Abstract
It has been postulated that for a binary mixture of phospholipid and cholesterol, phospholipid/cholesterol complexes are formed. Using grazing incidence x-ray diffraction, we have obtained evidence for lipid/cholesterol ordering in model membranes. Scattering features consistent with the existence of lipid/cholesterol complexes persist to high surface pressures even though fluorescence microscopy suggests a homogeneously fluid phase. Contrary to pure phospholipid and cholesterol systems, the resulting lattice spacing, integrated scattering intensity, and coherence lengths of these complexes are almost independent of surface pressure. Furthermore, the single peak observed in these mixed systems is very broad, suggesting that the extent of order for a single scattering structure only persists over a few molecules. This observation is consistent with these complexes being dynamic structures.
Mixtures of cholesterol (Chol) and phospholipids have been studied extensively for both their biological relevance and their nonideal mixing behavior. The nonideality of mixtures of Chol and egg lecithin monolayers was discovered when the average area for the mixtures measured was much lower than what would be expected from the individual components (3). The nonadditivity of the areas was termed the “condensing effect of cholesterol”. It was proposed that Chol and phospholipids form “complexes” with specific stoichiometries (4,5), and detailed lattice-based models for the structures of Chol lipid mixtures have been put forth (6).
A key observation in the field is the liquid-liquid immiscibility in phosphatidylcholine/Chol monolayer mixtures (5), which ultimately led to the discovery of phase diagrams with two liquid-liquid immiscibility regions separated by a sharp cusp point for certain mixtures of Chol and lipids (7). Such a phase diagram had been predicted (8) but never observed until 1999 for liquid mixtures. The shape of such a phase diagram can be explained by a thermodynamic model of “condensed complexes” where the complexes, with stoichiometry specified by the cusp point, are the result of a reversible reaction between Chol and phospholipid molecules (9,10). The complexes described may have relevance to biological membranes, as there is evidence that certain lipids in the plasma membrane promote the formation of “specialized regions” termed lipid rafts, thought to be enriched in Chol and sphingomyelin (SM). However, there is uncertainty concerning the nature of these domains including their size, function, and composition.
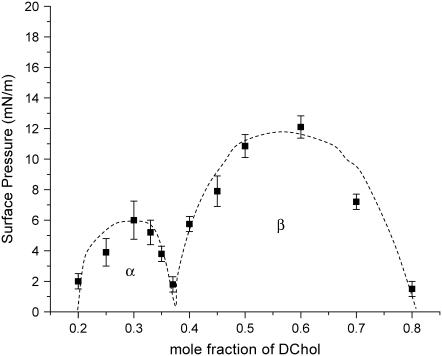
The phase diagram of certain mixed phospholipid-Chol monolayers, obtained by fluorescence microscopy (FM) (7), indeed shows two immiscibility regions (see Fig. 1). Denoting the two as α- and β-regions, the former corresponds to a lower mole fraction of Chol, and an excess of phospholipids, such that the observed immiscibility is envisioned to be due to lipid/Chol complexes in coexistence with a lipid-rich phase. Conversely, the complex is in coexistence with excess Chol in the β-region. Theoretical models that account for this behavior require a third species formed by the reaction of Chol with phospholipids with a stoichiometry given by the cusp point (7).
FIGURE 1.
Phase diagram of a mixed SM/DChol system at 30°C, on a pure water subphase. The dashed lines are to guide the eye. The α- and β-regions are marked on the diagram.
Several questions remain unresolved on the existence and characteristics of these complexes: Do complexes give rise to a packing order distinct from those of pure lipid and pure Chol? Although the phase diagram suggests their existence at pressures below the miscibility critical point, do these complexes persist to pressures relevant to biological membranes? How large are the complexes? Are they static or dynamic structures? To address these questions, we have used grazing incidence x-ray diffraction (GIXD) (12–14) to investigate structures occurring in mixed monolayers of phospholipids and dihydrocholesterol (DChol) in the 25–40 mN/m range. DChol, an excellent substitute for Chol, was used to avoid possible oxidative damage to the double bond in Chol.
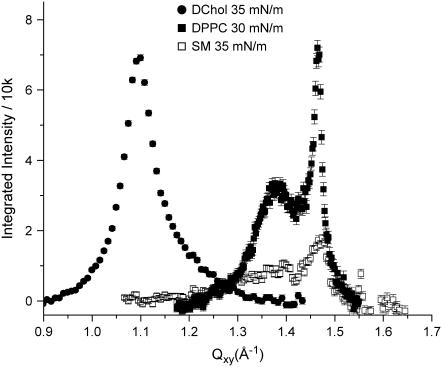
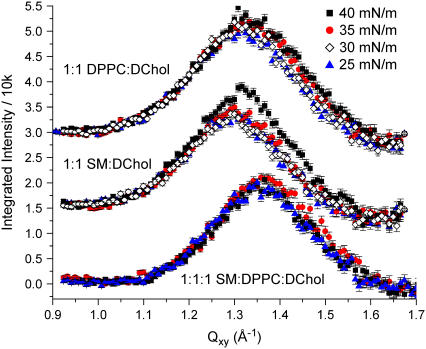
GIXD measurements on pure SM, dipalmitoylphosphatidylcholine (DPPC), and DChol reveal that all three exhibit order in this pressure range (Fig. 2), characteristic of a two-dimensional condensed phase (for SM, the condensed phase exists only above 30 mN/m). These pure systems have coherence lengths of the order of 100+ Å. When the phospholipids are mixed with DChol at the molar ratios examined, the GIXD peak becomes much broader (Fig. 3), pointing to scattering structures with order that is shorter ranged than that of the pure systems. Moreover, the peak position in the mixed lipid/DChol systems lies between those of pure DChol and pure phospholipid. Both the reduction in coherence lengths and the shifting of the peak to some intermediate values suggest that the scattering structures are neither due to DChol nor phospholipid alone, but a new species formed by the two. Our results are not inconsistent with the homogeneous phase observed by FM at surface pressures we have examined, as FM can only resolve structures in the micron length scales. GIXD, however, can detect order from nanoscale structures.
FIGURE 2.
Background-subtracted Bragg peaks at 30 mN/m for DPPC, 35 mN/m for SM, and 35 mN/m for DChol on a pure water subphase at 30°C.
FIGURE 3.
Background-subtracted Bragg peaks for lipid mixtures as a function of surface pressure. Bragg peaks corresponding to the different lipid compositions have been offset vertically for clarity.
The scattering structures from the mixed systems have coherence lengths of 21–25 Å, a range that lies between that of a liquid expanded phase with no detectable order, and a condensed phase where the coherence length is at least of the order of tens of molecules. This reduction in coherence length in the presence of DChol is consistent with the observed disordering effects of cholesterol on gel phase lipid bilayers. Because the d-spacing obtained in the mixed systems is in the range of 4.6–4.8 Å, the complexes are only four to five molecules across. This short-ranged order suggests that the scattering structures observed are dynamic in nature. The broad width of the Bragg peak could be due to a superposition of narrower peaks from species of close but different geometries, which would suggest that the ∼20 Å coherence length merely constitutes a lower limit. This points to the possibility that there can be a range of stoichiometries that gives rise to the broadening, and that the theorized stoichiometry given by the cusp point may correspond to the ensemble average of these values. A similarly broad peak has recently been reported to be in coexistence with the crystalline ceramide peaks for mixed ceramide/Chol monolayers at 5°C (11).
According to the two-region phase diagram, the α-region is predicted to be made up of phopholipid/DChol complex and excess phospholipid, whereas the β-region should have excess DChol. With the mole fractions of DChol presented in this study, we do not observe any separate Bragg peak for pure phospholipid or pure DChol that do not partake in complex formation. However, should the monolayer consist of many nanoscopic domains, the excess species could either be decorating the boundaries of or situated between complexes, thus not giving rise to any pure phospholipid or DChol peak. At very low DChol (or phospholipid) concentrations, we expect pure phospholipid (or DChol) peaks alongside with the broader mixed peak observed. Indeed, we observed additional phospholipid peaks for mixed films with DChol content up to 15%.
Bragg rod analysis suggests that the broad peak is due to a scatterer ∼11 Å long, corresponding to the ring structure dimensions of DChol as revealed by our x-ray reflectivity measurements (data not shown). However, the scattering cannot be due to DChol alone, because packed DChol has a d-spacing of 5.7 Å, too large for the 4.6–4.8 Å observed. This observed d-spacing is also larger than that of tightly packed phospholipid tailgroups (4.2 Å (15)). Instead, the observed peaks likely result from a fairly disordered mix of DChol molecules and phospholipid tails with the ∼11 Å tall entity consisting of the ring structure with intercalated acyl chain segments. In effect, DChol molecules are interspersed in the lipid matrix, acting as spacers between phospholipids.
Although FM shows, with micron resolution, a homogeneously mixed film for the phospholipid/DChol system at the pressures examined, GIXD, capable of accessing much smaller length scales, reveals order (albeit a very short range). There has been a recent report that for certain lipid mixtures, the β-region can extend to 40 mN/m (16) and therefore complexes formed at low pressures persist even at high pressures. For our systems, despite the termination of the β-region at ∼13 mN/m, we have evidence from GIXD for ordered lipid/DChol entities beyond this pressure.
Finally we wish to discuss our data in the context of two models: the umbrella model by Feigenson et al. (17) and the superlattice model by Chong (6), both for bilayers of phospholipid/Chol mixtures. In the umbrella model, the phospholipid headgroups shield the polar headgroup of Chol from water. As the mole fraction of Chol is increased beyond 0.67, the shielding breaks down, causing the excess Chol to precipitate out. In this study, we do not detect order from excess DChol though we have not explored mixtures with DChol mol fraction above 0.67. Like Chong, we envision that Chol intercalates into the lipid matrix by taking up the lattice site of a phospholipid tailgroup. However, due to a size mismatch, the resulting ordering can only be short ranged. Although the general concept of tailgroup site substitution by Chol can explain the variability of d-spacings, the sizes of the crystallites deduced from our GIXD study are too small to form any superlattices.
Acknowledgments
We acknowledge BW1 beamtime at HASYLAB, Deutsches Elektronen-Synchrotron, Hamburg, Germany.
We received financial support from the DanSync program of the Danish Natural Science Research Council and from the Carlsberg Foundation. C.E. was supported by the Alzheimer's Association (IIRG-9901175), the American Health Assistance Foundation (A-1999057), and The University of Chicago Materials Research Science and Engineering Center program of the National Science Foundation (DMR0213745). J.M. was supported by Los Alamos National Laboratory under Department of Energy (DOE) contract W7405-ENG-36, and by the DOE Office of Basic Energy Sciences. M.K.R. acknowledges the support of Burroughs Wellcome Fund Interfaces No. 1001774. K.Y.C.L. is grateful for support from the Packard Foundation (99-1465).
Canay Ege's present address is Intel Corporation, 2501 Elam Young Blvd., Hillsboro, OR 97124.
References
- 1.Reference deleted in proof.
- 2.Reference deleted in proof
- 3.Leathes, J. B. 1925. Condensing effect of cholesterol on monolayers. Lancet. 208:853–856. [Google Scholar]
- 4.Gershfeld, N. L. 1978. Equilibrium studies of lecithin-cholesterol interactions I. Stoichiometry of lecithin-cholesterol complexes in bulk systems. Biophys. J. 22:469–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subramaniam, S., and H. M. Mcconnell. 1987. Critical mixing in monolayer mixtures of phospholipid and cholesterol. J. Phys. Chem. 91:1715–1718. [Google Scholar]
- 6.Chong, P. L. 1994. Evidence for regular distribution of sterols in liquid crystalline phosphatidylcholine bilayers. Proc. Natl. Acad. Sci. USA. 91:10069–10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan, A., and H. M. McConnell. 1999. Condensed complexes of cholesterol and phospholipids. Biophys. J. 77:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales, L. R., and J. C. Wheeler. 1989. Chemical-reaction driven phase-transitions and critical-points. J. Chem. Phys. 91:7097–7112. [Google Scholar]
- 9.Radhakrishnan, A., X. M. Li, R. E. Brown, and H. M. McConnell. 2001. Stoichiometry of cholesterol-sphingomyelin condensed complexes in monolayers. Biochim. Biophys. Acta. 1511:1–6. [DOI] [PubMed] [Google Scholar]
- 10.McConnell, H. M., and M. Vrljic. 2003. Liquid-liquid immiscibility in membranes. Annu. Rev. Biophys. Biomol. Struct. 32:469–492. [DOI] [PubMed] [Google Scholar]
- 11.Scheffer, L., I. Solomonov, M. J. Weygand, K. Kjaer, L. Leiserowitz, and L. Addadi. 2005. Structure of cholesterol/ceramide monolayer mixtures: implications to the molecular organization of lipid rafts. Biophys. J. 88:3381–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen, T. R., and K. Kjaer. 2001. Structural properties and interactions of thin films at the air-liquid interface explored by synchrotron X-ray scattering. In Novel Methods to Study Interfacial Layers. (Studies in Interface Science, Vol. 11). D. Möbius and R. Miller, editors. Elsevier, Amsterdam, The Netherlands; New York, NY. 205–254.
- 13.Lee, K. Y. C., J. Majewski, T. L. Kuhl, P. B. Howes, K. Kjaer, M. M. Lipp, A. J. Waring, J. A. Zasadzinski, and G. S. Smith. 2001. Synchrotron x-ray study of lung surfactant-specific protein SP-B in lipid monolayers. Biophys. J. 81:572–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Als-Nielsen, J., and K. Kjaer. 1989. Reflectivity and diffraction studies of liquid surfaces and surfactant monolayers. In Phase Transitions in Soft Condensed Matter. Proc. NATO Advanced Study Institute, Plenum Publishing, New York. B211:113–138.
- 15.Ege, C., J. Majewski, G. Wu, K. Kjaer, and K. Y. Lee. 2005. Templating effect of lipid membranes on Alzheimer's amyloid beta peptide. ChemPhysChem. 6:226–229. [DOI] [PubMed] [Google Scholar]
- 16.Okonogi, T. M., and H. M. McConnell. 2004. Contrast inversion in the epifluorescence of cholesterol-phospholipid monolayers. Biophys. J. 86:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, J., and G. W. Feigenson. 1999. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76:2142–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]