberty, we examined whether interference in GAD67 and GAD65 synthesis in prepubertal monkeys modifies the LHRH release pattern (Mitsushima et al., 1996). Infusion of antisense oligodeoxynucleotides for GAD67 and GAD65 mRNAs into the S-ME of prepubertal monkeys results in a dramatic increase in LHRH release (Mitsushima et al., 1996; Kasuya et al., 1999), presumably due to the reduction in GABA synthesis and subsequent GABA release (Mitsushima et al., 1996; Terasawa et al., 1999). Scrambled oligodeoxynucleotides for GAD67 and GAD65 mRNAs as controls did not induce any significant effect. Observations from this experiment indicate that interference in GAD67 and GAD65 synthesis is effective in reducing tonic GABAergic inhibition, resulting in an increase in LHRH release.
Developmental changes in GABA and GAD in primates are not well studied, and there are conflicting data from the postnatal period. GAD activity in the human neocortex sharply increases at birth and continues to increase until 1 year of age, after which it declines gradually until pubertal age and then slightly increases at adulthood (Diebler et al., 1979; Johnston and Coyle, 1981). Urbanski et al. 1998 reported that the distribution pattern and concentration of GAD67 and GAD65 mRNAs in hypothalamic nuclei assessed by in situ hybridization in gonadally intact juvenile (~0.6 years of age) male rhesus monkeys were not different from those in adult (~10 years of age) male monkeys, and a report by Plant and his colleagues () indicates that GAD mRNA levels in the basal hypothalamus of juvenile castrated male rhesus monkeys did not differ from those in adult castrated male monkeys. Although these data do not appear to support the hypothesis that GAD plays an important role in puberty, more precise developmental studies with the exact regional distribution pattern of GABA neurons in non-human primates, including in females, are needed before conclusions can be drawn.
GAD67 and GAD65 exist in the enzymatically active holo form and inactive apo form, and conversion of holo-GAD67 or holo-GAD65 to and from apo-GAD67 or apo-GAD65 is determined by the presence of the cofactor, pyridoxal-5’-phosphate, which is influenced by physiological states as well as by experimental conditions (Erlander and Tobin, 1991; Kaufman et al., 1991). We do not have any data showing how the ratio of the active and inactive form changes during development.
Other possible factors associated with developmental changes in GABAergic function
GABA transporters
Elevated local concentrations of GABA in the synaptic cleft are actively removed by GABA transporters (GAT), located on presynaptic terminals and surrounding glial cells, where the neurotransmitters are recycled. Four GABA transporters (GAT-1, GAT-2, GAT-3 and BGT-1), classified as the Na+ and Cl−-coupled transporter family, have been described (Guastella et al., 1990; Nelson et al., 1990). GAT-1 is the predominant GABA transporter in the mammalian brain (Borden, 1996) and the GAT-1 transcript contains an estrogen responsive element (Herbison et al., 1995). Thus, it is possible that the reduction of GABA concentration in the S-ME at the onset of puberty might be due to a developmentally regulated increase in GABA transporter activity. At this time there is no information on the role of GAT activity in puberty.
GABAA receptors and their subunit composition
The developmental pattern of each GABAA receptor subunit is complex and differs from region to region or neuron to neuron, which insures the functional heterogeneity of GABA input. Nonetheless, it has been consistently reported that, in general, α2 subunit expression is very high before birth to shortly after birth, and decreases gradually toward adult levels, whereas α1 expression is minimal in prenates and then gradually increases after birth until adulthood (Brooks-Kayal and Pritchett, 1993; Hendrickson et al., 1994; Fritschy et al., 1994; Fritschy and Mohler, 1995; Laurie et al., 1992). In fact, it appears that GABAA receptors containing α1 subunits gradually replace GABAA receptors containing the α2 subunit during postnatal maturation in the rat, monkey and human brain, and that the increase in the α1 subunit is an indication of brain maturation, i.e., the onset of synaptic GABA inhibition, whereas β subunits do not generally undergo developmental changes (Brooks-Kayal and Pritchett, 1993; Hendrickson et al., 1994; Laurie et al., 1992). An example of developmental changes in subunit composition in association with function has been shown: Changes in GABAA receptor subunit composition in hippocampal neurons preceded the onset of epilepsy by weeks in epileptic rats (Brooks-Kayal et al., 1998).
It is, therefore, possible that developmental changes in the GABAA subunit composition in neuronal cells may occur prior to the onset of puberty. Analysis of the literature provides support for the hypothesis that GABA disinhibition of LHRH neurons through GABAA receptors at the onset of puberty in female monkeys (Mitsushima et al., 1994) is due to changes in GABAA receptor subunit composition. For example, a report using single cell RT-PCR (Sim et al., 2000) suggests that the pattern of LHRH neurons expressing GABA subunits in the POA and medial septum of sexually immature mice at neonatal and juvenile ages is more heterogeneous than that in adults, and it becomes homogeneous when the mice mature. Interestingly, the same authors have reported that sensitivity to GABA in LHRH neurons of juvenile mice is lower than that in adult mice and that the pattern of the dose response curve to GABA in prepubertal LHRH neurons is more heterogeneous than that in adult LHRH neurons (Sim et al., 2000). At this time, we have little knowledge of developmental changes in the GABAA subunit composition of LHRH neurons in non-human primates.
The pubertal reduction in GABAergic inhibition is followed by an increase in glutamatergic tone
Glutamate is profoundly involved in pulsatile LHRH release in vivo and in vitro through NMDA and kainate receptors. NMDA stimulates release of LH and LHRH in adult rats and monkeys in vivo (Price et al., 1978, Olney et al., 1976; ; Brann and Mahesh, 1997; van den Pol et al., 1994; Bourguignon et al., 1995), and glutamate, NMDA, and kainate all stimulate LHRH/LH release in sexually immature monkeys (Gay and Plant, 1987; Medhamurthy et al., 1990), rats (Bourguignon et al., 1989; Brann and Mahesh, 1992; Cicero et al., 1988), sheep (I’Anson et al., 1993), and fetal sheep (Bettendorf et al., 1999) in vitro and in vivo. Moreover, stimulation of NMDA receptors results in precocious puberty in rats and monkeys (Plant et al., 1989; Urbanski and Ojeda, 1990), whereas administration of the NMDA receptor blockers, MK-801 or 2-amino-5-phosphonovaleric acid (AP-5), delays the timing of puberty in rats (Meiji-Roelofs et al., 1991; Urbanski and Ojeda, 1990; Wu et al., 1990; MacDonald and Wilkinson, 1990). In contrast, the non-NMDA receptor antagonist, 6,7-dinitroquinoxaline-2,3-dione (DNQX), fails to change the timing of puberty (Brann and Mahesh, 1994). The excitatory action of glutamate on LHRH release may occur not only through NMDA receptors, but also through metabolic receptors. Therefore, the developmental changes in NMDA and kainate receptors are integrated parts of the mechanism of the onset of puberty.
We have measured glutamate release in the S-ME using the push-pull perfusion method (Terasawa et al., 1999). Glutamate levels during the prepubertal period are very low, but increase strikingly during the early pubertal period, and remain high during the midpubertal period, although midpubertal levels decline slightly from early pubertal levels (Fig. 1). However, this observation from monkeys at different ages (cross-sectional study) does not provide the exact timing of glutamate increase during puberty. Fortunately, there is an indication that the pubertal elevation in glutamate release may occur promptly following GABA reduction. The results of the antisense GAD67 infusion experiment in prepubertal monkeys suggest that the reduction in GABA release induced by the antisense GAD67 treatment is followed by an increase in glutamate release for several hours (Terasawa et al., 1999).
Sensitivity to glutamatergic stimulation increases after the onset of puberty. For example, 1) infusion of NMDA into the S-ME at 10 μM-100 μM stimulates LHRH release in pubertal monkeys, whereas only 100 μM NMDA results in LHRH release in prepubertal monkeys (Claypool et al., 2000), and 2) i.v. injection of NMDA at 10 mg/kg results in LHRH responses with a longer duration in pubertal monkeys than in prepubertal monkeys (Claypool et al., 2000). Although this increase in the responsiveness of LHRH neurons to NMDA after puberty may, in part, be due to an increase in circulating estrogen, glutamatergic tone is more elevated after the onset of puberty.
Conclusions
The mechanism of the onset of puberty is complex. As we discussed above, LHRH neurons are reasonably mature at birth and are already active during the neonatal period. However, in primates "central inhibition" suppresses pulsatile LHRH release during the juvenile period. Studies from this laboratory suggest that the GABAergic neuronal system appears to be a substrate for "central inhibition" in primates. When approaching puberty, this GABA inhibition is removed or diminished, and an increase in LHRH release occurs. Subsequently, increases in stimulatory input from glutamatergic neurons as well as new stimulatory input from norepinephrine and NPY neurons (which we did not discuss here, see Terasawa and Fernandez, 2001) and inhibitory input from β-endorphin neurons to the LHRH neuronal system become active to establish the adult type of regulatory mechanism for pulsatile LHRH release. This pubertal increase in LHRH release results in a cascade of events during puberty, such as increases in synthesis and release of gonadotropins, and increases in steroidogenesis and gametogenesis, followed by the appearance of secondary sexual characteristics.
The most important question still remains: What determines the timing to remove "GABA inhibition"? Because many genes in the brain are turned on or turned off to establish a complex series of events occurring during puberty, the timing of spontaneous puberty must be regulated by a master gene or genes, as part of a series of developmental events. We expect that future studies will include a search for genes determining events to remove "GABA inhibition" and genes which ultimately trigger the onset of puberty in primates.
Understanding the mechanism of the onset of puberty in detail is very important, as puberty is associated with onset of or changes in many neurodevelopmental disorders, including autism, schizophrenia, and affective disorder. At this time it is unclear whether the series of events in the hypothalamus with puberty also occur in the higher brain regions where cognitive function is controlled. Future studies to assess whether similar events in the hypothalamus also occur in the higher brain regions that are involved in autism and other disorders will be useful for treatment strategies.
Acknowledgments
The author thanks Kim Keen for her proofreading of the manuscript. This work is supported by NIH grants 5R01HD11355, 5R01HD15433, and 5P51RR00167.
References
- Appleton, R., and Gibbs, J. (1998). Epilepsy in Childhood and Adolescence. Martin Dunitz Publishers, London.
- Bettendorf M, de Zegher F, Albers N, Hart CS, Kaplan SL, Grumbach MM. Acute N-methyl-D,L-aspartate administration stimulates the luteinizing hormone releasing hormone pulse generator in the ovine fetus. Horm Res. 1999;51:25–30. doi: 10.1159/000023309. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Gérard A, Franchimont P. Direct activation of gonadotropin-releasing hormone secretion through different receptors to neuroexcitatory amino acids. Neuroendocrinology. 1989;49:402–408. doi: 10.1159/000125145. [DOI] [PubMed] [Google Scholar]
- Bourguignon JP, Gérard A, Gonzalez MLA, Purnelle G, Franchimont P. Endogenous glutamate involvement in pulsatile secretion of gonadotropin-releasing hormone: evidence from the effect of glutamine and developmental changes. Endocrinology. 1995;136:911–916. doi: 10.1210/endo.136.3.7867599. [DOI] [PubMed] [Google Scholar]
- Bourguignon J-P, Jaeken J, Gérard A, deZegher F. Amino acid neurotransmission and initiation of puberty: evidence from nonketotic hyperglycinemia in a female infant and gonadotropin-releasing hormone secretion by rat hypothalamic explants. J Clin Endocrinol Metab. 1997;82:1899–1903. doi: 10.1210/jcem.82.6.4018. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Excitatory amino acid neurotransmission. Evidence for a role in neuroendocrine regulation. Trends Endocrinol Metab. 1992;3:122–126. doi: 10.1016/1043-2760(92)90100-f. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol. 1994;15:3–49. doi: 10.1006/frne.1994.1002. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB. Excitatory amino acids: evidence for a role in the control of reproduction and anterior pituitary hormone secretion. Endocr Rev. 1997;18:678–700. doi: 10.1210/edrv.18.5.0311. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Pritchett DB. Developmental changes in human γ- aminobutyric acid A receptor subunit composition. Ann Neurol. 1993;34:687–693. doi: 10.1002/ana.410340511. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo LHRH release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. doi: 10.1111/j.1365-2826.1993.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Meyer ER, Bell RD. Characterization and possible opioid modulation of N-methyl-D-aspartic acid induced increases in serum luteinizing hormone levels in the developing male rat. Life Sci. 1988;42:1725–1732. doi: 10.1016/0024-3205(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Kasuya E, Saitoh Y, Marzban F, Terasawa E. N-methyl-D,L-aspartate induces the release of luteinizing hormone-releasing hormone in prepubertal and pubertal female rhesus monkeys as measured by in vivo push-pull perfusion in the stalk-median eminence. Endocrinology. 2000;141:219–228. doi: 10.1210/endo.141.1.7231. [DOI] [PubMed] [Google Scholar]
- Claypool LE, Watanabe G, Terasawa E. Effects of electrical stimulation of medial basal hypothalamus on the in vivo release of luteinizing hormone-releasing hormone in the prepubertal and peripubertal monkey. Endocrinology. 1990;127:3014–3022. doi: 10.1210/endo-127-6-3014. [DOI] [PubMed] [Google Scholar]
- Cohen BI. Use of GABA-transaminase agonist for treatment of infantile autism. Med Hypotheses. 2002;59:115–116. doi: 10.1016/s0306-9877(02)00157-3. [DOI] [PubMed] [Google Scholar]
- Cook JS, Bale JF, Hoffman RP. Pubertal arrest associated with valproic acid therapy. Pediatr Neurol. 1992;8:229–231. doi: 10.1016/0887-8994(92)90075-a. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Filicori M, Spratt DT, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Decavel C, van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Dhossche, D., Applegate, H., Abraham, A., Maertens, P., Bland, L., Bencsath, A., and Martinez, J. (2002). Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med. Sci. Monit. 8, PR1–6. [PubMed]
- Diebler MF, Farkas-Bargeton E, Wehrle R. Developmental changes of enzymes associated with energy metabolism and the synthesis of some neurotransmitters in discrete areas of human neocortex. J Neurochem. 1979;32:429–435. doi: 10.1111/j.1471-4159.1979.tb00367.x. [DOI] [PubMed] [Google Scholar]
- El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elian M. EEG, epilepsy, and precocious puberty. Electroencephalogr Clin Neurophysiol. 1970;28:642. [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: A review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay VL, Plant TM. N-Methyl-D,L-aspartate (NMDA) elicits hypothalamic GnRH release in prepubertal male rhesus monkeys (Macaca mulatta) Endocrinology. 1987;120:2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Autistic children growing up: problems during puberty and adolescence. Dev Med Child Neurol. 1984;26:125–9. doi: 10.1111/j.1469-8749.1984.tb04418.x. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent III TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella J, Nelson N, Nelson H, Czyzyk L, Keynan S, Miedel MC, Davidson N, Lester HA, Kanner BI. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, March D, Richards G, Erickson A, Shaw C. Coincidental appearance of the α1 subunit of the GABAA-receptor and the type I benzodiazepine receptor near birth in macaque monkey visual cortex. Int J Dev Neurosci. 1994;12:299–314. doi: 10.1016/0736-5748(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Augwood SJ, Simonian SX, Chapman C. Regulation of GABA transporter activity and mRNA expression by estrogen in rat preoptic area. J Neurosci. 1995;15:8302–8309. doi: 10.1523/JNEUROSCI.15-12-08302.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss, J., and Knobil, E. (1994). The menstrual cycle and its neuroendocrine control. In “The Physiology of Reproduction” (E. Knobil and J. D. Neill, Eds.), Second Edition, pp. 711–749. Raven Press, New York.
- Hussman JP. Suppressed gabaergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001;31:247–248. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- I’Anson H, Herbosa CG, Ebling FJ, Wood RI, Bucholtz DC, Mieher CD, Foster DL, Padmanabhan V. Hypothalamic versus pituitary stimulation of luteinizing hormone secretion in the prepubertal female lamb. Neuroendocrinology. 1993;57:467–475. doi: 10.1159/000126393. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Coyle JT. Development of central neurotransmitter systems. Ciba Found Symp. 1981;86:251–271. doi: 10.1002/9780470720684.ch12. [DOI] [PubMed] [Google Scholar]
- Kasuya E, Nyberg CL, Mogi K, Terasawa E. A role of γ-aminobutyric acid (GABA) and glutamate in control of puberty in female rhesus monkeys: effect of an antisense oligodeoxynucleotide for GAD67 messenger ribonucleic acid and MK801 on luteinizing hormone-releasing hormone release. Endocrinology. 1999;140:705–712. doi: 10.1210/endo.140.2.6574. [DOI] [PubMed] [Google Scholar]
- Kaufman DL, Houser CR, Tobin AJ. Two forms of the γ-aminobutyric acid synthetic enzyme glutamate decarboxylase have distinct intraneuronal distributions and cofactor interactions. J Neurochem. 1991;56:720–723. doi: 10.1111/j.1471-4159.1991.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABAA receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999;140:5257–5266. doi: 10.1210/endo.140.11.7139. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox, W. G., and Lennox, M. A. (1960). Epilepsy and Related Disorders. Little, Brown and Company, Boston.
- Lewis DA. Development of the prefrontal cortex during adolescence-insights into vulnerable neural circuits in schizophrenia. Neuropsycopharmacol. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lundberg B, Nergardh A, Ritzen E, Samuelson K. Influence of valproic acid on the gonadotropin releasing hormone test in puberty. Acta Pediatr Scand. 1986;75:787–792. doi: 10.1111/j.1651-2227.1986.tb10291.x. [DOI] [PubMed] [Google Scholar]
- MacDonald MC, Wilkinson M. Peripubertal treatment with N-methyl-Daspartic acid (NMDA) or neonatally with monosodium glutamate (MSG) accelerates sexual maturation in female rats, an effect reversed by MK801. Neuroendocrinology. 1990;52:143–149. doi: 10.1159/000125565. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of γ-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Medhamurthy R, Dichek HL, Plant TM, Bernardini I, Cutler Jr GB. Stimulation of gonadotropin secretion in prepubertal monkeys after hypothalamic excitation with aspartate and glutamate. J Clin Endocrinol Metab. 1990;71:1390–1392. doi: 10.1210/jcem-71-5-1390. [DOI] [PubMed] [Google Scholar]
- Meiji-Roelofs HMA, Kramer P, van Leeuwen ECM. The N-methyl-D-aspartate receptor antagonist MK-801 delays the onset of puberty and may acutely block the first spontaneous LH surge and ovulation in the rat. J Endocrinol. 1991;131:435–441. doi: 10.1677/joe.0.1310435. [DOI] [PubMed] [Google Scholar]
- Menold MM, Shao Y, Wolpert CM, Donnelly SL, Raiford KL, Martin ER, Ravan SA, Abramson RK, Wright HH, Delong GR, Cuccaro ML, Pericak-Vance MA, Gilbert JR. Association analysis of chromosome 15 GABA receptor subunit genes in autistic disorder. J Neurogenet. 2001;15:245–259. doi: 10.3109/01677060109167380. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Hei DL, Terasawa E. GABA is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci USA. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Marzban F, Luchansky LL, Burich AJ, Keen KL, Durning M, Golos TG, Terasawa E. Role of glutamic acid decarboxylase in the prepubertal inhibition of the luteinizing hormone-releasing hormone release in prepubertal female monkeys. J Neurosci. 1996;16:2563–2573. doi: 10.1523/JNEUROSCI.16-08-02563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen SE, Rich B, Isager T. Epilepsy in disintegrative psychosis and infantile autism: a long-term validation study. Dev Med Child Neurol. 1999;41:110–114. doi: 10.1017/s0012162299000213. [DOI] [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics. 2004;113:e472–e486. doi: 10.1542/peds.113.5.e472. [DOI] [PubMed] [Google Scholar]
- Nelson H, Mandiyan S, Nelson N. Cloning of the human brain GABA transporter. FEBS Lett. 1990;269:181–184. doi: 10.1016/0014-5793(90)81149-i. [DOI] [PubMed] [Google Scholar]
- Nurmi EL, Dowd M, Tadevosyan-Leyfer O, Haines JL, Folstein SE, Sutcliffe JS. Exploratory subsetting of autism families based on savant skills improves evidence of genetic linkage to 15q11-q13. J Am Acad Child Adolesc Psychiatry. 2003;42:856–863. doi: 10.1097/01.CHI.0000046868.56865.0F. [DOI] [PubMed] [Google Scholar]
- Olney JW, Cicero TJ, Meyer ER, De Gubareff T. Acute glutamateinduced elevations in serum testosterone and luteinizing hormone. Brain Res. 1976;12:420–424. doi: 10.1016/0006-8993(76)90298-5. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Avoli M. GABA and epileptogenesis. Epilepsia. 1997;38:399–407. doi: 10.1111/j.1528-1157.1997.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Plant, T. M. (1994). Puberty in Primates. In “The Physiology of Reproduction” (E. Knobil and J. D. Neill, Eds.), pp. 453–485. Raven Press, New York.
- Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci USA. 1989;86:2506–2510. doi: 10.1073/pnas.86.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MT, Olney JW, Cicero TJ. Acute elevations of serum luteinizing hormone induced by kainic acid, N-methyl-aspartic acid, or homosysteic acid. Neuroendocrinology. 1978;26:352–358. doi: 10.1159/000122790. [DOI] [PubMed] [Google Scholar]
- Prosser J, Hughes CW, Sheikha S, Kowatch RA, Kramer GL, Rosenbarger N, Trent J, Petty F. Plasma GABA in children and adolescents with mood, behavior, and comorbid mood and behavior disorders: a preliminary study. J Child Adolesc Psychopharmacol. 1997;7:181–199. doi: 10.1089/cap.1997.7.181. [DOI] [PubMed] [Google Scholar]
- Rando RR, Bangerter FW, Farb DH. The inactivation of γ- aminobutyric acid transaminase in dissociated neuronal cultures from spinal cord. J Neurochem. 1981;36:985–990. doi: 10.1111/j.1471-4159.1981.tb01690.x. [DOI] [PubMed] [Google Scholar]
- Richter TA, Terasawa E. Inhibitory neural mechanisms underlying changes in LHRH release at the onset of puberty in the rhesus monkey. Trends Endocrinol Metab. 2001;12:353–359. doi: 10.1016/s1043-2760(01)00442-8. [DOI] [PubMed] [Google Scholar]
- Rimvall K, Martin DL. The level of GAD67 protein is highly sensitive to small increases in intraneuronal gamma-aminobutyric acid levels. J Neurochem. 1994;62:1375–1381. doi: 10.1046/j.1471-4159.1994.62041375.x. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Morrish DW, Wheler GH, Grace MG. Neonatal encephalopathy: an indicator of early sexual maturation in girls. Pediatr Neurol. 1990;6:102–108. doi: 10.1016/0887-8994(90)90042-y. [DOI] [PubMed] [Google Scholar]
- Shenoy SN, Raja A. Hypothalamic hamartoma with precocious puberty. Pediatr Neurosurg. 2004;40:249–252. doi: 10.1159/000082302. [DOI] [PubMed] [Google Scholar]
- Sim JA, Skynner MJ, Pape JR, Herbison AE. Late postnatal reorganization of GABAA receptor signaling in native GnRH neurons. Eur J Neurosci. 2000;12:3497–3504. doi: 10.1046/j.1460-9568.2000.00261.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Terasawa, E. (1994). In vivo measurement of pulsatile release of neuropeptides and neurotransmitters in rhesus monkeys using push-pull perfusion. In “Methods in Neurosciences: Pulsatility in Neuroendocrine System” (J. E. Levine, Ed.), pp 184–202. Academic Press, New York.
- Terasawa, E. (1995). Mechanisms controlling the onset of puberty in primates: the role of GABAergic neurons. In: “The Neurobiology of Puberty” (T.M. Plant and P.A. Lee, eds), pp. 139–151. Journal of Endocrinology Limited, Bristol.
- Terasawa, E. (2000). The Control of the onset of puberty by neurotransmitters in female rhesus monkeys. In: “The 5th International Conference on the Control of the Onset of Puberty” (J.P. Bourguignon and T.M. Plant, eds), pp.131–143. Elsevier Science, Amsterdam.
- Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. doi: 10.1210/edrv.22.1.0418. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Luchansky LL, Kasuya E, Nyberg CL. An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in female rhesus monkeys. J Neuroendocrinol. 1999;11:275–282. doi: 10.1046/j.1365-2826.1999.00325.x. [DOI] [PubMed] [Google Scholar]
- Terasawa, E., Nass, T. E., Yeoman, R. R., Loose, M. D., and Schultz, N. J. (1983). Hypothalamic control of puberty. In “Neuroendocrine Aspects of Reproduction” (R. L. Norman, Ed.), pp. 149–182. Academic Press, New York.
- Urbanski HF, Ojeda SR. A role for N-methyl-D-aspartate (NMDA) receptors in the control of LH secretion and initiation of female puberty. Endocrinology. 1990;126:1774–1776. doi: 10.1210/endo-126-3-1774. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Rodrigues SM, Garyfallou VT, Kohama SG. Regional distribution of glutamic acid decarboxylase (GAD65 and GAD67) mRNA in the hypothalamus of male rhesus before and after puberty. Mol Brain Res. 1998;57:86–91. doi: 10.1016/s0169-328x(98)00070-9. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Kogelman L, Ghost P, Liljelund P, Blackstone C. Developmental regulation of the hypothalamic metatropic glutamate receptor mGluR1. J Neurosci. 1994;14:3816–3834. doi: 10.1523/JNEUROSCI.14-06-03816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe G, Terasawa E. In vivo release of luteinizing hormone-releasing hormone (LHRH) increases with puberty in the female rhesus monkey Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- Wildt L, Marshall G, Knobil E. Experimental induction of puberty in the infantile rhesus monkey. Science. 1980;207:1373–1375. doi: 10.1126/science.6986658. [DOI] [PubMed] [Google Scholar]
- Wilson RC, Knobil E. Acute effects of N-methyl-DL-aspartate on the release of pituitary gonadotropins and prolactin in the adult female rhesus monkey. Brain Res. 1982;248:177–179. doi: 10.1016/0006-8993(82)91160-x. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FCW, Howe DC, Naylor AM. N-methyl-D-aspartate (NMDA) receptor antagonism by D-2-amino-5-phosphonovaleric acid delays onset of puberty in female rat. J Neuroendocrinol. 1990;2:627–631. doi: 10.1111/j.1365-2826.1990.tb00457.x. [DOI] [PubMed] [Google Scholar]


