Abstract
Dynein interacts with microtubules through an ATP-sensitive linkage mapped to a structurally complex region of the heavy chain following the fourth P-loop motif. Virtually nothing is known regarding how binding affinity is achieved and modulated during ATP hydrolysis. We have performed a detailed dissection of the microtubule contact site, using fragment expression, alanine substitution, and peptide competition. Our work identifies three clusters of amino acids important for the physical contact with microtubules; two of these fall within a region sharing sequence homology with MAP1B, the third in a region just downstream. Amino acid substitutions within any one of these regions can eliminate or weaken microtubule binding (KK3379,80, E3385, K3387, K3397, KK3410,11, W3414, RKK3418–20, F3426, R3464, S3466, and K3467), suggesting that their activities are highly coordinated. A peptide that actively displaces MAP1B from microtubules perturbs dynein binding, supporting previous evidence for similar sites of interaction. We have also identified four amino acids whose substitutions affect release of the motor from the microtubule (E3413, R3444, E3460, and C3469). These suggest that nucleotide-sensitive affinity may be locally controlled at the site of contact. Our work is the first detailed description of dynein–tubulin interactions and provides a framework for understanding how affinity is achieved and modulated.
INTRODUCTION
Dynein is a high-molecular-weight motor protein important for microtubule-based motility in eukaryotic cells (Holzbaur and Vallee, 1994; Hirokawa, 1998). It moves along a tubulin polymer through repetitive binding and release cycles that are tightly coordinated with force generation and nucleotide hydrolysis (Johnson, 1985). The dynein heavy chains (DHCs) contain a highly conserved region just downstream of the fourth P-loop motif that is predicted to encode two extended α-helices (Holzbaur and Vallee, 1994; Mitchell and Brown, 1994). Gee et al. (1997) have proposed that the two α-helices form an antiparallel coiled-coil stalk and that the intervening ∼125 aa form the region that physically contacts the microtubule in an ATP-sensitive manner. Polypeptide fragments containing these regions colocalize with microtubules in transiently transfected eukaryotic cells and cosediment with microtubules when expressed in vitro (Gee et al., 1997; Koonce, 1997). These data support long-standing electron microscopy images showing a slender connection between the globular head and microtubule (Goodenough and Heuser, 1982, 1984).
To probe the details of how dynein interacts with microtubules and how its affinity is coordinated with nucleotide hydrolysis, we have produced a series of alanine substitutions within the contact region. Many of these mutations generate distinct changes in microtubule binding activity. These not only enhance or reduce binding but also affect nucleotide-stimulated release. The distribution of functionally active amino acids reveals the existence of at least three regions within the microtubule-binding domain that act together to bind the motor to its substrate. Two of these regions share sequence homology with MAP1B, supporting previous observations that dynein and fibrous MAPs share similar binding domains (Paschal et al., 1989; Lopez and Sheetz, 1993; Hagiwara et al., 1994). We also show that a peptide that displaces MAP1B (Joly and Purich, 1990) partially perturbs the dynein–microtubule interaction. The third region important for binding does not have any obvious sequence homology with MAPs, suggesting a separate contact site on the tubulin polymer (e.g., Rodionov et al., 1990). Because at least one single-headed dynein can make processive movements along a microtubule (Sakakibara et al., 1999), our work lends structural insight into how dynein could both move along and remain tethered to microtubules.
MATERIALS AND METHODS
Construct Design and Dictyostelium Expression
Most of the in vivo expression work is based on a 380-kDa head domain fragment (aa 1384–4725; Koonce and Samsó, 1996) of the DHC. The fragment is subcloned between the native DHC promoter and the actin 8 terminator sequence, on a plasmid that also contains a G418-selectable marker (Ostrow et al., 1994). Smaller DHC fragments were generated through restriction endonuclease digestion and similarly subcloned into the same host plasmid.
Oligonucleotide-mediated site-directed substitutions were performed using the Stratagene (La Jolla, CA) QuikChange kit on a 675-bp BglII–StyI fragment (aa 3297–3520) that covers the microtubule contact domain. After PCR amplification, the plasmid insert was sequenced to confirm that only the appropriate substitution had been made and then subcloned in two steps into the head domain expression construct (details upon request). The purified plasmid was introduced into Dictyostelium AX-2 cells by Ca2+PO4-mediated transformation. Individual transformants were selected for growth in G418 and cloned as described by Koonce and Samsó (1996). At least three independent clones for each substitution were isolated and characterized.
Microtubule-binding Assay
High speed supernatant (HSS) was prepared in PMEG buffer (100 mM 1,4-piperazinediethanesulfonic acid, 4 mM MgCl2, 5 mM EGTA, 0.1 mM EDTA, 0.9 M glycerol, pH 7.5) as described by Koonce and McIntosh (1990). Typically, paclitaxol-stabilized purified bovine microtubules (0.25 mg/ml final concentration) were added to 3 ml of HSS and incubated for 30 min at room temperature. Microtubules were pelleted at 75,000 × g for 10 min, resuspended in 0.5 ml of buffer, and repelleted through a 0.5-ml 20% sucrose cushion. The washed pellet was resuspended in 50 μl of buffer containing 10 mM MgATP and recentrifuged. The supernatant (ATP extract) was removed, and the final microtubule pellet was suspended in 100 μl of buffer. Aliquots of HSS, microtubule pellet, and ATP extract were separated on a 7.5% low-bis polyacrylamide gel. For UV-vanadate cleavage, HSS was supplemented with 1 mM MgATP and 100 μM vanadate and irradiated with 365 nm light for 1 h on ice. Immunostaining and immunoblotting were as performed by Koonce and McIntosh (1990).
Peptide Synthesis and Use
Peptides were synthesized following standard solid-phase techniques using Fmoc chemistry and an automated synthesizer (model 631; Applied Biosystems, Foster City, CA). Purity was determined by HPLC, and amino acid sequence was confirmed by mass spectrometry. Four sequences were chosen to mimic different regions of the heavy chain or fibrous MAPs. Sequence 1 (KKEIKKEERKELKKEVK) contains four repeat elements of the murine MAP1B microtubule-binding domain (aa 683–699; Noble et al., 1989). Sequence 2 covers the highly conserved non–MAP-like region of the DHC microtubule-binding domain (ETVNRASKACGPLVKW, aa 3460–3475; Koonce et al., 1992). Sequence 3 is a second highly conserved region of the DHC just downstream of the microtubule-binding region (LPSDDLCTENAIMLKRFNRYPLIIDPSGQA, aa 3647–3676). The fourth peptide (m2′: VTSKCGSLKNIRHRPGGGRVK, aa 1705–1725 of mouse MAP2; Lewis et al., 1988) has been characterized in detail to promote microtubule assembly and actively displace fibrous MAPs from microtubules (Joly and Purich, 1990).
Lyophilized peptides were dissolved in water then diluted into PMEG for use. To assess dynein–microtubule binding, peptides were mixed with purified microtubules. After a 15-min preincubation, HSS from wild-type AX-2 cells was added to 1-ml volume and incubated at room temperature for 30 min. The final peptide and microtubule concentrations (2.5 mM, 0.8 mg/ml) were as described by Joly and Purich (1990). Samples were then underlayed with 0.5 ml of 20% sucrose in PMEG and centrifuged at 75,000 × g for 10 min. Pellets were suspended in 50 μl of buffer, mixed with an equal volume of SDS sample buffer, and boiled.
RESULTS
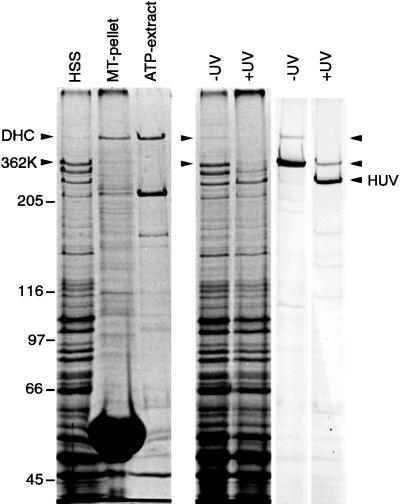
Previously, we have shown that the 380-kDa fragment of the Dictyostelium DHC encodes the monomeric head domain and that it binds to microtubules in an ATP-sensitive manner indistinguishable from the full-length, dimeric molecule (Koonce and Samsó 1996; Samsóet al., 1998). Here, we report that a smaller head domain fragment (362 kDa), lacking the region between the two predicted α-helices (aa 3358–3518), fails to cosediment with microtubules in cellular extracts (Figure 1). Moreover, the fragment retains its ability to UV-vanadate cleave, suggesting that the nucleotide catalytic activity remains intact, and the motor is otherwise properly folded (Gibbons et al., 1987). This is the strongest evidence to date that indicates an intact dynein motor has a single ATP-sensitive microtubule-binding domain. To understand how binding occurs and how affinity is coordinated with nucleotide hydrolysis, we have performed a detailed dissection of this DHC region.
Figure 1.
Coomassie blue-stained gels showing tubulin binding and UV cleavage activity of the native DHC and 362-kDa fragment (362K) lacking the microtubule contact site. The HSS shows that substantially more 362K is expressed relative to the native DHC, but the polypeptide is not detectable in either the pellet or supernatant after incubation, sedimentation, and ATP extraction. The native DHC serves as an internal binding control and is substantially enriched in the ATP extract. The lanes marked UV show aliquots of HSS in the absence (−) and presence (+) of UV irradiation. The left panel shows a Coomassie blue-stained gel; the right panel shows a corresponding immunoblot probed with a rabbit antibody raised against a 62-kDa fragment covering the entire microtubule-binding domain (see Figure 2). Both the native DHC and the 362K fragment nearly completely disappear in the irradiated sample, evidence of efficient photocleavage for both polypeptides.
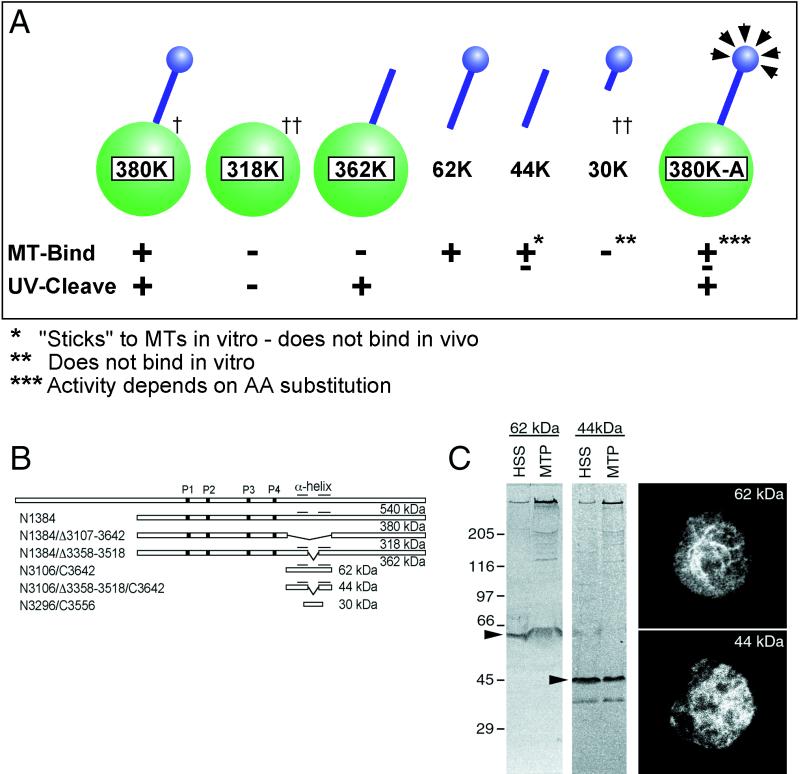
Expression work involving fragments of the microtubule-binding domain from Dictyostelium are summarized in Figure 2. Although these results are generally consistent with recently published mapping data (Gee et al., 1997; Koonce, 1997), they also reveal two important differences. First, we fail to see in vitro microtubule binding of the contact region (30K) alone (data from Koonce, 1997). This argues that the α-helical stalks contain critical determinants of tertiary structure and thus may make important contributions to modulating ATP-sensitive affinity. Second, the stalk structure itself (44K) is capable of ATP-insensitive “sticking” to polymeric tubulin in vitro, but it does not bind microtubules in vivo (Figure 2C). Therefore, activity from fragments expressed out of context of an intact motor domain must be interpreted with caution.
Figure 2.
(A) Summary diagram of microtubule binding for several fragments of the dynein motor domain. The 380K diagrams a complete head; the green represents the motor domain; and the blue represents the stalk and microtubule contact site. The relative positions of these fragments within the DHC are schematically shown in B. †, data from Koonce and Samsó (1996); ††, data from Koonce (1997). (C) In vitro and in vivo microtubule affinity of the 62- and 44-kDa fragments. Both copellet with microtubules as shown on the left immunoblot panels (MTP), probed with the antibody against the 62-kDa fragment. However, only the 62-kDa fragment decorates microtubules in vivo (right). The right panels show two cells fixed and stained with the c-myc antibody, recognizing epitope tags placed on the C terminus of both constructs. Care was taken not to overextract the cells and drive dynein onto the microtubules. Despite the high background, a clear microtubule pattern can be discerned in the 62-kDa–expressing cells, whereas only diffuse cytoplasmic staining can be seen in the 44-kDa–expressing cells.
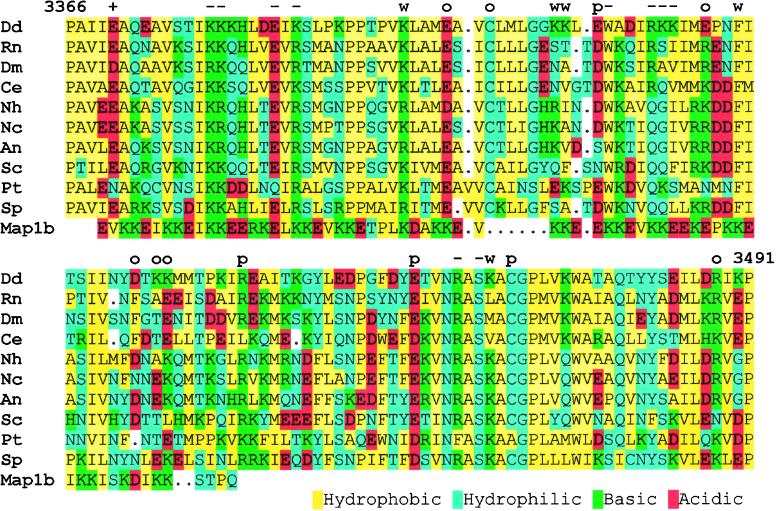
The rationale behind the alanine substitution approach, one that targets single or a few amino acids within the microtubule contact region, is to minimize structural perturbation while analyzing amino acid function in an otherwise complete motor. An alignment of the contact region of several cytoplasmic DHCs is shown in Figure 3 (also see Gee and Vallee, 1998). Overall, the sequences range from 40 to 50% identity with Dictyostelium and include several highly conserved charged positions. Axonemal dyneins are generally less related (25–35% identity) but retain several of the most highly conserved positions. Interestingly, an alignment can also be made in the first half of this region with a portion of the microtubule-binding domain of MAP1B (Noble et al., 1989). The sequence (aa 680–743) is 38% identical, 44% similar to the Dictyostelium DHC. Although dynein does not show the highly repeated motif characteristic of the stable MAP interactions, several of the charged positions we show below as important for the dynein–microtubule interaction are conserved.
Figure 3.
Sequence alignment of the microtubule contact site for several cytoplasmic dyneins and MAP1B. The comparison begins at the conserved proline (aa 3366 in Dd) that is thought to mark the end of the first helical region and ends at proline 3491 that begins the second helical region. The Dictyostelium sequence is shown on top; the characters above mark those residues changed to alanine. +, enhanced binding; −, no binding; w, weak binding; o, no effect; P, poor extraction with ATP. Dd, Dictyostelium discoideum; Rn, Rattus norvegicus; Dm, Drosophila melanogaster; Ce, Caenorhabditis elegans; Nh, Nectria hematococca; Nc, Neurospora crassa; An, Aspergillus nidulans; Sc, Saccharomyces cerevisiae; Pt, Paramecium tetraurelia; Sp, Schizosaccharomyces pombe.
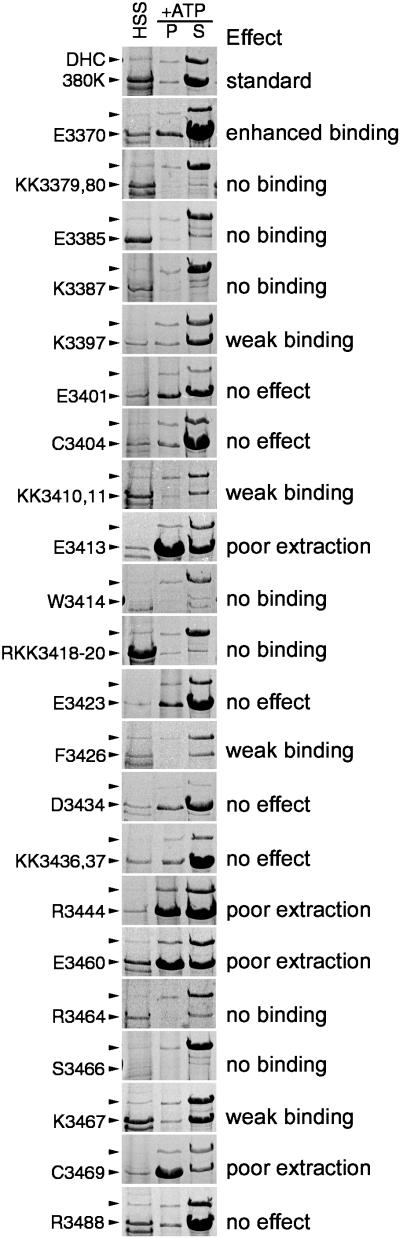
Because transient interactions of kinesin and the more stable binding of structural MAPs with microtubules likely occur through ionic interactions (Mandelkow and Mandelkow, 1995; Woehlke et al., 1997), we were particularly interested in the contributions of the conserved charged residues to microtubule binding. Results of microtubule-binding experiments from 22 different substitutions are shown in Figure 4. These fall into four groups: substitutions that have no significant effect (6), that enhance binding (1), that decrease binding (11), and those that appear to slow nucleotide-stimulated release (4). None of substitutions that decrease binding affect UV-vanadate cleavage (our unpublished results), indicating that the motor's catalytic activity remained intact. Three substitutions resulted in transformed cells that grew poorly (KK3410,11, F3426, and S3466). Their phenotypic changes are under investigation.
Figure 4.
Each block shows a portion of three Coomassie blue-stained gel lanes representing the HSS and the microtubule pellet (P) and supernatant (S) after ATP extraction. The top (380K) panel shows the relative binding of the control, unaltered head domain fragment; the remaining panels are representative of the amino acid substitution listed on the left. Positions of both the native DHC and the 380-kDa head domain fragment are marked with arrowheads. Although the levels of polypeptide overexpression appear to vary between the substitutions, they are generally consistent among clones isolated for each transformation and remain stable over time. In comparing the affinity, the relative amount of the 380 kDa fragment to the native DHC is important. For example, in clone KK3379,80, the altered 380-kDa polypeptide is much more abundant in the HSS but is virtually absent in the ATP extract. Two substitutions showed reproducibly low levels of polypeptide expression (W3414 and S3466). Their pattern of binding was confirmed by immunoblotting.
Of the 11 substitutions that decrease binding, 5 are basic residues within the first half of the domain that are highly conserved among both the DHCs and MAP1B. Of the five changes that enhance binding or perturb release, three are acidic residues. This pattern of acidic and basic residue function is similar to the effects of mutagenesis on the kinesin microtubule-binding domain (Woehlke et al., 1997). Moreover, the positions of inhibitory substitutions reveal at least three clusters of activity important for binding, two within the MAP1B-like region and one just downstream.
Four substitutions in the contact region also appear to affect motor release from the microtubule. In simple pelleting assays, there does not appear to be an increased affinity for the polymer (our unpublished results), rather, just a slow or incomplete ATP-stimulated release. A similar uncoupling of the kinesin ATPase and microtubule-binding domains has also been reported (Song and Endow, 1998). In addition, mutant clones fixed and stained with antibodies reveal a dynein motor domain distribution along cytoplasmic microtubules in vivo (our unpublished results), a result supporting the increased retention seen in vitro. A microtubule pattern is not seen in similarly treated wild-type or unaltered 380K cells.
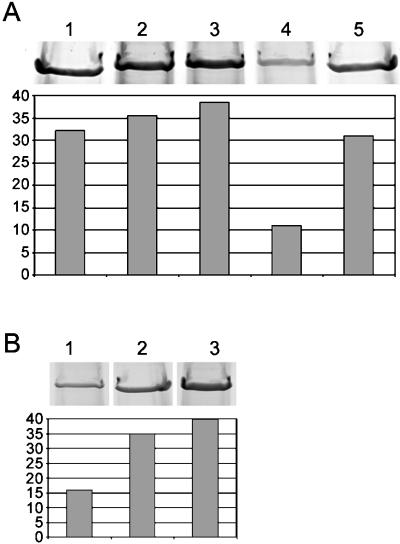
To further address the significance of the MAP1B similarity within this region, we tested four peptides for their ability to interfere with native dynein binding to tubulin. Their results are shown in Figure 5. The first three (two experimental and one control peptide) had no significant effect on dynein–tubulin cosedimentation. Because we know nothing of how these peptides are folded, the results do not conclude that these regions are unimportant for binding. However, the peptides are useful here as controls for nonspecific charge effects. The fourth peptide (m2′) has been previously characterized and shown to bind tubulin and displace both MAP1 and 2 polypeptides (Joly and Purich, 1990). M2′ also has a pronounced effect on the dynein–tubulin interaction (Figure 5). Approximately two-thirds less dynein cosediments with tubulin in the presence of 2.5 mM m2′ peptide than in control experiments. Although at higher concentrations the m2′ peptide became insoluble, a partial effect was seen with lower amounts (Figure 5B). These results strengthen the idea that dynein shares a microtubule-binding domain with fibrous MAPs.
Figure 5.
Microtubule cosedimentation of the DHC in the presence of synthetic peptides. (A) The Coomassie blue-stained portion of the gel containing the DHC band is shown above densitometric quantitation of the amount of DHC present in each lane. Lane 1 contains the repeated MAP1B motif peptide, lane 2 the non–MAP-like DHC sequence, lane 3 the control DHC sequence following the MT-binding region, and lane 4 the m2′ sequence (see MATERIALS AND METHODS). Lane 5 shows a no-peptide control. Only the peptide in lane 4 produces a marked effect on dynein cosedimentation. For densitometry, the pelleted actin band was used to normalize loading. The y-axis measures total pixel density. (B) Similar to A, except the amount of m2′ peptide was varied: 2.5, 0.5, and 0 mM in lanes 1–3, respectively.
DISCUSSION
We provide evidence here that the dynein motor contains a single ATP-sensitive microtubule binding domain and, consistent with previous reports, that it is located within the predicted α-helical region downstream of the fourth P-loop (Gee et al., 1997; Koonce, 1997). Furthermore, the microtubule-binding activity of fragments of this domain expressed both in vitro and in vivo suggests that this region is structurally complex, a physical property that might be predicted for an activity delicately regulated by nucleotide hydrolysis. To minimize structural perturbations, we have targeted many of the conserved residues within the contact site for alanine substitution and have analyzed their effect on microtubule binding in the context of a complete head. In the absence of an atomic structure, it is not possible to determine which residues are solvent exposed and thus likely to make physical contact with the microtubule and which ones contribute to the domain's structural organization. Nonetheless, our work highlights at least three clusters of functional activity within this domain that are particularly sensitive to alanine substitutions; two within a MAP1B-like region in the first half of the sequence and a third in a smaller, highly conserved ∼15-aa region downstream (NRASKAC). Changes in any one of these regions can have a dramatic effect on microtubule binding, whereas modifications in the less conserved areas are less obvious.
Several substitutions in the contact region also affect nucleotide-stimulated dynein release. This suggests that we are impacting a coupling mechanism between nucleotide hydrolysis and affinity, and that there are structural changes at the tip that modulate binding. This could argue that affinity is locally controlled at the site of contact and that structural information is transmitted from the nucleotide-binding pocket through the predicted coiled-coil domain. Although only subtle changes would be tolerated by a coiled coil, the strategy is not without precedent. The primary dimer contact of DNA topoisomerase II lies at one end of an anti-parallel coiled coil (Berger et al., 1996). Dimerization activity (e.g., binding) is regulated through an ATPase domain located at the other end of the molecule. Perhaps similar, nucleotide-sensitive binding strategies have been adopted by these two different proteins.
The sequence similarities in this domain to MAP1B are particularly interesting in light of several reports that indicate fibrous MAPs (MAP1, 2, and tau) and motors (dynein and kinesin) compete for the same binding region on the tubulin polymer (Paschal et al., 1989; Hagiwara et al., 1994; Trinczek et al., 1999). Although steric hindrance is likely a contributing factor (Lopez and Sheetz, 1993), it is also clear that the MAP–microtubule-binding domains alone can inhibit dynein interactions. Subtilisin-cleaved microtubules that do not bind MAP1 and 2 also show a significantly reduced ability to stimulate dynein's ATPase activity, suggesting a common interaction site on the tubulin polymer (Paschal et al., 1989). Our sequence comparison, substitution data, and peptide competition work reported here provide direct molecular support for a MAP-like dynein–tubulin interaction.
However, similar subtilisin-treated microtubules retain the ability to bind dynein in vitro (Rodionov et al., 1990). Although this may seem to contradict a common MAP–dynein-binding site, it could also suggest a second, distinct mechanism important for dynein–tubulin interactions (a possibility mentioned by Paschal et al., 1989). Indeed, several in situ structural studies have indicated that axonemal dynein remains physically tethered to a microtubule throughout its catalytic cycle, even in the presence of ATP-vanadate, a treatment that should act to release the motor (summarized by Goodenough and Heuser, 1989). These observations are supported by biochemical evidence for both strong and weak binding states (e.g., Vale et al., 1989) as well as a recent demonstration that a single-headed dynein can make processive movements along a microtubule (Sakakibara et al., 1999). Because dynein has a low duty ratio, this indicates that it must somehow remain bound to the microtubule during multiple rounds of ATP hydrolysis. Similar activities have also been noted for a single-headed kinesin (Okada and Hirokawa, 1999). Our substitution results have identified a highly conserved sequence outside of the MAP-like region that is also important for microtubule binding. This strengthens the possibility of at least two functional regions within the dynein–microtubule-binding domain, one that is MAP1B-like and one that is unique. Although both appear essential for tubulin binding, it is possible that they contribute to different parts of the interaction cycle and may account for the strong and weak binding states. Further correlation among mutant analysis, binding, and ATPase activity is in progress and should help determine the contributions of each region for dynein–microtubule binding.
ACKNOWLEDGMENTS
We thank Drs. Alexey Khodjakov, Susan Baxter, Patrick Van Roey, and Andrea Habura for helpful discussions. We also gratefully acknowledge the efforts of Tim Moran, Angelo Lobo, and Jim Seeger of the Wadsworth Center Core Facilities in performing the DNA sequencing, oligonucleotide and peptide synthesis. This work was supported in part by National Institutes of Health grant GM-51532.
REFERENCES
- Berger JM, Gamblin SJ, Harrison SC, Wang JC. Structure and mechanism of DNA topoisomerase II. Nature. 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- Gee M, Vallee R. The role of the dynein stalk in cytoplasmic and flagellar motility. Eur Biophys J. 1998;27:466–473. doi: 10.1007/s002490050157. [DOI] [PubMed] [Google Scholar]
- Gibbons IR, Lee-Eiford A, Mocz G, Phillipson A, Tang W-JY, Gibbons BH. Photosensitized cleavage of dynein heavy chains. J Biol Chem. 1987;262:2780–2786. [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. Substructure of the outer dynein arm. J Cell Biol. 1982;95:798–815. doi: 10.1083/jcb.95.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. Structural comparison of purified dynein proteins with in situ dynein arms. J Mol Biol. 1984;180:1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. Structure of the soluble and in situ ciliary dyneins visualized by quick-freeze deep-etch microscopy. In: Warner FD, Satir P, Gibbons IR, editors. Cell Movement, vol. 1, The Dynein ATPases. New York: Alan R. Liss; 1989. pp. 121–140. [Google Scholar]
- Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. Competition between motor molecules (kinesin and cytoplasmic dynein) and fibrous microtubule-associated proteins in binding to microtubules. J Biol Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- Holzbaur ELF, Vallee RB. Dyneins: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Johnson KA. Pathway of the microtubule-dynein ATPase and the structure of dynein: a comparison with myosin. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- Joly JC, Purich DL. Peptides corresponding to the second repeated sequence in MAP-2 inhibit binding of microtubule-associated proteins to microtubules. Biochemistry. 1990;29:8916–8920. doi: 10.1021/bi00490a006. [DOI] [PubMed] [Google Scholar]
- Koonce MP. Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. J Biol Chem. 1997;272:19714–19718. doi: 10.1074/jbc.272.32.19714. [DOI] [PubMed] [Google Scholar]
- Koonce MP, Grissom PG, McIntosh JR. Dynein from Dictyostelium: primary structure comparison between a cytoplasmic motor enzyme and flagellar dynein. J Cell Biol. 1992;119:1597–1604. doi: 10.1083/jcb.119.6.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonce MP, McIntosh JR. Identification and immunolocalization of cytoplasmic dynein in Dictyostelium. Cell Motil Cytoskeleton. 1990;15:51–62. doi: 10.1002/cm.970150108. [DOI] [PubMed] [Google Scholar]
- Koonce MP, Samsó M. Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol Biol Cell. 1996;7:935–948. doi: 10.1091/mbc.7.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Wang DH, Cowan NJ. Microtubule-associated protein MAP2 shares a microtubule binding motif with tau protein. Science. 1988;242:936–939. doi: 10.1126/science.3142041. [DOI] [PubMed] [Google Scholar]
- Lopez LA, Sheetz MP. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil Cytoskeleton. 1993;24:1–16. doi: 10.1002/cm.970240102. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow EM. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Brown KS. Sequence analysis of the Chlamydomonas alpha and beta dynein heavy chain genes. J Cell Sci. 1994;107:635–644. doi: 10.1242/jcs.107.3.635. [DOI] [PubMed] [Google Scholar]
- Noble M, Lewis SA, Cowan NJ. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J Cell Biol. 1989;109:3367–3376. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Hirokawa N. A processive single-headed motor: kinesin superfamily protein KIF1A. Science. 1999;283:1152–1157. doi: 10.1126/science.283.5405.1152. [DOI] [PubMed] [Google Scholar]
- Ostrow BD, Chen P, Chisholm R. Expression of a myosin regulatory light chain phosphorylation site mutant complements the cytokinesis and developmental defects of Dictyostelium RMLC null cells. J Cell Biol. 1994;127:1945–1955. doi: 10.1083/jcb.127.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Obar RA, Vallee RB. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature. 1989;342:569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Gyoeva FK, Kashina AS, Kuznetsov SA, Gelfand VI. Microtubule-associated proteins and microtubule-based translocators have different binding sites on tubulin molecule. J Biol Chem. 1990;265:5702–5707. [PubMed] [Google Scholar]
- Sakakibara H, Kojima H, Sakai Y, Katayama E, Oiwa K. Inner-arm dynein c of Chlamydomonas flagella is a single-headed processive motor. Nature. 1999;400:586–590. doi: 10.1038/23066. [DOI] [PubMed] [Google Scholar]
- Samsó M, Radermacher M, Frank J, Koonce MP. Structural characterization of a dynein motor domain. J Mol Biol. 1998;276:927–937. doi: 10.1006/jmbi.1997.1584. [DOI] [PubMed] [Google Scholar]
- Song H, Endow SA. Decoupling of nucleotide- and microtubule-binding sites in a kinesin mutant. Nature. 1998;396:587–590. doi: 10.1038/25153. [DOI] [PubMed] [Google Scholar]
- Trinczek B, Ebneth A, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112:2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]
- Vale RD, Soll DR, Gibbons IR. One-dimensional diffusion of microtubules bound to flagellar dynein. Cell. 1989;59:915–925. doi: 10.1016/0092-8674(89)90614-4. [DOI] [PubMed] [Google Scholar]
- Woehlke G, Ruby AK, Hart CL, Ly B, Hom-Booher N, Vale RD. Microtubule interaction site of the kinesin motor. Cell. 1997;90:207–216. doi: 10.1016/s0092-8674(00)80329-3. [DOI] [PubMed] [Google Scholar]