Abstract
In contrast to Madin–Darby canine kidney cells, Fischer rat thyroid cells deliver the majority of endogenous glycosylphosphatidyl inositol (GPI)–anchored proteins to the basolateral surface. However, we report here that the GPI proteins Placental Alkaline Phosphatase (PLAP) and Neurotrophin Receptor–Placental Alkaline Phosphatase (NTR-PLAP) are apically localized in transfected Fischer rat thyroid cells. In agreement with the “raft hypothesis,” which postulates the incorporation of GPI proteins into glycosphingolipids and cholesterol-enriched rafts, we found that both of these proteins were insoluble in Triton X-100 and floated into the lighter fractions of sucrose density gradients. However, disruption of lipid rafts by removal of cholesterol did not cause surface missorting of PLAP and NTR-PLAP, and the altered surface sorting of these proteins after Fumonisin B1 treatment did not correlate with reduced levels in Triton X-100 –insoluble fractions. Furthermore, in contrast to the GPI-anchored forms of both of these proteins, the secretory and transmembrane forms (in the absence of a basolateral cytoplasmic signal) were sorted to the apical surface without association with lipid microdomains. Together, these data demonstrate that the GPI anchor is required to mediate raft association but is not sufficient to determine apical sorting. They also suggest that signals present in the ectodomain of the proteins play a major role and that lipid rafts may facilitate the recognition of these signals in the trans-Golgi network, even though they are not required for apical sorting.
INTRODUCTION
The plasma membrane of polarized epithelial cells is divided into apical and basolateral domains that display specialized functions as a result of different protein and lipid compositions (Rodriguez-Boulan and Powell, 1992; Eaton and Simons, 1995; Drubin and Nelson, 1996). The asymmetric distribution of lipids and proteins on the surface is achieved by continuous sorting of newly synthesized components and by their regulated internalization (Drubin and Nelson, 1996). Plasma membrane proteins are synthesized in the endoplasmic reticulum and transported through the Golgi complex, where, in the trans-Golgi network (TGN), they are incorporated by selective sorting signals into different vesicles and separately sorted to the apical or basolateral domain of the plasma membrane (Wandinger-Ness et al., 1990; Rodriguez-Boulan and Powell, 1992).
Most studies of protein trafficking have been carried out in the Madin–Darby canine kidney (MDCK) cell line (Rodriguez-Boulan and Powell, 1992), in which it has been shown that sorting of transmembrane proteins to the basolateral domain is mediated by short amino acid sequences found in their cytoplasmic tails (Matter and Mellman, 1994; Mellman, 1996). These basolateral sorting signals are often dominant over apical determinants (and/or cryptic apical signals) present in the extracellular domain of the protein (Matter and Mellman, 1994; Fiedler and Simons, 1995).
Protein sorting to the apical domain of the plasma membrane is controlled by at least three different mechanisms: 1) the N- and/or O-glycosylation of the ectodomain, possibly recognized by cellular lectins (Fiedler and Simons, 1995; Rodriguez-Boulan and Gonzalez, 1999); 2) apical sorting determinants present in the cytoplasmic tail of seven transmembrane proteins (Chuang and Sung, 1988; Sun et al., 1998); and 3) the incorporation of apically sorted proteins into lipid microdomains, called rafts, in the Golgi complex (Harder and Simons, 1997; Simons and Ikonen, 1997). N- and O-glycosylation appear to play a role in apical sorting of both secretory and single-transmembrane-spanning proteins. It has been shown that N-glycosylation functions as an apical signal for a subset of secretory proteins, such as erythropoietin (Kitagawa et al., 1994) and growth hormone (Scheiffele et al., 1995), but not for others, such as the hepatitis B surface antigen (Marzolo et al., 1997) or the p75 neurotrophin receptor (NTR) ectodomain (Yeaman et al., 1997). Similar data have been obtained for transmembrane proteins. O-Glycosylation is required for apical sorting of p75NTR in MDCK (Yeaman et al., 1997) and Caco2 (Monlauzeur et al., 1998) cells, whereas N-glycosylation is needed for apical sorting of occludin, ERGIC-53, and the Fc-LDL receptor (Gut et al., 1998). An exception is CD3-ε, an unglycosylated protein, which is nonetheless similarly sorted to the apical membrane (Alonso et al., 1997).
Recently, a putative apical sorting signal has been found independently by two different laboratories in the cytoplasmic tail of two seven-transmembrane-spanning proteins, rhodopsin (Chuang and Sung, 1998) and the apical Na+-dependent bile acid transporter (Sun et al., 1998). In fact, 39 and 40 amino acids, respectively, of the cytoplasmic tail of these two proteins were able to redirect two different basolateral proteins to the apical surface in MDCK cells. Interestingly, these findings are peculiar to these specific classes of proteins, and there is no sequence conservation of residues between the two signals of the two cytoplasmic tails. These studies indicate that a cytoplasmic sorting machinery analogous to the one described for basolateral proteins also might exist for apically targeted proteins.
Inclusion into rafts and subsequent apical sorting has been shown for some transmembrane proteins (Kundu et al., 1996; Lin et al., 1998) and for all proteins anchored to the plasma membrane via the glycosylphosphatidyl inositol (GPI) anchor (Harder and Simons, 1997). Rafts are membrane microdomains enriched in glycosphingolipids (GSLs) and cholesterol that are thought to originate in the Golgi apparatus and that have been proposed to function as a sorting platform for the apical delivery of plasma membrane proteins (Simons and van Meer, 1988; Simons and Ikonen, 1997).
Raft association is mediated either by the transmembrane domains of proteins, e.g., in the case of the influenza virus proteins hemagglutinin (Lin et al., 1998) and neuraminidase (Kundu et al., 1996), or by the GPI moiety (Harder and Simons, 1997). It has been shown that all GPI-anchored proteins are sorted to the apical membrane in several epithelial cell lines (Brown et al., 1989; Lisanti et al., 1989, 1990) and to the axonal region of neuronal cells (Dotti et al., 1991). This correlation between apical sorting and possession of a GPI tail (Brown et al., 1989; Lisanti et al., 1989; Soole et al., 1995) was strong enough to postulate that the GPI moiety by itself might be an apical sorting signal. The mechanism by which this signal works, according to the raft model, is via the lateral association of the long saturated acyl chain of the GPI moiety with sphingomyelin and GSLs in the Golgi apparatus (Simons and Ikonen, 1997). Experimental evidence for the association of GPI-anchored proteins with lipid rafts has been derived from the observation that these proteins are found in detergent-insoluble glycosphingolipid complexes (DIGs) at 4°C that also contain sphingomyelin, GSLs, and cholesterol, which float to lighter fractions on sucrose density gradients (Brown and Rose, 1992). In agreement with the “raft hypothesis,” in MDCK cells reduction of GSL levels by treatment with Fumonisin B1 (FB1) leads to missorting of GPI-anchored proteins (Mays et al., 1995). However, there is no proof that proteins that are associated with DIGs at 4°C are incorporated into rafts in vivo and that proteins that are Triton X-100 (TX-100) soluble are not associated into membrane lipid microdomains. Therefore, an important point that needs to be addressed is the correlation between DIGs and rafts.
To complicate this scenario, some GPI-anchored proteins, such as DAF, Thy-1.2, and Placental Alkaline Phosphatase (PLAP), contain a protein-encoded apical sorting signal, as shown by the apical secretion of their ectodomains (Brown et al., 1989; Lisanti et al., 1989; Powell et al., 1991). Furthermore, several transmembrane proteins have been found to be completely soluble in nonionic detergents, suggesting that they are not incorporated in lipid microdomains during their transport to the apical (Zurzolo et al., 1994; Lipardi et al., 1999; Zheng et al., 1999) or axonal (Tienari et al., 1996) surface. Similarly, apical secretory proteins have been shown not to be associated with these TX-100–insoluble domains (Graichen et al., 1996; Marzolo et al., 1997). In summary, then, it is not clear whether one or more mechanisms exist in polarized epithelial cells to sort proteins to the apical surface and what the role of the GPI anchor and lipid rafts is in this phenomenon.
Fischer rat thyroid (FRT) cells are the only known epithelial cells that sort most of their endogenous GPI-anchored proteins to the basolateral surface (Zurzolo et al., 1993). Although they have TX-100–insoluble microdomains, which are enriched in sphingomyelin, GSLs, and cholesterol (Zurzolo et al., 1994; Sarnataro and Zurzolo, personal communication), gD1-DAF, a chimeric GPI-anchored protein, is also basolaterally targeted in transfected FRT cells and is soluble in TX-100, suggesting that it does not associate with rafts during its transport to the basolateral surface (Zurzolo et al., 1994). Because a few endogenous GPI-anchored proteins are apically localized in FRT cells (Zurzolo et al., 1993), we asked whether apical GPI-anchored proteins associate with rafts in this cell line and whether this association is required for apical sorting of GPI and/or transmembrane and secretory proteins.
We show here that, in contrast to gD1-DAF, two transfected GPI-anchored proteins, PLAP and NTR-PLAP, are apically localized and cosegregate with detergent-insoluble microdomains during their transport from the TGN to the plasma membrane in FRT cells. The ectodomains of both of these proteins are also sorted to the apical surface but are not incorporated into lipid microdomains, regardless of whether they are secreted or attached to the membrane via a transmembrane domain. We also show that FB1 treatment, but not cholesterol depletion, reverts the apical sorting of GPI-anchored PLAP and NTR-PLAP. Surprisingly, FB1 treatment does not affect the rate of TX-100 solubility of these proteins, which become more resistant to extraction in this detergent only after cholesterol depletion. In summary, these data demonstrate that GPI is not an apical sorting signal, although it is required for association with detergent-insoluble microdomains of GPI-anchored proteins. They also indicate that lipid rafts do not provide an exclusive mechanism driving apical sorting of GPI-anchored proteins.
MATERIALS AND METHODS
Reagents and Antibodies
Cell culture reagents were purchased from GIBCO (Grand Island, NY). Protein A–Sepharose was from Pharmacia (Uppsala, Sweden), and sulfo-N-hydroxylsulfosuccinimide derivatives and streptavidin– agarose beads were from Pierce (Rockford, IL). The polyclonal antibody against PLAP was from Rockland (Gilbertsville, PA), and anti-p75NTR mAb was a gift from Andrè Le Bivic (Institut de Biologie du Développement de Marseille, Marseille, France). Affinity-purified rabbit anti-mouse immunoglobulin G antibodies were purchased from Cappel (Westchester, PA). FB1, methyl-β-cyclodextrin (M-β-CD), and all other reagents were obtained from Sigma (St. Louis, MO). Mevinolin was a kind gift of Maurizio Bifulco (Naples University, Italy).
Cell Culture and Drug Treatment
FRT cells stably expressing different proteins were grown in F12 Coon's modified medium containing 5% FBS. FB1, mevinolin, and M-β-CD treatments were carried out as described elsewhere (Mays et al., 1995; Keller and Simons, 1998). Briefly, FRT cells were plated on filters or on dishes, and FB1 (25 μg/ml) was added 4 h after plating of cells in F12 Coon's modified medium. Culture medium was removed after 48 h, and fresh FB1 was added to the medium for the subsequent 24 h. In experiments performed with mevinolin and M-β-CD, mevinolin (10 μM) was added to the cells 24 h after plating in F12 Coon's modified medium supplemented with 2.5% delipidated calf serum and cells were allowed to grow for another 48 h in this medium. A total of 10 mM M-β-CD was added to medium containing 10 mM HEPES, pH 7.5, and 0.2% BSA for 1 h at 37°C to cells pretreated with mevinolin for 48 h.
Constructs, Transfection, and Clonal Selection
FRT cells were transfected with cDNAs encoding PLAP, NTR-PLAP, p75NTR, PLAP-PS321, PLAP-sec, and NTR-sec, as described previously, with the use of a modification of the calcium phosphate precipitation procedure (Zurzolo et al., 1993). All of these cDNAs were a kind gift of Andrè Le Bivic. PLAP and PLAP-sec have been described by Berger et al. (1989), p75NTR and NTR-sec by Le Bivic et al. (1991), and PLAP-PS321 and NTR-PLAP by Monlauzeur et al. (1995, 1998). Stable clones were selected by G418 resistance.
Biotinylation Assays
Confluent monolayers on transwells were labeled overnight with the use of 1 mCi/ml [35S]met–cys or [35S]cys (Amersham, Arlington Heights, IL) and were biotinylated and processed for immunoprecipitation, as described previously (Zurzolo et al., 1993). Briefly, cells were lysed in buffer containing 1% TX-100 and immunoprecipitated against specific antibodies. Biotinylated antigens were then precipitated with streptavidin–agarose beads. After boiling the beads in Laemmli buffer, supernatants were analyzed by SDS-PAGE and fluorography with the use of preflashed films. Densitometry analysis was carried out within the linear range of the films. Phospholipase C digestion and Triton X-114 partitioning were performed as described previously (Lisanti et al., 1990).
Pulse Chase and TX-100 Extraction
TX-100 extractability during pulse-chase experiments was assayed as described previously (Brown and Rose, 1992; Zurzolo et al., 1994). Cells in 35-mm dishes were starved of met and cys for 30 min and pulse labeled for 5 min with 100 μl of pulse medium containing ∼500 μCi/ml trans-35S label and then incubated in chase medium (DMEM containing 10% FBS and 100× met and cys) for different times. After each time point, cells were washed twice with PBS containing calcium and magnesium on ice and lysed for 20 min on ice in 1 ml of TNE/TX-100 buffer (Brown and Rose, 1992; Zurzolo et al., 1994). Lysates were collected and centrifuged at 13,000 rpm for 2 min at 4°C. Supernatants, representing the soluble material, were separated from pellets that were solubilized in 100 μl of solubilization buffer (50 mM Tris-HCl, pH 8.8, 5 mM EDTA, 1% SDS); DNA was sheared through a 22-gauge needle. Both soluble and insoluble materials were adjusted to 0.1% SDS before immunoprecipitation with specific antibodies, as described previously (Brown and Rose, 1992).
Sucrose Gradients
Sucrose gradient analysis of TX-100 lysates was performed according to previously published protocols (Brown and Rose, 1992; Zurzolo et al., 1994). Briefly, cells were grown to confluence in 100-mm dishes, labeled for 30 min with 500 μCi/ml [35S]met–cys or [35S]cys, and incubated in chase medium for 3 h. Monolayers were then rinsed in PBS CM and lysed for 20 min in Tris, NaCl, and EDTA (TNE)/TX-100 buffer on ice. Lysates were scraped from the dishes, brought to 40% sucrose, and placed at the bottom of a centrifuge tube. A step sucrose gradient (5–35% in TNE) was layered on top of the lysates, and the samples were centrifuged at 39,000 rpm for 18–20 h in a Beckman (Fullerton, CA) SW41 rotor. One-milliliter fractions were harvested from the top of the gradient. Immunoprecipitation of distinct proteins was performed on the different fractions after bringing them up to ∼20% sucrose and 1% TX-100. Samples were solubilized in Laemmli buffer and boiled for 5 min before running on SDS-PAGE and analysis by autoradiography. Similarly, unlabeled cells were lysed and layered on sucrose gradients. Collected fractions were trichloroacetic acid (TCA) precipitated and run on SDS-PAGE. After transfer to nitrocellulose, proteins were detected by hybridization with specific antibodies and revealed by the ECL detection system (Amersham).
RESULTS
PLAP and NTR-PLAP Are Directly Sorted to the Apical Domain in Transfected FRT Cells
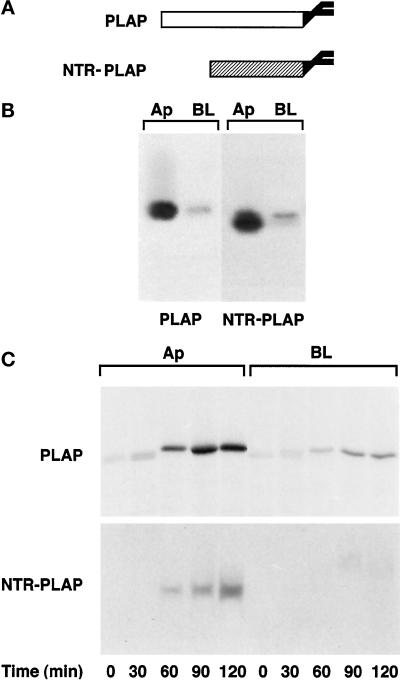
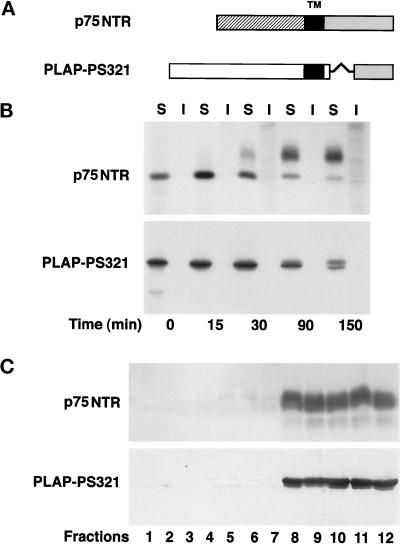
We previously reported that most endogenous GPI-anchored proteins, as well as a transfected protein, gD1-DAF, are delivered to the basolateral surface in FRT cells (Zurzolo et al., 1993). However, because a few endogenous GPI-anchored proteins are localized on the apical surface (Zurzolo et al., 1993), we asked whether GPI could be a functional apical sorting signal in these cells as well. To address this question, we transfected FRT cells with cDNAs encoding two GPI-anchored proteins that were previously shown to be apically localized in MDCK and Caco2 cells (Brown et al., 1989; Monlauzeur et al., 1998): PLAP and NTR-PLAP, a chimeric protein formed by the ectodomain of p75NTR and the GPI-attachment signal of PLAP (Figure 1A). After selecting different clones expressing either PLAP or NTR-PLAP, we determined and quantified their surface distribution by a domain-selective biotinylation assay (Zurzolo et al., 1992). We found that 80 and 90%, respectively, of surface-expressed PLAP and NTR-PLAP was localized on the apical membrane in transfected FRT cells (Figure 1B), which was different from what we had shown previously for gD1-DAF and most endogenous GPI proteins. Both proteins were sensitive to phospholipase C treatment from the apical and basolateral sides, indicating that they were GPI anchored on both domains of the plasma membrane (our unpublished results).
Figure 1.
Steady-state distribution and delivery to the plasma membrane of PLAP and NTR-PLAP in transfected FRT cells. (A) Schemes of PLAP and NTR-PLAP (a fusion protein containing the ectodomain of p75NTR and the C-terminal GPI attachment signal of PLAP). (B) FRT cells stably expressing PLAP or NTR-PLAP were grown to confluence and pulsed overnight with [35S]met–cys or [35S]cys, respectively. After surface biotinylation from the apical (Ap) and basolateral (BL) domains, cells were lysed, immunoprecipitated against specific antibodies, and reprecipitated against streptavidin. (C) FRT cells were labeled for 20 min with [35S]met–cys or [35S]cys. At different times of the chase, surface proteins were biotinylated from the apical (Ap) or the basolateral (BL) side. Cells were then lysed and immunoprecipitated with antibodies against PLAP or NTR and with streptavidin beads. Samples were run on 10% SDS-PAGE and detected by fluorography. PLAP and NTR-PLAP are directly sorted to the apical surface in FRT cells, whereas a small percentage of the two proteins is delivered with similar kinetics to the basolateral domain.
Using a biotin-targeting assay (Zurzolo et al., 1992), we found that newly synthesized PLAP and NTR-PLAP were initially detected on the apical surface after 60 min of chase and accumulated there for up to 2 h (Figure 1C), whereas a small amount of both proteins was missorted to the basolateral domain. These data indicate that both proteins were sorted directly from the TGN to the apical surface without passing through the basolateral membrane.
Both PLAP and NTR-PLAP Are Incorporated into Detergent-insoluble Microdomains in Transfected FRT Cells
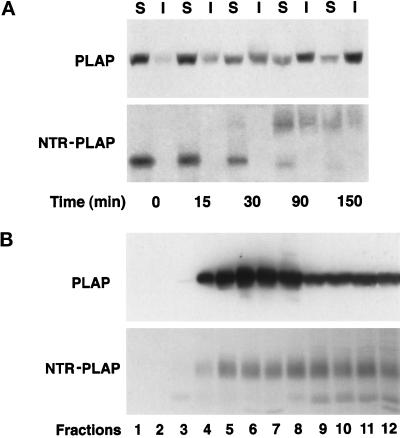
It has been postulated that in MDCK cells, GPI-anchored proteins are sorted to the apical surface via their incorporation into lipid microdomains in the Golgi complex (Simons and van Meer, 1988; Simons and Ikonen, 1997). To determine whether PLAP and NTR-PLAP were associated with TX-100–insoluble microdomains and whether this association occurred during transport to the apical surface, we performed a TX-100 extraction experiment after pulse chase, as described previously (Brown and Rose, 1992; Zurzolo et al., 1994). We found that after only 15 min of chase, PLAP had shifted from the TX-100–soluble to the TX-100–insoluble fraction; by 30 min, ∼50% of the protein was insoluble; and after 150 min, it became almost totally insoluble (Figure 2A, top). Similarly, NTR-PLAP also became progressively insoluble at increasing chase times (Figure 2A, bottom), but the insoluble amount of this protein was only ∼50% of the total at 150 min, which was less than what we had observed for PLAP (compare top and bottom panels of Figure 2A).
Figure 2.
Pulse-chase analysis and purification on sucrose density gradients of PLAP and NTR-PLAP in transfected FRT cells. (A) FRT cells stably expressing PLAP or NTR-PLAP were grown to confluence and pulsed for 5 min with [35S]met–cys or [35S]cys, followed by incubation in chase medium for the indicated times. After extraction in TNE/1% TX-100 buffer at 4°C, both the soluble (S; supernatant) and insoluble (I; pellet) fractions were collected after centrifugation, and PLAP or NTR-PLAP were subsequently immunoprecipitated and analyzed by SDS-PAGE and fluorography. PLAP and NTR-PLAP are mostly insoluble at increasing chase times during their transport to the cell surface. (B) FRT cells expressing PLAP were lysed in TNE/TX-100 buffer and run through a linear 5–40% sucrose gradient. FRT cells expressing NTR-PLAP were labeled overnight with [35S]cys and after lysis run on the gradient. Fractions of 1 ml were collected from top to bottom after centrifugation to equilibrium, and proteins from cells expressing PLAP were TCA precipitated, whereas labeled NTR-PLAP was immunoprecipitated from all fractions. Western blotting of PLAP and fluorography of NTR-PLAP indicate that both proteins are able to float, albeit at different rates, to lighter fractions on sucrose density gradients.
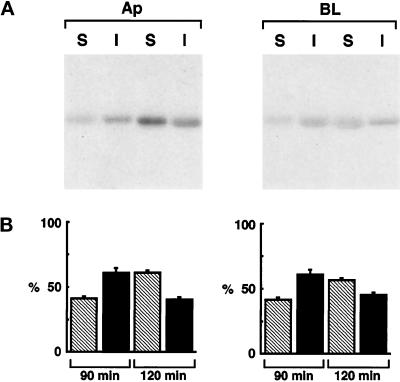
We then purified TX-100–insoluble fractions by centrifugation to equilibrium on sucrose density gradients and immunoprecipitated PLAP and NTR-PLAP from all fractions (Figure 2B). Both GPI-anchored proteins were enriched in the lighter fractions of the gradients. Although PLAP was enriched in fractions 4–7 (15–30% sucrose) compared with fractions 8–12 (40% sucrose), indicating that the major part of the protein was floating (Figure 2B, top), NTR-PLAP was present in equal amounts in fractions 4–7 and fractions 8–12 (Figure 2B, bottom). These results are in complete agreement with the pulse-chase TX-100 insolubility assay (compare top and bottom panels of Figure 2, A and B) and indicate that both PLAP and NTR-PLAP associate with TX-100–insoluble domains, albeit at different rates, during their transport to the plasma membrane. However, we found that after 90 min of chase PLAP was largely insoluble in TX-100 (∼60%) at both the apical and the basolateral surfaces, and that after 120 min of chase the insoluble fraction of PLAP was reduced to ∼40% on both surfaces (Figure 3). These results indicate that both the predominant apically targeted PLAP and the minor basolaterally targeted material were equally insoluble in TX-100 (∼60%) upon their arrival at the plasma membrane. Once at the plasma membrane, both apical and basolateral fractions show reduced association with detergent-insoluble microdomains. These experiments, therefore, reveal the presence of lipid microdomains on both surfaces and indicate that once a protein is incorporated into these microdomains it stays there, regardless of whether it is correctly transported to the apical membrane or is missorted to the basolateral side.
Figure 3.
Extraction in TX-100 of apical and basolateral PLAP in transfected FRT cells. (A) FRT cells expressing PLAP were labeled with [35S]met–cys for 30 min and chased for 90 or 120 min in chase medium. Cells were then biotinylated from the apical (Ap) and basolateral (BL) domains, lysed in TNE/1% TX-100 buffer at 4°C, and separated by centrifugation into soluble (S; supernatant) and insoluble (I; pellet) fractions that were immunoprecipitated with a specific antibody against PLAP and reprecipitated with streptavidin. Insoluble PLAP is present on the apical and basolateral surfaces at similar rates. (B) Quantification of three independent experiments is shown as mean ± SD. Hatched bars represent soluble proteins, and black bars represent insoluble proteins.
Ectodomains of PLAP and NTR-PLAP Are Apically Secreted but Are Not Present in TX-100–insoluble Microdomains
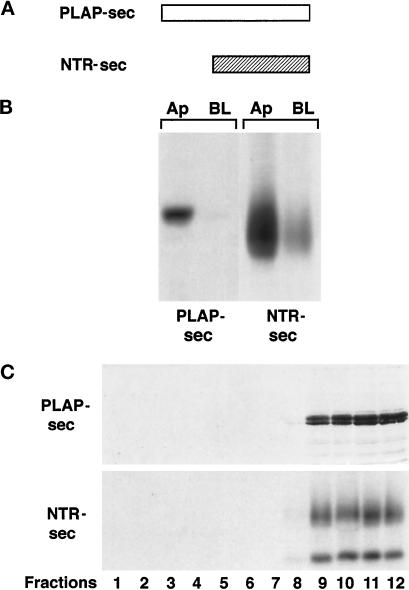
Data presented here together with our previous work on gD1-DAF (Zurzolo et al., 1994) show a strong correlation between GPI protein incorporation in DIGs and their apical delivery in FRT cells. Nonetheless, it is not clear whether this association is sufficient to determine apical sorting, because apical distribution of PLAP and NTR-PLAP in FRT cells could be accounted for by a dominant apical signal present in the protein ectodomains (Brown et al., 1989; Lisanti et al., 1989; Powell et al., 1991; Arreaza and Brown, 1995). Therefore, we analyzed whether the PLAP and NTR ectodomains contain apical sorting determinants recognized in FRT cells. After stable transfection of FRT cells with constructs encoding secretory forms, denoted PLAP-sec and NTR-sec (Figure 4A), we labeled cells growing on filters and collected separately the apical and basolateral media that were immunoprecipitated with specific antibodies. Both PLAP-sec and NTR-sec were almost totally secreted into the apical medium (Figure 4B), indicating that the ectodomains of PLAP and NTR-PLAP indeed contained apical sorting information.
Figure 4.
Secretion and purification on sucrose density gradients of PLAP-sec and NTR-sec in transfected FRT cells. (A) Schemes of the secretory forms of PLAP (PLAP-sec) and NTR-PLAP (NTR-sec). (B) Immunoprecipitation of PLAP-sec and NTR-sec from the apical and basolateral media after overnight labeling of transfected FRT cells grown on filters. (C) PLAP-sec– and NTR-sec–enriched fractions were purified on sucrose density gradients. FRT cells expressing NTR-sec, after labeling overnight with [35S]cys, were lysed and layered on a sucrose density gradient. Twelve fractions were collected from top to bottom after centrifugation to equilibrium. FRT cells expressing PLAP-sec were lysed, and after lysis they were purified on sucrose density gradients. Proteins of cells expressing PLAP-sec were TCA precipitated, run on SDS-PAGE, transferred to nitrocellulose, and blotted with an antibody against the ectodomain of PLAP. Both PLAP-sec and NTR-sec are almost exclusively restricted to the bottom fractions.
To determine whether lipid microdomains were involved in the sorting of the secretory forms to the apical membrane, we examined their incorporation into DIGs and their ability to float on sucrose density gradients. We found that both PLAP-sec and NTR-sec were completely soluble in TX-100 in pulse-chase experiments (our unpublished results) and that they fractionated almost completely to the bottom of sucrose density gradients in fractions 9–12 (40% sucrose) (Figure 4C), indicating that they do not segregate in lipid microdomains during their transport to the apical surface.
Transmembrane Forms of PLAP and NTR-PLAP Are Not Incorporated into TX-100–insoluble Microdomains
The data described above suggest that two different pathways could exist for sorting of apical proteins in FRT cells: one that is raft mediated and involving signals present in the membrane-associated portion of the protein, and another raft-independent pathway involving signals present in the ectodomain. To determine whether there might be a hierarchy between these signals, we studied whether the apical determinants present in the PLAP and NTR ectodomains could promote the association of transmembrane forms of these proteins with DIGs. Therefore, we stably expressed in FRT cells the cDNA encoding the wild-type p75NTR, a single-membrane-spanning glycoprotein, and the chimeric protein PLAP-PS321 (Figure 5A), which is formed by the ectodomain of PLAP and the transmembrane and the cytosolic tail of a mutant p75NTR (PS321), which has the same transmembrane domain as the wild-type protein but a different cytosolic tail containing a basolateral sorting signal (Le Bivic et al., 1991). We found that both transmembrane proteins remained completely soluble in TX-100 at increasing chase times in pulse-chase experiments (Figure 5B) and that they remained at the bottom of sucrose density gradients (Figure 5C). These data show that neither protein was incorporated into DIGs, indicating that the apical signal(s) present in the ectodomain of both proteins is not able to mediate DIG association (even if the protein is already associated with the membrane via a transmembrane domain). They also suggest that this association is exclusively dependent on the presence of a GPI anchor or of a specific transmembrane domain (as shown in the case of neuraminidase [Kundu et al., 1996] and hemagglutinin [HA] [Lin et al., 1998] in MDCK cells).
Figure 5.
Pulse-chase analysis and purification on sucrose gradients of transmembrane forms of PLAP and p75NTR in transfected FRT cells. (A) Schemes of p75NTR and PLAP-PS321 (a fusion protein containing the ectodomain of PLAP and the transmembrane and the cytoplasmic domain of the PS321 NTR mutant). (B) Pulse-chase and TX-100 extraction of p75NTR and PLAP-PS321. FRT cells expressing p75NTR and PLAP-PS321 were labeled with [35S]cys or [35S]met–cys, respectively, and subsequently treated as described in Figure 2A. (C) Purification of p75NTR- and PLAP-PS321–enriched fractions on sucrose density gradients. Labeled proteins of FRT cells expressing p75NTR and PLAP-PS321 were solubilized in TX-100 and purified as described in Figure 2B. Both transmembrane proteins are soluble in TX-100 and do not float on sucrose density gradients.
FB1 Treatment Affects Only Apical but Not Basolateral Sorting of GPI Proteins and Not the Sorting of Transmembrane and Secretory Forms
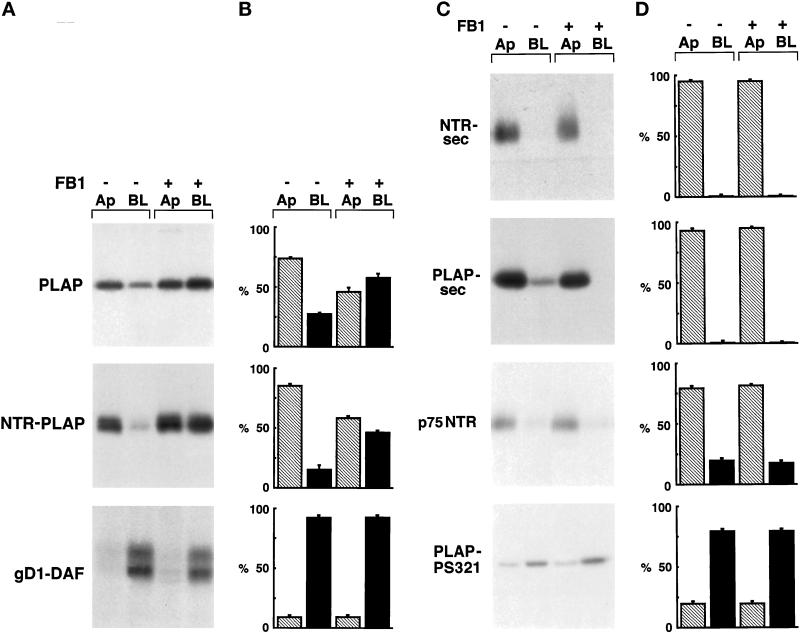
To further analyze the importance of raft association for the apical sorting of GPI-anchored proteins, we followed the incorporation into DIGs and the plasma membrane sorting of GPI and secretory and transmembrane proteins in cells treated with compounds that affect the intracellular levels of GSLs and cholesterol. FB1, a toxin derived from the fungus Fusarium moniliforme, specifically competes with sphingosine as a substrate of ceramide synthase and inhibits GSL synthesis (Wang et al., 1991). It was shown previously that in MDCK cells this fungal metabolite inhibits the biosynthesis of GSLs and alters apical sorting of the GPI-anchored protein GP-2 (Mays et al., 1995). To determine whether the apical distribution of PLAP and NTR-PLAP in FRT cells correlated with intracellular levels of GSLs, we treated FRT cells with FB1 and analyzed sorting to the plasma membrane of these two apical GPI-anchored proteins. FRT cells were grown on filters to confluence for 72 h in the presence of FB1 (25 μg/ml). This treatment does not induce morphological alterations, as revealed by the observation of treated cells in phase contrast and by analysis of the actin cytoskeleton. Furthermore, the values of transepithelial resistance were identical to those of control cells, indicating that treatment with this compound does not induce opening of the junctional complexes. After FB1 treatment, cells were pulsed for 30 min and chased for 120 min, and apical and basolateral proteins were biotinylated and revealed as usual. In these conditions, PLAP and NTR-PLAP were missorted to the basolateral membrane, in contrast to gD1-DAF, which maintained its basolateral distribution (Figure 6, A and B). These results indicate that FB1 alters apical but not basolateral sorting of GPI-anchored proteins in transfected FRT cells.
Figure 6.
Surface expression of GPI-anchored, secretory, and transmembrane proteins after FB1 treatment. (A) FRT cells stably expressing PLAP, NTR-PLAP, or gD1-DAF were grown to confluence in the absence (−) or presence (+) of FB1, as described in MATERIALS AND METHODS. After 30 min of labeling with [35S]met–cys or [35S]cys and a 2-h chase, cells were surface biotinylated from the apical (Ap) or the basolateral (BL) domain, lysed, immunoprecipitated against specific antibodies, and reprecipitated against streptavidin. (B) Amounts of labeled proteins were quantified from three independent experiments and are shown as means ± SD. Hatched bars represent apical proteins, and black bars represent basolateral proteins. Apical PLAP and NTR-PLAP, but not basolateral gD1-DAF, became unpolarized after FB1 treatment. (C) FRT cells expressing PLAP-sec, NTR-sec, p75NTR, or PLAP-PS321 were grown to confluence in the absence (−) or presence (+) of FB1, as described in MATERIALS AND METHODS. After labeling with [35S]met–cys or [35S]cys for 20 min and a chase of 2 h, apical and basolateral media were collected separately and cells were surface biotinylated from apical or basolateral domains. Polarized secretion of the secretory forms was revealed by immunoprecipitation in the apical and basolateral medium (top two panels), whereas biotinylated transmembrane proteins were revealed by double immunoprecipitation with specific antibody and streptavidin beads (bottom two panels). (D) Amounts of labeled proteins were quantified from three independent experiments and are shown as means ± SD. Hatched bars represent apical proteins, and black bars represent basolateral proteins. FB1 does not alter the sorting of transmembrane and secretory proteins.
The effect of FB1 on the apical sorting of PLAP and NTR-PLAP could be specific for the apical sorting of GPI-anchored proteins or it could be a phenomenon generally affecting apical transport. To distinguish between these two possibilities, we analyzed the polarized sorting of the transmembrane and the secretory forms after FB1 treatment. FB1 affected neither the apical sorting of the secretory PLAP-sec and NTR-sec or of the transmembrane p75NTR nor the basolateral delivery of transmembrane PLAP-PS321 (Figure 6, C and D), which is dependent on a dominant basolateral sorting signal contained in the PS321 cytosolic tail (Lipardi, Ruggiano, Monlauzeur, Nitsch, Le Bivic, Zurzolo, unpublished data). The effect of FB1, therefore, appears to be specific for apical GPI proteins and again suggests the existence of two apical pathways, one sensitive and the other insensitive to FB1 treatment, that are not discriminated by membrane association.
Cholesterol Depletion Does Not Affect Surface Localization of GPI-anchored and Transmembrane Proteins
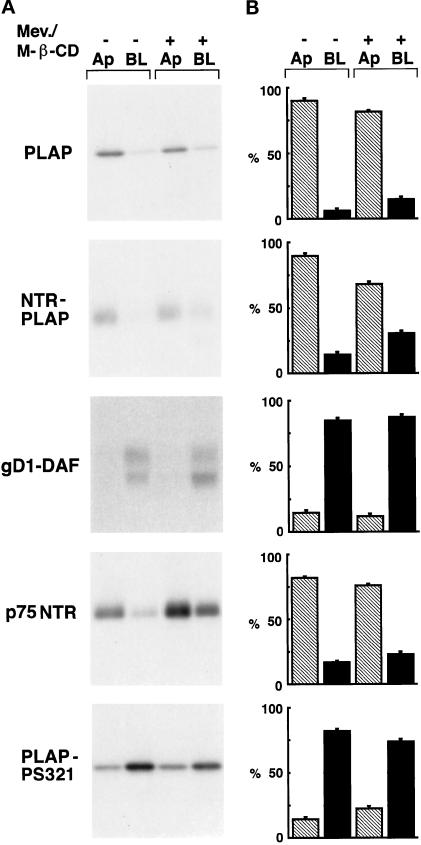
To examine the role of cholesterol in the sorting of PLAP and NTR-PLAP to the plasma membrane, we analyzed the sorting of these proteins in FRT cells in the presence of drugs that specifically decrease intracellular levels of cholesterol. Using [3H]cholesterol, as described by Keller and Simons (1998), we found that FRT cells have similar total amounts of cholesterol as MDCK cells (our unpublished results). A combined treatment of these cells with mevinolin (10 μM, 48 h), which inhibits cholesterol synthesis, and M-β-CD (10 mM, 60 min), which extracts cholesterol from the plasma membrane (Keller and Simons, 1998), results in an ∼60% removal of cholesterol (our unpublished results), similar to what was shown previously in MDCK cells (Keller and Simons, 1998). We analyzed the sorting of newly synthesized PLAP, NTR-PLAP, and gD1-DAF by pulse chase and domain-selective biotinylation in cells depleted of cholesterol. We found that neither the basolateral sorting of gD1-DAF nor the apical delivery of PLAP and NTR-PLAP was affected by cholesterol depletion (Figure 7, A and B, top three panels). Depletion of cholesterol, therefore, does not appear to affect either the apical or the basolateral sorting of GPI-anchored proteins in FRT cells. Similarly, cholesterol depletion did not affect the sorting of transmembrane forms (Figure 7, A and B, bottom two panels).
Figure 7.
Surface expression of GPI-anchored and transmembrane proteins after cholesterol depletion. (A) FRT cells expressing PLAP, NTR-PLAP, gD1-DAF, p75NTR, or PLAP-PS321 were grown on filters and treated with mevinolin (Mev.; 10 μM) for 48 h and M-β-CD (10 mM) for 60 min. Cells were then pulse labeled for 30 min, chased for 2 h, and biotinylated as usual. (B) Amounts of labeled proteins were quantified in three independent experiments and are shown as means ± SD. Hatched bars represent apical (Ap) proteins, and black bars represent basolateral (BL) proteins. Removal of cholesterol does not alter the surface expression of apical and basolateral GPI-anchored and transmembrane proteins.
TX-100 Extraction of PLAP and NTR-PLAP after Drug Treatment
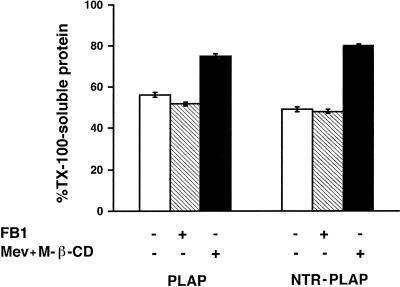
To rule out the possibility that the inability of cholesterol depletion to affect polarized sorting of GPI-anchored proteins was because it was not sufficient to modify their association with detergent-resistant microdomains, we examined the effect of mevinolin and M-β-CD on TX-100 extraction of PLAP and NTR-PLAP. We found that, compared with control cells, cholesterol depletion increased significantly (up to 80%) the amount of soluble PLAP and NTR-PLAP in TX-100 (Figure 8). Surprisingly, FB1 treatment did not have a significant effect on the TX-100 solubility of the two proteins (Figure 8). These experiments, therefore, indicate that the effect of FB1 on the surface sorting of PLAP and NTR-PLAP may not be related directly to a different partitioning of these proteins in TX-100–resistant microdomains. They also show that a reduction in cholesterol levels leads to an alteration in the TX-100 insolubility of PLAP and NTR-PLAP in FRT cells but does not affect apical sorting. These data indicate that DIG association is one feature of the apical pathway taken by GPI proteins and by some transmembrane proteins but that additional signals are required for the sorting of these proteins to the apical membrane. They also pose the important question of the relationship between lipid microdomains occurring in vivo and DIGs revealed in vitro by means of TX-100 extraction.
Figure 8.
TX-100 insolubility of PLAP and NTR-PLAP in FB1- or mevinolin (Mev)- and M-β-CD–treated cells. FRT cells expressing PLAP and NTR-PLAP were grown on dishes in the absence or presence of FB1 or mevinolin and M-β-CD, as described in MATERIALS AND METHODS. Cells were then labeled overnight, lysed in buffer containing 1% TX-100, and soluble and insoluble fractions were separated by centrifugation and immunoprecipitated with antibodies against PLAP and NTR-PLAP. Bars represent TX-100–soluble fractions as percentage of total protein. Although FB1 does not alter TX-100 behavior of PLAP and NTR-PLAP, the continued treatment with mevinolin and M-β-CD increases the TX-100 solubility of both proteins.
DISCUSSION
Although protein apical sorting is currently being intensively investigated, the mechanisms responsible are not yet known. Putative apical sorting signals have been found throughout the length of plasma membrane proteins: the ectodomains, the membrane-associated portion, and the cytosolic tail. N- and O-glycosylation groups have been shown to work as apical signals for some secretory and transmembrane proteins (Yeaman et al., 1997; Gut et al., 1998; Monlauzeur et al., 1998) but not for others (Alonso et al., 1997; Rodriguez-Boulan and Gonzalez, 1999), whereas signals within the membrane-associated portion are either specific amino acid sequences within the transmembrane-spanning regions, as shown in the case of HA (Lin et al., 1998) and neuraminidase (Kundu et al., 1996), or the GPI anchor of proteins anchored to the membrane via this glycolipid (Brown et al., 1989; Lisanti et al., 1989, 1990). The cytosolic tails of two seven-transmembrane-spanning proteins were recently implicated in the sorting of these proteins to the apical membrane in transfected MDCK cells (Chuang and Sung, 1998; Sun et al., 1998). Overall, a mechanism for apical sorting has been proposed that suggests that apical proteins are selectively incorporated into lipid microdomains or rafts in the TGN and are then transported to the apical membrane. This model is particularly attractive in the case of GPI proteins, which have been shown to be raft associated and apically delivered in the majority of epithelial cells studied (Lisanti et al., 1990; Harder and Simons, 1997; Brown and London, 1998). However, because the only proof of raft association is the insolubility of a given protein in a nonionic detergent and partition with DIGs, the existence of these microdomains in living cells has been heavily debated (Jacobson and Dietrich, 1999). Recent work from two independent laboratories has shown, by means of different techniques, that GPI-anchored proteins are indeed localized in cholesterol-dependent submicrometer-sized domains at the cell surface of MDCK and Chinese hamster ovary cells (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998). Therefore, a key question that must now be addressed regards the relationship between DIGs and the microdomains that occur in vivo.
In this work, we have studied whether GPI is an apical sorting signal and whether the association of GPI proteins with DIGs is necessary and sufficient for apical sorting. We found that, in contrast to gD1-DAF, which is TX-100 soluble and basolaterally sorted in FRT cells, two other transfected GPI-anchored proteins (PLAP and NTR-PLAP) are sorted directly to the apical domain in these cells (Figure 1, B and C) and associate with TX-100–insoluble microdomains during their transport to the apical membrane (Figure 2, A and B).
To determine whether rafts were required for the apical sorting of GPI proteins in FRT cells, we used FB1 and a combined treatment with mevinolin and M-β-CD to deplete the cells of sphingolipids and cholesterol, respectively. It was shown previously that FB1 affects apical sorting of the GPI-anchored protein GP-2 in MDCK cells (Mays et al., 1995). However, because this missorting was not correlated with a decrease in the association of the protein with DIGs, the authors could not exclude the possibility that this effect was a consequence of the disruption of ceramide signaling, similar to what has been suggested by Keller and Simons (1998). We now clearly show that in FRT cells, FB1 affects exclusively the apical delivery of newly synthesized PLAP and NTR-PLAP but not the basolateral delivery of gD1-DAF (Figure 6A). However, this effect was not dependent on reduced partitioning of the protein with DIGs, which remained unaffected in all cases (Figure 8). Interestingly, we observed that the effect of FB1 was rather specific for the apical delivery of newly synthesized GPI proteins and that this drug did not affect the apical sorting of newly synthesized transmembrane and secretory proteins (Figure 6, A–D). There are at least two possible explanations for these data. One is that the effect of FB1, although specific for raft-associated proteins, is not caused by a perturbation of their organization but rather by an indirect effect on ceramide signaling. The other possibility is that the effect of FB1 is a result of raft misorganization but that this effect is not revealed in the TX-100 extraction assay, implying that the rafts that are involved in sorting are not revealed by this technique, e.g., that they are different from DIGs.
Although cholesterol is a coorganizer of sphingolipid cholesterol domains, its role in protein sorting has not been clearly established. Keller and Simons (1998) reported increased solubility in TX-100 and partial missorting of HA in cholesterol-depleted MDCK cells, but Lin et al. (1998) found that in similar conditions the increased solubility of HA in TX-100 does not correlate with its missorting, and Hannan and Edidin (1996) have shown no missorting of gD1-DAF in cholesterol-depleted MDCK cells. However, it seems clear that cholesterol depletion affects the organization of both DIGs and submicrometer-sized rafts (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998), at least within the plasma membrane. We show here that cholesterol depletion of up to 60% does not affect apical sorting of PLAP and NTR-PLAP, although it increases their solubility in TX-100. From these data, we can conclude that DIGs are not involved in apical sorting of GPI-anchored proteins, but we cannot exclude the possibility that the conditions we used for cholesterol depletion did not affect microraft organization in the TGN. Two preliminary observations may suggest that this is indeed the case. One is that, in these conditions, we found a relocalization of the cholesterol-binding protein caveolin 1 from the plasma membrane to the Golgi apparatus, indicating the efficient removal of cholesterol from the plasma membrane but not from the Golgi. Second, in cholesterol-depleted cells, we observed strong labeling of the Golgi area with the cholesterol-binding drug filipin (our unpublished results). Together, these data indicate that there are major differences between DIGs obtained in vitro after TX-100 extraction and submicrometer-sized rafts that have been demonstrated to occur in vivo. Furthermore, although they exclude the involvement of DIGs in the apical sorting of GPI-anchored proteins, it is possible that TGN submicrometer-sized domains have a role in this event (see below).
Another related question concerns the role of the GPI anchor in apical sorting. It has been postulated that GPI is an apical sorting signal and that it acts by mediating raft association (Lisanti et al., 1990; Simons and Ikonen, 1997). However, we have shown previously that gD1-DAF is basolateral in FRT cells and is TX-100 soluble, in contrast to what we have reported here for PLAP and NTR-PLAP. One possible explanation is that these proteins possess different GPI anchors. However, preliminary data indicate that both PLAP and gD1-DAF GPI anchors contain saturated fatty acid chains of similar length (Rietveld, Benting, Zurzolo, and Simons, unpublished results). Another possibility is that the ectodomains of these proteins have an effect on raft association and/or apical sorting. We found previously that the gD1 ectodomain is secreted without polarity in FRT cells (Zurzolo et al., 1993), whereas we show here that PLAP and p75NTR contain apical sorting signals within their ectodomains (Figure 4B) that may be important for the apical sorting of the GPI-anchored forms. Interestingly, we found that PLAP-sec, NTR-sec, and two other transmembrane forms carrying PLAP and NTR ectodomains were completely soluble in TX-100 and were unable to float on sucrose density gradients (Figures 4 and 5), indicating that the ectodomains of these proteins are not capable of mediating the association with DIGs. Together, these data clearly indicate that GPI is not responsible for apical sorting; rather, it mediates raft association of GPI-anchored proteins, whereas a signal present in the proteinaceous portion of the molecule plays a major role in the sorting event. A similar conclusion was reached recently with another model GPI-anchored protein expressed in MDCK cells via adenovirus infection (Benting et al., 1999). We postulate a two-step mechanism for apical sorting of GPI proteins. The first step is association with rafts, which is mediated by the GPI anchor; the second step is stabilization of the protein into rafts, which may be dependent on the recognition of an apical sorting signal present within the ectodomain of the protein. Preliminary data with tunicamycin indicate that in some (but not all) cases this signal could be dependent on correct glycosylation of the protein (Lipardi and Zurzolo, unpublished results). Interestingly, we found that there is a difference in the amount of protein association with DIGs (Figure 2, A and B) between PLAP and NTR-PLAP that could be the result of the difference in the ectodomains of these two proteins. Therefore, raft association could increase the efficiency of recognition of an apical sorting signal in the ectodomain of the protein by recruiting the sorting machinery. The model that we propose here could also explain why gD1-DAF is not found in TX-100–insoluble microdomains and is basolaterally sorted in FRT cells, even though it possesses a similar GPI anchor to PLAP but not an apical signal in the ectodomain.
Our TX-100 extraction and FB1 experiments appear to have identified two apical sorting pathways in FRT cells, one that is raft dependent that is used by PLAP and NTR-PLAP and one that is raft independent that is used by their secretory and transmembrane forms. Two different apical vesicles may exist, one enriched in raft-associated proteins and the other enriched in TX-100–soluble proteins. However, it is also possible that one class of apical vesicles includes both raft-associated and non-raft-associated proteins. Raft association could simply be an intrinsic characteristic of a protein resulting from its chemical and physical properties. However, the fact that to date only apical proteins have been found associated with rafts suggests that rafts may be used to recruit components of the apical sorting machinery that have to recognize a signal exposed in the vesicle lumen. Rafts, therefore, may have a mainly organizational role in the apical sorting of GPI-anchored proteins, whereas additional requirements are needed in the ectodomains of proteins for recognition by putative components of the apical machinery.
ACKNOWLEDGMENTS
This work is dedicated to the memory of Prof. Gaetano Salvatore, to whom C.Z. will be always grateful for having introduced her to science. We thank Dr. Andrè Le Bivic for the generous gifts of various cDNAs and for helpful discussions. We also thank Dr. Chris Bowler for critical revision of the manuscript and Mario Belardone for photographic reproductions. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (1998), the Ministero per l'Università e la Ricerca Scientifica e Tecnologica, the Consiglio Nazionale delle Ricerche (Target Project on Biotechnology), and the European Union (BIO 4-CT-986055).
Abbreviations used:
- DIG
detergent-insoluble glycosphingolipid complex
- FB1
Fumonisin B1
- FRT
Fischer rat thyroid
- GPI
glycosylphosphatidyl inositol
- GSL
glycosphingolipid
- HA
hemagglutinin
- MDCK
Madin–Darby canine kidney
- M-β-CD
methyl-β-cyclodextrin
- NTR
Neurotrophin Receptor
- PLAP
Placental Alkaline Phosphatase
- TCA
trichloroacetic acid
- TGN
trans-Golgi network
- TNE
Tris, NaCl, and EDTA
- TX-100
Triton X-100
REFERENCES
- Alonso MA, Fan L, Alarcon B. Multiple sorting signals determine apical localization of a nonglycosylated integral membrane protein. J Biol Chem. 1997;272:30748–30752. doi: 10.1074/jbc.272.49.30748. [DOI] [PubMed] [Google Scholar]
- Arreaza G, Brown DA. Sorting and intracellular trafficking of a glycosylphosphatidylinositol-anchored protein and two transmembrane proteins with the same ectodomain in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1995;270:23641–23647. doi: 10.1074/jbc.270.40.23641. [DOI] [PubMed] [Google Scholar]
- Benting JH, Rietveld AG, Simons K. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J Cell Biol. 1999;146:313–320. doi: 10.1083/jcb.146.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Micanovic R, Greenspan RJ, Udenfriend S. Conversion of placental alkaline phosphatase from a phosphatidylinositol-glycan-anchored protein to an integral transmembrane protein. Proc Natl Acad Sci USA. 1989;86:1457–1460. doi: 10.1073/pnas.86.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Parton RG, Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991;349:158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Science. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell. 1995;82:5–8. doi: 10.1016/0092-8674(95)90045-4. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by cross-linking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- Graichen R, Losch A, Appel D, Koch-Brandt C. Glycolipid-independent sorting of a secretory glycoprotein to the apical surface of polarized epithelial cells. J Biol Chem. 1996;271:15854–15857. doi: 10.1074/jbc.271.27.15854. [DOI] [PubMed] [Google Scholar]
- Gut A, Balda MS, Matter K. The cytoplasmic domains of a β1 integrin mediate polarization in Madin-Darby canine kidney cells by selective basolateral stabilization. J Biol Chem. 1998;273:29381–29388. doi: 10.1074/jbc.273.45.29381. [DOI] [PubMed] [Google Scholar]
- Hannan LA, Edidin M. Traffic, polarity, and detergent solubility of glycosylphosphatidylinositol-anchored protein after LDL-deprivation of MDCK cells. J Cell Biol. 1996;133:1265–1276. doi: 10.1083/jcb.133.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Sano Y, Ueda M, Higashio K, Narita H, Okano M, Matsumoto S, Sasaki R. N-Glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp Cell Res. 1994;213:449–457. doi: 10.1006/excr.1994.1222. [DOI] [PubMed] [Google Scholar]
- Kundu A, Avalos RT, Sanderson CM, Nayak DP. Transmembrane domain of influenza virus neuramidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E. An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol. 1991;115:607–618. doi: 10.1083/jcb.115.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Naim HY, Rodriguez C, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Nitsch L, Zurzolo C. Mechanisms of protein apical sorting in thyroid polarized epithelial cells. Biochimie. 1999;81:347–353. doi: 10.1016/s0300-9084(99)80080-7. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Le Bivic A, Saltiel AR, Rodriguez-Boulan E. Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J Membr Biol. 1990;113:155–167. doi: 10.1007/BF01872889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo MP, Bull P, Gonzalez A. Apical sorting of hepatitis B surface antigen (HBsAg) is independent of N-glycosylation and glycosylphosphatidylinositol-anchored protein segregation. Proc Natl Acad Sci USA. 1997;94:1834–1839. doi: 10.1073/pnas.94.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Monlauzeur L, Breuza L, Le Bivic A. Putative O-glycosylation sites and a membrane anchor are necessary for apical delivery of the human neurotrophin receptor in Caco-2 cells. J Biol Chem. 1998;273:30263–30270. doi: 10.1074/jbc.273.46.30263. [DOI] [PubMed] [Google Scholar]
- Monlauzeur L, Rajasekaran A, Chao M, Rodriguez-Boulan E, Le Bivic A. A cytoplasmic tyrosine is essential for the basolateral localization of mutants of human Nerve Growth Factor receptor in Madin-Darby canine kidney cells. J Biol Chem. 1995;270:12219–12225. doi: 10.1074/jbc.270.20.12219. [DOI] [PubMed] [Google Scholar]
- Powell SK, Lisanti M, Rodriguez-Boulan E. Thy-1 expresses two signals for apical localization in epithelial cells. Am J Physiol. 1991;260:C715–C720. doi: 10.1152/ajpcell.1991.260.4.C715. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Gonzalez A. Glycans in postGolgi apical targeting: sorting signals or structural props? Trends Cell Biol. 1999;9:291–294. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-Glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Soole KL, Jepson MA, Hazlewood GP, Gilbert HJ, Hirst BH. Epithelial sorting of a glycosylphosphatidylinositol-anchored bacterial protein expressed in polarized renal MDCK and intestinal Caco-2 cells. J Cell Sci. 1995;108:369–377. doi: 10.1242/jcs.108.1.369. [DOI] [PubMed] [Google Scholar]
- Sun AQ, Ananthanarayanan M, Soroka CJ, Thevananther S, Shneider BL, Suchy FJ. Sorting of rat liver and ileal sodium-dependent bile acid transporters in polarized epithelial cells. Am J Physiol. 1998;275:G1045–G1055. doi: 10.1152/ajpgi.1998.275.5.G1045. [DOI] [PubMed] [Google Scholar]
- Tienari PJ, et al. The beta-amyloid domain is essential for axonal sorting of amyloid precursor protein. EMBO J. 1996;15:5218–5229. [PMC free article] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111:987–1000. doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon W, Riley RT, Merrill AH., Jr Inhibition of sphingolipid biosynthesis by fumonisins: implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Lu D, Sadler JE. Apical sorting of bovine enteropeptidase does not involve detergent-resistant association with sphingolipid-cholesterol rafts. J Biol Chem. 1999;274:1596–1605. doi: 10.1074/jbc.274.3.1596. [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Le Bivic A, Quaroni A, Nitsch L, Rodriguez-Boulan E. Modulation of transcytotic and direct targeting pathways in a polarized thyroid cell line. EMBO J. 1992;11:2337–2344. doi: 10.1002/j.1460-2075.1992.tb05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C, Lisanti MP, Caras IW, Nitsch L, Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993;121:1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurzolo C, van't Hof W, van Meer G, Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994;13:42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]