Abstract
We have studied the wavelength dependence of retinal Schiff base absorbencies on the protonation state of the chromophore at the multiconfigurational level of theory using second order perturbation theory (CASPT2) within an atomic natural orbital basis set on MP2 optimized geometries. Quantitative agreement between calculated and experimental absorption maxima was obtained for protonated and deprotonated Schiff bases of all-trans- and 11-cis-retinal and intermediate states covering a wavelength range from 610 to 353 nm. These data will be useful as reference points for the calibration of more approximate schemes.
Retinal is the chromophore in several photosensitive proteins where it converts light energy into structural changes (1): in the visual pigments or rhodopsins, the light-induced isomerization of 11-cis-retinal to all-trans initiates the visual cycle. In bacteriorhodopsin, all-trans-retinal is transformed by light into the 13-cis isomer, which starts the proton pumping cycle across the bacterial cell wall of Halobacterium salinarium.
One particular aspect in retinal protein chemistry concerns the ultraviolet-visible spectral changes in these pigments, which serve specific needs: from ancient bacteria that use sensory rhodopsins to test the composition of light (2) to the human eye where three different rhodopsins enable the perception of colors (3). Understanding the physical origin of these changes has been a major challenge to theory ever since the original concept of the external point charge model has been introduced in the literature (4). Advances in x-ray crystallography have provided a multitude of bacteriorhodopsin structures, including intermediates of the proton pumping cycle (5) and have culminated recently in the three-dimensional structure of bovine rhodopsin (6) and its first photointermediate, bathorhodopsin (7). These structures, which reveal the geometry of the retinal chromophore and its environment in atomic detail, have been instrumental for theoretical studies of retinal protein spectral shifts using diverse quantum-mechanical schemes (8–13). The dilemma that these studies face is exemplified by the fact that two of them arrive at very reasonable values for the theoretically calculated absorbance of rhodopsin, yet their results for the simple 11-cis-retinal protonated Schiff base (pSb), which forms the basis for the ensuing quantum mechanical and molecular mechanical (QM/MM) calculations, differ by 0.56 eV or 176 nm.
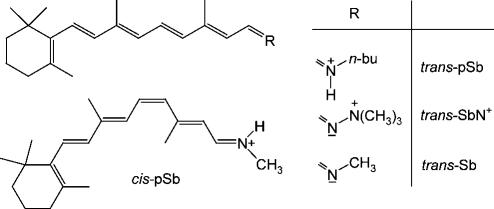
Recently the gas phase absorption spectra of several retinal Schiff bases in different protonation states have been determined (14,15) and found to peak at 610/620 nm (trans-pSb in Scheme 1), 487 nm (trans-SbN+), and 610 nm (cis-pSb). These data define much needed reference points for the calculation of retinal protein spectra, both for the protonated chromophores in vacuo and for the effect of a positive charge in a defined relative orientation to the chromophore. To cover the short-wavelength region of retinal Schiff base spectra, we also include the neutral species trans-Sb whose absorbance in the nonpolar solvent 3-methylpentane peaks at 353 nm (16). In the following, we show that CASPT2 theory at a very high level of sophistication is able to quantitatively reproduce these data.
SCHEME 1.
Retinal Schiff base chromophores and their short term notations.
In view of the huge computational requirements, the n-butyl group in Scheme 1 was reduced to methyl (the solvent spectra of the two pSbs are essentially identical) (17) and 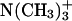 to
to 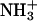 . Geometry optimization at the CASPT2 level for systems of this size is still prohibitive in computer resources. We therefore resorted to MP2 and its analytical gradients, which allow for an efficient geometry search with a correlated wave function. Starting with the DFT-optimized structures (18), the chromophores were reoptimized with MP2 using a 6-31G** basis set (19).
. Geometry optimization at the CASPT2 level for systems of this size is still prohibitive in computer resources. We therefore resorted to MP2 and its analytical gradients, which allow for an efficient geometry search with a correlated wave function. Starting with the DFT-optimized structures (18), the chromophores were reoptimized with MP2 using a 6-31G** basis set (19).
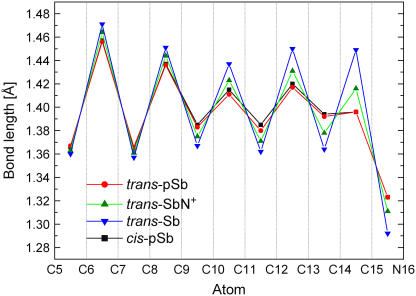
All four chromophores exhibit strong bond alternation (Fig. 1), which is, however, significantly reduced between C9 and N16 in the three positively charged systems. A further reduction is observed in trans- and cis-pSb, where the positive charge is part of the π-system. From C6 to N16, all chromophores are essentially planar with the exception of cis-pSb, which is twisted by 7° and 3° about the C11=C12 and the C12-C13 bonds, respectively, and moves the C13-N16 fragment away from the bulky β-ionone ring.
FIGURE 1.
MP2-optimized bond lengths along the conjugated carbon chain of the retinal Schiff bases shown in Scheme 1.
Ground and excited state energies were calculated with the CASSCF method as provided by the MOLCAS set of routines (20). Six-root state-averaged wave functions were expanded in an atomic natural orbital basis set (21) with the contraction C,N[4s3p1d]/H[2s]. The active space was (12,12), i.e., all pseudo π-electrons and valence pseudo π-orbitals were considered. Second-order corrections to the CASSCF energies were calculated with CASPT2. All core orbitals were kept frozen during the calculations. To avoid the effect of intruder states, the level shift was set uniformly to 0.3 au. These parameters are identical to the ones we used in recent studies on retinal model chromophores (22) and on the rhodopsin binding pocket (23,24). A summary of the calculations is given in Table 1.
TABLE 1.
CASPT2 energies, oscillator strengths, main contributing configurations with weight, and dipole moments for the ground state S0 and two main excited states of the four retinal chromophores shown in Scheme 1 and experimental wavelengths (in bold, nm) of the optical transitions
| Structure | State | CASPT2* | f | Configuration† | μ‡ | λexp |
|---|---|---|---|---|---|---|
| trans-pSb | S0 | −871.2380 | – | (6a)2(7a)075 | 24.45 | |
| S1 | 2.07 (600) | 1.3 | (6a)1(7a)161 | 7.17 | 610/620§¶ | |
| S2 | 2.85 (435) | 0.1 | (6a)0(7a)228 | 20.04 | 385 | |
| trans-SbN+ | S0 | −887.2138 | – | (6a)2(7a)067 | 35.48 | |
| S1 | 2.56 (484) | 0.9 | (6a)1(7a)145 | 19.82 | 487§ | |
| S2 | 2.76 (449) | 0.2 | (6a)0(7a)227 | 30.02 | ||
| trans-Sb | S0 | −870.8470 | – | (6a)2(7a)069 | 2.02 | |
| S1 | 3.47 (357) | 1.2 | (6a)1(7a)168 | 3.94 | 353‖ | |
| S2 | 3.62 (342) | 0.0 | (6a)0(7a)227 | 1.96 | ||
| cis-pSb | S0 | −871.2306 | – | (6a)2(7a)075 | 22.75 | |
| S1 | 2.05 (606) | 1.1 | (6a)1(7a)161 | 6.70 | 610¶ | |
| S2 | 2.84 (436) | 0.1 | (6a)0(7a)228 | 19.45 | 390 |
The agreement between calculated and experimental S0 → S1 energies is extremely good, especially in view of the fact that the calculations cover a wavelength range from 350 nm for the neutral Schiff base to 600 nm for the protonated chromophore. Why another study (12) using the same CASPT2 methodology as described here arrives at results significantly different from ours—and from the experiment—is a matter of speculation. It is possible that the smaller 6-31G* basis set employed there has not sufficient flexibility for these kinds of systems. It may be interesting to note that the first ever calculations on retinal Schiff bases more than 30 years ago using empirical and π-electron calculations converged already on an absorbance near 600 nm for the protonated species (25–27).
The hypsochromic shift of 116 nm, or 0.49 eV, from trans-pSb to trans-SbN+ is a consequence of the positive charge being moved from the Schiff base nitrogen to the neighboring atom where it is no longer part of the conjugated system and can interact only electrostatically with the chromophore. However, this interaction is very effective judging from the further 0.91 eV energy shift for the trans-SbN+ to trans-Sb transformation. These data highlight the extraordinary sensitivity of the retinal chromophore to the environment, where the movement of one positive charge suffices to shift the absorbance maximum by almost 1.4 eV or 243 nm.
With respect to the formally forbidden S2 state, the situation is less clear-cut. In the protonated species trans- and cis-pSb, we find this state ∼0.8 eV above S1, somewhat smaller than the value (1.2 eV) determined by Andersen (15). In the neutral trans-Sb, this gap is significantly reduced, to 0.15 eV, but S2 is still above S1. In their study of a six double bond polyene Schiff base, Palmer et al. found this state 0.4 eV below S1 (28), in line with results on other polyene hydrocarbons (29). Grossjean and Tavan, in their semiempirical studies on retinal models (30) have demonstrated the importance of electron correlation for the correct description of the S1/S2 gap. It appears that the setup that we have applied in our CASPT2 treatment (size of the active space; number of roots) is not sufficient to correctly describe the S2 state, especially in the neutral species (31).
A key to understanding the spectral shifts is the change in the dipole moment as the chromophore is promoted to an excited state (Table 1). This change is small in the case of the neutral system trans-Sb but large in the presence of a positive charge and when the excitation is to S1: In trans-SbN+, the moment decreases by almost 50%, and in trans- and cis-pSb the decrease is even larger. This loss in Coulomb energy stabilizes the excited state and results in huge bathochromic shifts. Excitation to S2 states leads to a small but almost constant decrease of the dipole moment, and accordingly the S0 to S2 absorbance is largely unaffected by differences in the charged environment.
Three mechanisms are generally discussed in connection with the phenomenon of spectral tuning in retinal proteins: the effect of the counterion, internal twisting of the chromophore, and the polar environment due to the binding pocket. In this report, we have concentrated on the latter aspect. We have shown that the CASPT2 method with a basis set that optimally treats correlation and polarization effects is able to quantitatively reproduce the response of retinal Schiff base absorption spectra to polar perturbations. The huge computational resources necessary to perform this kind of calculations will be prohibitive for their use as standard application. However, these calculations provide the necessary benchmark data for the calibration of the embedded QM part in any of the widely used QM/MM schemes.
Acknowledgments
This work was funded by the DFG Forschergruppe “Retinal Protein Action”.
References
- 1.Birge, R. R., C. M. Einterz, H. M. Knapp, and L. P. Murray. 1988. The nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biophys. J. 53:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoff, W. D., K. H. Jung, and J. L. Spudich. 1997. Molecular mechanism of photosignaling by archaeal sensory rhodopsins. Annu. Rev. Biophys. Biomol. Struct. 26:223–258. [DOI] [PubMed] [Google Scholar]
- 3.Shichi, H. 1983. Biochemistry of Vision. Academic Press, New York.
- 4.Nakanishi, K., V. Balogh-Nair, M. Arnaboldi, K. Tsujimoto, and B. Honig. 1980. An external point charge model for bacteriorhodopsin to account for its purple color. J. Am. Chem. Soc. 102:7945–7951. [Google Scholar]
- 5.For a summary, see W. Kühlbrandt. 2000. Bacteriorhodopsin—the movie. Nature. 406:569–570. [DOI] [PubMed] [Google Scholar]
- 6.Palczewski, K., T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. Le Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto, and M. Miyano. 2000. Crystal structure of rhodopsin: a G-protein coupled receptor. Science. 289:739–745. [DOI] [PubMed] [Google Scholar]
- 7.Okada, T., and H. Nakamichi. 2006. Crystallographic analyses of the primary visual photochemistry. Angew. Chem. Int. Ed. Engl. 45. [DOI] [PubMed]
- 8.Houjou, H., Y. Inoue, and M. Sakurai. 2001. Study of the opsin shift of bacteriorhodopsin: Insight from QM/MM calculations with electronic polarization effects of the protein environment. J. Phys. Chem. B. 105:867–879. [Google Scholar]
- 9.L. Ren; C. H. Martin, K. J. Wise, N. B. Gillespie, H. Luecke, J. K. Lanyi, J. L. Spudich, and R. R. Birge. 2001. Molecular mechanism of spectral tuning in sensory rhodopsin II. Biochemistry. 46:13906–13914. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, S., E. Tajkhorshid, E. Pebay-Peyroula, A. Royant, E. M. Landau, J. Navarro, and K. Schulten. 2001. Structural determinants of spectral tuning in retinal proteins: bacteriorhodopsin vs. sensory rhodopsin II. J. Phys. Chem. B. 105:10124–10131. [Google Scholar]
- 11.Rajamani, R., and J. Gao. 2002. Combined QM/MM study of the opsin shift in bacteriorhodopsin. J. Comput. Chem. 23:96–105. [DOI] [PubMed] [Google Scholar]
- 12.Andruinow, T., N. Ferre, and M. Olivucci. 2004. Structure, initial excited-state relaxation, and energy storage of rhodopsin resolved at the multiconfigurational perturbation theory level. Proc. Natl. Acad. Sci. USA. 101:17908–17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto, K., J. Hasegawa, S. Hayashi, S. Kato, and H. Nakatsuji. 2005. Mechanism of color tuning in retinal protein: SAC-CI and QM/MM study. Chem. Phys. Lett. 414:239–242. [Google Scholar]
- 14.Andersen, L. H., I. B. Nielsen, M. B. Kristensen, M. O. A. El Ghazaly, S. Haake, M. Brondsted Nielsen, and M. A. Petersen. 2005. Absorption of Schiff-base retinal chromophores in vacuo. J. Am. Chem. Soc. 127:12347–12350. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen, I. B., L. Lammich, and L. H. Andersen. 2006. S1 and S2 Excited states of gas-phase Schiff-base retinal chromophores. Phys. Rev. Lett. 96:018304. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer, A. M., W. H. Waddell, and R. S. Becker. 1973. Experimental and theoretical investigation of the absorption spectra of retinal Schiff bases and retinals. J. Am. Chem. Soc. 96:2063–2068. [DOI] [PubMed] [Google Scholar]
- 17.Waddell, W. H., A. M. Schaffer, and R. S. Becker. 1977. Experimental and theoretical investigations of the absorption spectral properties of protonated retinal Schiff bases and implications for the bathochromic shift in visual pigments. J. Am. Chem. Soc. 99:8456–8460. [DOI] [PubMed] [Google Scholar]
- 18.Terstegen, F., and V. Buss. 1998. All-trans- and 11-cis-retinal, their N-methyl Schiff base and N-methyl protonated Schiff base derivatives: a comparative ab initio study. J. Mol. Struct. THEOCHEM. 430:209–218. [Google Scholar]
- 19.Frisch, M. J., G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, and others. 2003. Gaussian Revision B.04. Gaussian Inc., Pittsburgh, PA.
- 20.Andersson, K., M. Barysz, A. Bernhardsson, M. R. A. Blomberg, D. L. Cooper, M. P. Fülscher, C. de Graaf, B. A. Hess, G. Karlström, R. Lindh, P.-Å. Malmqvist, T. Nakajima, P. Neogrády, and others. 2002. MOLCAS Version 5.4; University of Lund, Lund, Sweden.
- 21.Pierloot, K., B. Dumez, P. O. Widmark, and B. O. Roos. 1995. Density matrix averaged atomic natural orbital (ANO) basis sets for correlated molecular wave functions. Theor. Chim. Acta. 90:87–114. [Google Scholar]
- 22.Hufen, J., M. Sugihara, and V. Buss. 2004. How the counterion affects ground and excited state properties of the rhodopsin chromophore. J. Phys. Chem. B. 108:20419–20426. [Google Scholar]
- 23.Sugihara, M., J. Hufen, and V. Buss. 2006. Origin and consequences of steric strain in the rhodopsin binding pocket. Biochemistry. 45:801–810. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber, M., M. Sugihara, T. Okada, and V. Buss. 2006. Quantum-mechanical studies on the crystallographic model of bathorhodopsin. 2006. Angew. Chem. Int. Ed. Engl. 45. [DOI] [PubMed]
- 25.Blatz, P. E., J. H. Mohler, and H. V. Navangul. 1972. Anion-induced wavelength regulation of absorption maxima of Schiff bases of retinal. Biochemistry. 11:848–855. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki, H., T. Komatsu, and H. Kitajima. 1972. Theory of the optical property of visual pigment. J. Phys. Chem. Jpn. 37:177–185. [Google Scholar]
- 27.Honig, B., A. D. Greenberg, U. Dinur, and T. Ebrey. 1976. Visual-pigment spectra: Implications of the protonation of the retinal Schiff base. Biochemistry. 15:4593–4599. [DOI] [PubMed] [Google Scholar]
- 28.Palmer, B., B. Jumper, W. Hagan, J. C. Baum, and R. L. Christensen. 1982. Optical studies of a simple polyene Schiff base: Low-lying electronic levels in the free, hydrogen-bonded, and protonated species. J. Am. Chem. Soc. 104:6907–6913. [Google Scholar]
- 29.Christensen, R. L., M. Goyette, L. Gallagher, J. Duncan, B. DeCoster, J. Lugtenburg, F. J. Jansen, and I. van der Hoef. 1999. S1 and S2 States of apo-and diapocarotenes. J. Phys. Chem. A. 103:2399–2407. [Google Scholar]
- 30.Grossjean, M. F., and P. Tavan. 1987. Wavelength regulation in bacteriorhodopsin and halorhodopsin: A PPP multireference double excitation configuration interaction study of retinal dyes. J. Chem. Phys. 88:4884–4896. [Google Scholar]
- 31.We have found the switch of the S1 and S2 states in five double bond model retinal Schiff bases (22) using the same computational setup as described in this report. Apparently this is sufficient for treating the S2 state in the reduced retinal model compounds but needs enlargement in more extended systems.