Abstract
Molecular transport in avascular collagenous tissues such as articular cartilage occurs primarily via diffusion. The presence of ordered structures in the extracellular matrix may influence the local transport of macromolecules, leading to anisotropic diffusion depending on the relative size of the molecule and that of extracellular matrix structures. Here we present what we believe is a novel photobleaching technique for measuring the anisotropic diffusivity of macromolecules in collagenous tissues. We hypothesized that macromolecular diffusion is anisotropic in collagenous tissues, depending on molecular size and the local organization of the collagen structure. A theoretical model and experimental protocol for fluorescence imaging of continuous point photobleaching was developed to measure diffusional anisotropy. Significant anisotropy was observed in highly ordered collagenous tissues such as ligament, with diffusivity ratios >2 along the fiber direction compared to the perpendicular direction. In less-ordered tissues such as articular cartilage, diffusional anisotropy was dependent on site in the tissue and size of the diffusing molecule. Anisotropic diffusion was also dependent on the size of the diffusing molecule, with greatest anisotropy observed for larger molecules. These findings suggest that diffusional transport of macromolecules is anisotropic in collagenous tissues, with higher rates of diffusion along primary orientation of collagen fibers.
INTRODUCTION
Diffusive transport through the extracellular matrix is crucial to the healthy function of most tissues, and in particular, avascular connective tissues such as cartilage or ligament. Solute diffusion, however, is impeded by the presence of the structural macromolecules, such as collagen and proteoglycans, that comprise the extracellular matrix (1–8). Thus the apparent diffusion coefficient of molecules can vary with site (inhomogeneity) (2,7,9) or with direction (i.e., anisotropy) within a material or tissue. Previous theoretical models have suggested that an oriented fibrous microstructure can lead to more rapid diffusion parallel to the primary fiber direction (10–13). Magnetic resonance imaging techniques have been used to demonstrate diffusional anisotropy of small molecules such as ATP and phosphocreatine in muscle (14), as well as water in nerves (15), brain (16), cardiac muscle (17), skeletal muscle (18), tendon (19), and intervertebral disc (20). In addition, diffusional anisotropy at the cellular level has been examined with fluorescence techniques such as fluorescence correlation spectroscopy (21,22) or photobleaching (23,24). However, little is known regarding the potential anisotropic diffusion of macromolecules through the extracellular matrix of hydrated, collagenous tissues.
Most connective tissues such as articular cartilage, ligament, fascia, or intervertebral disk exhibit a highly oriented and site-specific collagenous microstructure that also appears to influence the structure and distribution of cells within the tissue. For example, articular cartilage possesses a stratified structure whereby collagen fibers in the superficial zone are closely packed and are oriented parallel to the surface (25,26). In addition, the surface of the tissue exhibits a preferential direction for orientation of the collagen fibers, termed the “split line direction” based on the observation that the surface defect preferentially splits in an anisotropic format (27,28). The deep zone collagen fibers are oriented perpendicular to the surface, but they are large and relatively widely spaced, making diffusional anisotropy less likely (25,26).
In this study, we introduce what to our knowledge is a new technique termed fluorescence imaging of continuous point photobleaching (FICOPP) to quantify site-specific diffusional anisotropy in connective tissues or other anisotropic materials. Based on a theoretical analysis of anisotropic macromolecular diffusion, an experimental protocol for FICOPP was developed and applied to determine site-specific anisotropy in the diffusion properties of fluorescently labeled dextran molecules in specimens of cartilage and ligament.
METHODS
The principle of the FICOPP method is based on continuous photobleaching (microphotolysis) of a fluorescently labeled solute. Photobleaching is performed by a diffraction-limited, intense laser focused on a single point within the specimen. Over time, the bleached spot expands at a rate dependent on the diffusion coefficient. If the diffusion coefficient is anisotropic, the spot will expand at different rates in each principal direction, revealing the extent of diffusional anisotropy in the tissue.
Model development
A mathematical model of the FICOPP process was developed to relate the ratio of the radii of a spot, centered at the origin, to the ratio of the diffusion coefficients. The experiment can be described mathematically by solving the following diffusion equation in the x,y plane,
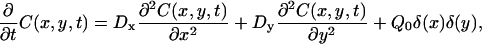 |
(1) |
where C is the concentration of bleached or unbleached fluorophores, t is time, Dx is the diffusion coefficient in the x direction, Dy is the diffusion coefficient in the y direction, and Qo is the bleaching power of the laser. It is assumed that an infinite supply of freely diffusing, fluorescent molecules is available at the sample boundaries and that, before photobleaching, the initial concentration is uniform. The solution to this problem is given by
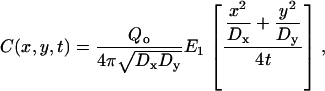 |
(2) |
where 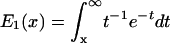 is the exponential integral function.
is the exponential integral function.
For the reduced case of isotropic diffusion (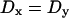 ), this solution predicts a circular bleached spot with increasing diameter proportional to
), this solution predicts a circular bleached spot with increasing diameter proportional to 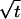 , and that the rate of increase in size of the spot will be a function of the diffusion coefficient. With diffusional anisotropy (
, and that the rate of increase in size of the spot will be a function of the diffusion coefficient. With diffusional anisotropy ( ), the model predicts a bleach spot that is elliptical with the long axis being in the direction of the larger diffusion coefficient (Fig. 1). At fixed time t*,
), the model predicts a bleach spot that is elliptical with the long axis being in the direction of the larger diffusion coefficient (Fig. 1). At fixed time t*,
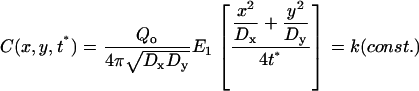 |
(3) |
when
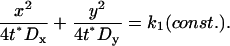 |
(4) |
This solution is represented by an ellipse, which can be written in standard form as
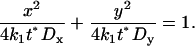 |
(5) |
Hence the semi-axes of the ellipse are
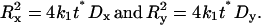 |
(6) |
Thus, all intensity contours of the bleached image form ellipses with increasing radii proportional to 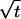 , where the ratio of the radii squared is equal to the ratio of the diffusion coefficients
, where the ratio of the radii squared is equal to the ratio of the diffusion coefficients
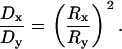 |
(7) |
FIGURE 1.
Model predictions of effects of anisotropy on size of bleached spot over time. The spot radius is greater in the direction of the higher diffusion coefficient.
The nondimensional model (Eq. 7) holds independent of time, the particular intensity level at which the elliptical image contour is measured, and the bleaching power, which is assumed to be independent of direction. This finding implies that a single measurement of the ratio of the major axes of the elliptical bleached region can reveal the extent of diffusional anisotropy. Furthermore, the radii can be measured and averaged over time to decrease the noise/error in the signal. Thus, the model can be used to translate measured ratios of radii into ratios of diffusion coefficients.
To test the effects of image noise on the measured anisotropy, various levels of Gaussian noise were added to the simulated images, and the error introduced into the ratio of diffusion coefficients was calculated.
Measuring diffusional anisotropy in collagenous tissues
Diffusional anisotropy was measured in agarose gel, ligament, and articular cartilage tissue, which show a wide range of fibrous alignment and mechanical anisotropy. Disks of 3% agarose gel, 5 mm in diameter, were allowed to soak in FITC-conjugated 3 kDa or 500 kDa dextran for 24 h. Lateral collateral ligaments were dissected from porcine knee joints and allowed to soak in 3 kDa or 500 kDa dextran solution at 4°C for 3 days. FICOPP experiments were performed in a plane parallel to the presumed fiber orientation for collagenous tissues and in random orthogonal directions for the agarose. In tissues, all measurements were taken at sites that were several cell diameters away from any cell.
All experiments were performed on a Zeiss 510 laser scanning confocal microscope using a 15 mW Argon laser with a 63×/1.2 numerical aperture objective, with a 166 μm pinhole, which yielded a 1.2 μm optical slice. This high numerical aperture objective does produce a nonuniform bleaching distribution in the z direction; however, diffusion in and out of the imaging plane may increase the radius measurements but did not change the ratio of the radii. A single, central pixel (0.29 μm × 0.29 μm) was set as the bleach region. Experiments were performed ∼25 μm in from the surface of the tissue to avoid the cut surface. Six images (512 × 512 pixels, 0.29 μm/pixel) were taken every 28.3 s. Images took 4 s to acquire, thus the imaging time was very short relative to the bleaching time. In preliminary studies, we performed six separate experiments at the same spot where a single image was taken at the end of experiments that were run for different lengths of time. These experiments yielded the same anisotropy ratios as those determined from images during the course of the experiment, which suggests that the imaging time did not significantly affect the results.
The same laser was used for bleaching (at 100% power) and imaging (at 1% power). No significant bleaching occurred during the imaging phase as the background intensity did not vary significantly during the course of the experiment. An initial prebleach image was taken and subtracted from all subsequent images to clarify the bleached region from background patterns. All images were smoothed with a 3 × 3 median filter to reduce noise. Radii of the bleached spot were measured at the point where the intensity fell to e−2 of the initial value. The radii were measured at the two central rows and columns in all four directions and averaged in the two orthogonal directions. The ratio of the radii was then calculated. This ratio was measured in each of the six images and averaged for a single ratio.
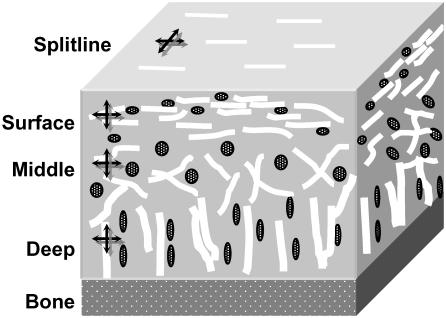
In additional experiments, full thickness cartilage explants were removed from the femoral condyles of pigs and incubated in concentrated solutions of 3 or 500 kDa FITC-dextrans. FICOPP experiments were performed perpendicular to the cut, full-thickness surface in the surface, middle, and deep zones (Fig. 2). Fiber orientation was determined from the surface direction, and zone was determined by cell morphology. In addition, for split line specimens, the surface of the cartilage was pricked with a pin dipped in India ink, and a small explant with the split line intact was removed. FICOPP experiments were performed on the surface with split line sitting on the coverslip, adjacent to the known split line direction (Fig. 2). Analyses were performed as in the previous section.
FIGURE 2.
Schematic of cartilage showing collagen orientation and cell shape in the three zones in the thickness direction and on the surface in the split line direction (black line shows pin prick; white lines show collagen fibers). The crossed arrows show the direction of anisotropy measurements in each region.
Statistical analysis
For material where diffusivity is faster in the primary fiber direction, the diffusivity ratio should be >1. Paired t-tests were used to determine whether the diffusivity ratios (parallel versus perpendicular to the primary fiber direction) were significantly different from unity. As the Han and Herzfeld model (10) predicts that diffusional anisotropy should be greater with a larger molecule, the effect of molecular size on the degree of anisotropy was assessed by comparing the diffusivity ratios obtained with the 3 and 500 kDa dextrans with a t-test.
RESULTS
Model testing and validation
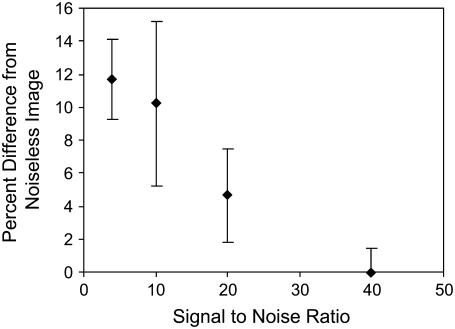
Theoretical modeling of anisotropic diffusion predicts that photobleaching of a spot creates an elliptical bleach pattern with the major axis in the direction of the higher diffusion coefficient (Fig. 1). The addition of Gaussian noise to simulated images showed that whereas the percent error increased with increasing noise levels (decreasing signal/noise ratio) (Fig. 3), the maximum error, even in very noisy images, was only 15%, which suggests that this technique is relatively robust even for relatively poor quality images. For the low signal/noise ratio images, this technique tended to underestimate the degree of anisotropy. Thus, the technique may be slightly biased toward underestimating anisotropy when high levels of noise are present in the images. In addition, agarose showed no significant anisotropy, with all diffusivity ratios being not significantly different from one and exhibiting approximately circular photobleached spots (Fig. 4). No effect of molecular size was detected on the photobleached pattern.
FIGURE 3.
Percent difference in measured anisotropy decreases as signal/noise ratio increases, but even with relatively noisy images error is only ∼12% (mean ± SE).
FIGURE 4.
(A) Images of photobleached spots from agarose and ligament after 170 s of bleaching. Fiber direction in ligament is vertical. (B) Diffusivity ratio parallel and perpendicular to the primary fiber direction for agarose gel and ligament (mean + SE). (*) indicates a mean significantly different from 1 (p < 0.01); (#) indicates 500 kDa mean is significantly >3 kDa mean (p < 0.05).
Anisotropy in collagenous tissues
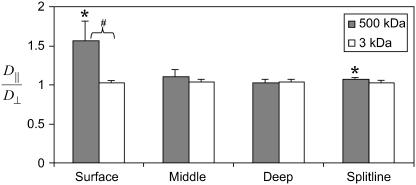
The diffusivity ratios for ligament were significantly >1 for both the 3 and 500 kDa dextrans, indicating significant anisotropy of the diffusion coefficients (Fig. 4). The photobleached spots were generally elliptical. The diffusivity ratio for 500 kDa, however, was significantly greater than that of 3 kDa, indicating a significant effect of molecular size.
In articular cartilage, the diffusivity ratio in the superficial zone was significantly >1 with the 500 kDa dextran, but not with the 3 kDa dextran (Fig. 5). In addition, the diffusivity ratio of the 500 kDa dextran was significantly greater than that of the 3 kDa dextran, indicating that there is significant diffusional anisotropy in the superficial zone for very large molecules, but not for smaller ones. In both the middle and deep zones, the diffusivity ratios for both 3 and 500 kDa were not significantly different from one or from each other. With respect to the split line direction of the superficial zone, the 500 kDa dextran had a diffusivity ratio that was slightly >1 (p < 0.005); however, no anisotropy was detected for the 3 kDa dextran, nor was there a significant difference between the 3 and 500 kDa dextrans.
FIGURE 5.
Diffusivity ratio parallel and perpendicular to the primary fiber direction for cartilage zones (mean + SE). (*) indicates a mean significantly different from 1 (p < 0.05); (#) indicates 500 kDa mean is significantly >3 kDa mean (p < 0.05).
DISCUSSION
We have applied what we believe is a new microscopy imaging technique, FICOPP, to demonstrate the presence of significant anisotropy in the diffusion coefficients of macromolecules in oriented collagenous tissues. Diffusion coefficients were significantly greater in a direction parallel to the collagen fibers, and diffusion anisotropy was greater at higher molecular weights. No anisotropy was observed in agarose gel, which was expected to be isotropic and homogeneous. Our experimental findings are consistent with a previous theoretical model (10) that predicts an anisotropic diffusivity ratio of ∼3 for a molecule of similar size to the fiber diameter of the matrix, and a fiber volume fraction of 0.6, as would be the case for 500 kDa dextran in ligament. Our measured value of 2.2 is slightly lower than this prediction, but given that the ligament is not solely comprised of perfectly oriented fibers, a slightly lower value might be expected. The model also predicts a dramatic decrease in anisotropy as the size of the diffusing molecule decreases relative to the fibers through which it moves, consistent with our observations on the 3 kDa dextran as compared to the 500 kDa dextran. However, the technique may be slightly biased toward underestimating anisotropy when high levels of noise are present in the images. This technique may also underestimate anisotropy when measurements are made too close to the surface of the tissue, due to changes in the boundary conditions at the tissue edge. Ultimately, the maximum penetration distance from the cut edge will depend on the power of the laser and the working distance of the objective to allow bleaching and confocal imaging of the region of interest.
An important point in the application of this method is that FICOPP allows measurement of the anisotropy without quantification of the individual diffusion coefficients. This point represents both an advantage and a potential disadvantage, as the measurement process is easier but does not provide absolute values for the diffusion coefficients in different directions, only their ratio in principal directions. We have used FICOPP to examine diffusional anisotropy in a structure with known fiber directions where we are interested in transport across a broad zone. FICOPP can also be used to examine how closely diffusional anisotropy correlates with fiber direction by measuring the direction of the long axis of the ellipse relative to fiber direction. Alternatively, this method could be used to determine the principal directions of the diffusion tensor, which presumably are aligned with the local fiber orientation in the tissue. However, it is important to note that the anisotropy measurements are made within a single plane, and therefore three-dimensional diffusion anisotropy cannot be determined using this method.
Articular cartilage is a highly stratified tissue that exhibits significant collagen fiber orientation in the superficial-most and deep-most zones of the tissue, with decreased fiber orientation in the middle zone of the tissue. Consistent with this structure, we observed significant diffusional anisotropy of 500 kDa dextran in the superficial zone, but not in the middle zone. By the Han and Herzfeld model (10), the 500 kDa dextran would exhibit little interaction with the widely spaced collagen fibers in the deep zone (200 nm apart (29)), whereas the 3 kDa dextran is too small to interact strongly with the collagen fibers, even in the superficial zone (60 nm apart (29)). Thus, these molecules may not be expected to show significant diffusional anisotropy in these settings.
The presence of such diffusional anisotropy appears to reflect the underlying collagenous structure of the tissue. Although the overall implications of this property are not clear, anisotropic diffusion properties may have significant implications on the transport of matrix macromolecules or solutes. The size-dependence of diffusional anisotropy may allow nutrients and other small molecules to move easily into cartilage from the synovial fluid, but prevent diffusion of larger structural molecules out of the cartilage. In addition, it is likely that compression, which has been shown to decrease overall diffusivity (30), could further increase the anisotropy by increasing the packing and alignment of superficial zone collagen fibers (31,32). Conversely, loss or degradation of the superficial zone as occurs with osteoarthritis could eradicate this diffusional anisotropy, allowing further loss of structural molecules from the cartilage surface.
The molecules used for this study were uncharged, inert dextrans. The large, 500 kDa molecule is similar in size to some matrix macromolecules such as hyaluronic acid, cartilage oligomeric protein, or fibronectin, whereas the small dextran, 3 kDa, is similar in size to signaling molecules such as insulin or epidermal growth factor (33,34). A charged or bioactive molecule that interacts directly with the matrix or with cells could have different diffusion characteristics. The dextran molecules are also linear, and a molecule with a more globular configuration may also have different diffusive characteristics (35).
Using other techniques, there have been limited reports of anisotropic diffusion in cells. In neurons, the diffusion of a 10 kDa protein has been found to be faster along the length of the axon rather than across the axon (21), likely due to the dense microtubules oriented parallel to the length of the axon. On the cell surface, diffusion in the cell membrane has been shown to be anisotropic over oriented stress fibers of the cytoskeleton (23). This phenomenon could facilitate formation of membrane signaling complexes, such as focal adhesions, in a manner analogous to the formation of lipid rafts (36). In muscle, diffusion of ATP and phosphocreatine is faster along muscle fibers, but the need for these molecules is to move radially, across the muscle fiber (14). At a larger tissue scale, the formation of stripe patterns on fishes, crucial for finding a mate or hiding from predators, are thought to form from anisotropic diffusion, possibly due to the structure of the scales (37,38). Measurements of diffusional anisotropy at a tissue scale (19,20) have largely focused on the movement of water, which interacts differently with collagen fibers than larger molecules because the water can move within the fibers.
In summary, FICOPP provides a relatively straightforward and robust method for measuring the diffusional anisotropy of molecules. The method can be applied on a standard confocal laser scanning microscope, and the diffusion of any fluorescently labeled macromolecule can be determined. This method has potential implications for the study of diffusional transport at the tissue level, and may provide further insight on the role of molecular structure in governing solute diffusion.
Acknowledgments
This study was supported by the American Association of University Women, National Institutes of Health (AG15768, AR48182, AR50245, and GM08555), National Aeronautics and Space Administration (NNJ04HC72G), the Whitaker Foundation (RG-020933), and the National Science Foundation (DMS-0211154).
References
- 1.Bursac, P. M., L. E. Freed, R. J. Biron, and G. Vunjak-Novakovic. 1996. Mass transfer studies of tissue engineered cartilage. Tissue Eng. 2:141–150. [DOI] [PubMed] [Google Scholar]
- 2.Leddy, H. A., and F. Guilak. 2003. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31:753–760. [DOI] [PubMed] [Google Scholar]
- 3.Maroudas, A. 1976. Transport of solutes through cartilage: permeability to large molecules. J. Anat. 122:335–347. [PMC free article] [PubMed] [Google Scholar]
- 4.Maroudas, A. 1970. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 10:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torzilli, P. A. 1993. Effects of temperature, concentration and articular surface removal on transient solute diffusion in articular cartilage. Med. Biol. Eng. Comput. 31:S93–S98. [DOI] [PubMed] [Google Scholar]
- 6.Torzilli, P. A., T. C. Adams, and R. J. Mis. 1987. Transient solute diffusion in articular cartilage. J. Biomech. 20:203–214. [DOI] [PubMed] [Google Scholar]
- 7.Torzilli, P. A., J. M. Arduino, J. D. Gregory, and M. Bansal. 1997. Effect of proteoglycan removal on solute mobility in articular cartilage. J. Biomech. 30:895–902. [DOI] [PubMed] [Google Scholar]
- 8.Mauck, R. L., C. T. Hung, and G. A. Ateshian. 2003. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J. Biomech. Eng. 125:602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maroudas, A. 1979. Physicochemical properties of articular cartilage. In Adult Articular Cartilage. M. A. R. Freeman, editor. Pitman Medical, Kent, UK. 215–290.
- 10.Han, J., and J. Herzfeld. 1993. Macromolecular diffusion in crowded solutions. Biophys. J. 65:1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clague, D. S., and R. J. Phillips. 1996. Hindered diffusion of spherical macromolecules through dilute fibrous media. Phys. Fluids. 8:1720–1731. [Google Scholar]
- 12.Powles, J. G., M. J. D. Mallett, G. Rickayzen, and W. A. B. Evans. 1992. Exact analytic solutions for diffusion impeded by an infinite array of partially permeable barriers. Proc. Mathematical and Physical Sciences. 436A:391–403. [Google Scholar]
- 13.Rigby, S. 2000. Macroscopic diffusional anisotropy in porous media. Chaos Solitons Fractals. 11:1297–1301. [Google Scholar]
- 14.de Graaf, R. A., A. van Kranenburg, and K. Nicolay. 2000. In vivo (31)P-NMR diffusion spectroscopy of ATP and phosphocreatine in rat skeletal muscle. Biophys. J. 78:1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiltunen, J., T. Suortti, S. Arvela, M. Seppa, R. Joensuu, and R. Hari. 2005. Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin. Neurophysiol. 116:2315–2323. [DOI] [PubMed] [Google Scholar]
- 16.Parker, G. J. 2004. Analysis of MR diffusion weighted images. Br. J. Radiol. 77 Spec. No. 2:S176–185. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Y., K. Pandya, O. Smithies, and E. W. Hsu. 2004. Three-dimensional diffusion tensor microscopy of fixed mouse hearts. Magn. Reson. Med. 52:453–460. [DOI] [PubMed] [Google Scholar]
- 18.Bonny, J. M., and J. P. Renou. 2002. Water diffusion features as indicators of muscle structure ex vivo. Magn. Reson. Imaging. 20:395–400. [DOI] [PubMed] [Google Scholar]
- 19.Han, S., S. J. Gemmell, K. G. Helmer, P. Grigg, J. W. Wellen, A. H. Hoffman, and C. H. Sotak. 2000. Changes in ADC caused by tensile loading of rabbit Achilles tendon: evidence for water transport. J. Magn. Reson. 144:217–227. [DOI] [PubMed] [Google Scholar]
- 20.Hsu, E. W., and L. A. Setton. 1999. Diffusion tensor microscopy of the intervertebral disc anulus fibrosus. Magn. Reson. Med. 41:992–999. [DOI] [PubMed] [Google Scholar]
- 21.Gennerich, A., and D. Schild. 2002. Anisotropic diffusion in mitral cell dendrites revealed by fluorescence correlation spectroscopy. Biophys. J. 83:510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gennerich, A., and D. Schild. 2000. Fluorescence correlation spectroscopy in small cytosolic compartments depends critically on the diffusion model used. Biophys. J. 79:3294–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, B. A., W. R. Clark, and H. M. McConnell. 1979. Anisotropic molecular motion on cell surfaces. Proc. Natl. Acad. Sci. USA. 76:5641–5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsay, T. T., and K. A. Jacobson. 1991. Spatial Fourier analysis of video photobleaching measurements. Principles and optimization. Biophys. J. 60:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang, W. S., B. Li, L. H. Jin, K. Ngo, N. S. Schachar, and G. N. Hughes. 1992. Collagen fibril structure of normal, aging, and osteoarthritic cartilage. J. Pathol. 167:425–433. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery, A. K., G. W. Blunn, C. W. Archer, and G. Bentley. 1991. Three-dimensional collagen architecture in bovine articular cartilage. J. Bone Joint Surg. Br. 73:795–801. [DOI] [PubMed] [Google Scholar]
- 27.Kamalanathan, S., and N. D. Broom. 1993. The biomechanical ambiguity of the articular surface. J. Anat. 183:567–578. [PMC free article] [PubMed] [Google Scholar]
- 28.Meachim, G., D. Denham, I. H. Emery, and P. H. Wilkinson. 1974. Collagen alignments and artificial splits at the surface of human articular cartilage. J. Anat. 118:101–118. [PMC free article] [PubMed] [Google Scholar]
- 29.Comper, W. D. 1991. Physicochemical aspects of cartilage extracellular matrix. In Cartilage: Molecular Aspects. B. Hall and S. Newman, editors. CRC Press, Boston. 59–96.
- 30.Quinn, T. M., P. Kocian, and J. J. Meister. 2000. Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch. Biochem. Biophys. 384:327–334. [DOI] [PubMed] [Google Scholar]
- 31.Schmid, T. M., J. Li, C. Muehleman, M. Wimmer, and T. C. Irving. 2004. Cartilage compression changes collagen fiber orientation as measured by small angle x-ray diffraction. Trans. Ortho. Res. Soc. 29:0230. [Google Scholar]
- 32.Xia, Y., and H. A. Alhadlaq. 2004. Response of collagen fibrils to compression in individual histological zones in articular cartilage. Trans. Ortho. Res. Soc. 29:0052.
- 33.Heinegard, D., and A. Oldberg. 1989. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 3:2042–2051. [DOI] [PubMed] [Google Scholar]
- 34.Seyedin, S. M., and D. M. Rosen. 1991. Cartilage growth and differentiation factors. In Cartilage: Molecular Aspects. B. Hall and S. Newman, editors. CRC Press, Boston. 131–151.
- 35.Pluen, A., P. A. Netti, R. K. Jain, and D. A. Berk. 1999. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys. J. 77:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietrich, C., B. Yang, T. Fujiwara, A. Kusumi, and K. Jacobson. 2002. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys. J. 82:274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoji, H., Y. Iwasa, A. Mochizuki, and S. Kondo. 2002. Directionality of stripes formed by anisotropic reaction-diffusion models. J. Theor. Biol. 214:549–561. [DOI] [PubMed] [Google Scholar]
- 38.Shoji, H., A. Mochizuki, Y. Iwasa, M. Hirata, T. Watanabe, S. Hioki, and S. Kondo. 2003. Origin of directionality in the fish stripe pattern. Dev. Dyn. 226:627–633. [DOI] [PubMed] [Google Scholar]