Abstract
This national surveillance study presents the in vitro activities of the main antimicrobial agents against 1,331 S. pneumoniae isolates as tested by an agar dilution method according to the guidelines of the Clinical and Laboratory Standards Institute (formerly NCCLS). The strains were isolated in several regions of Portugal from cases of invasive disease over an 11-year period (1994 to 2004). This study shows that the percentage of penicillin-nonsusceptible strains increased from 12% in 1994 to 28.5% in 2000. Then the rate declined to 17.7% in 2003 but increased again to 23.2% in 2004. Nevertheless, the rate of highly resistant isolates declined consistently, to 0.9% in 2001 to 2004. Ceftriaxone- and cefotaxime-nonsusceptible isolates became less frequent, from 4% and 8%, respectively, in 1994 to ≤1% in 2004. The macrolide-lincosamide-streptogramin B phenotype was the predominant macrolide phenotype found. The increase in the percentage of isolates that were only nonsusceptible to erythromycin (3.7% in 1994 to 1998 to 9.1% in 2002 to 2004) was similar to that for isolates with coresistance to penicillin and erythromycin (3.3% in 1994 to 1998 to 9.1% in 2002 to 2004). The nonsusceptibility to ciprofloxacin increased during recent years, from 0.5% in 2002 to 3.5% in 2004. Multidrug resistance also increased in recent years: from 7.9% in 2002 to 15.6% in 2004. The increasing use of macrolides could be causing the increase in penicillin and multidrug resistance, due to the coresistance to macrolides. The use of penicillin to treat empirical invasive pneumococci infections may need to be reconsidered.
Streptococcus pneumoniae is an important pathogen responsible for serious invasive diseases, including meningitis and septicemia. The spread of multidrug-resistant (MDR) pneumococci has become a worldwide problem, making treatment more difficult (18). Indeed, in addition to resistance to penicillin, resistance to other antibiotics, including erythromycin, tetracycline, and chloramphenicol, has emerged and is spreading (10).
Since 1989, the National Institute of Health Dr. Ricardo Jorge reference laboratory has been continually monitoring the in vitro activity of antimicrobial agents against S. pneumoniae collected from invasive sources. This program for monitoring susceptibility to antibiotics in Portugal (ARSIP) provides a unique collection of Portuguese pneumococcal isolates. This national surveillance study reported that 4.6% of isolates were penicillin nonsusceptible in 1989, and this value remained generally stable until 1991 (6.4%) (32). Fully penicillin-resistant isolates (MIC of 2 μg/ml) were reported for the first time in 1992 (0.8%) and made up 5.5% of isolates in the following year (32).
Here, we describe the surveillance of pneumococci by the reference laboratory in Portugal. We report the in vitro activities of different antimicrobial agents used against S. pneumoniae isolated from invasive sources over 11 years (from 1994 to 2004).
MATERIALS AND METHODS
Patients and bacterial isolates.
Between 1 January 1994 and 31 December 2004, the ARSIP survey conducted by the Antibiotic Resistance Unit (ARU) from the National Institute of Health Dr. Ricardo Jorge constantly monitored pneumococcal isolates from cases of invasive disease in various regions of Portugal. The national laboratory-based surveillance system collected 1,331 invasive pneumococcal strains, which were isolated in 24 bacteriology laboratories in hospitals and public health institutions. In the period 1994 to 1998, 12 hospitals participated in the study, and since 1999, 12 more hospitals have been added to the network. Isolates were included if they were nonrepetitive or consecutive blood, cerebrospinal fluid (CSF), or pleural fluid samples from patients with symptoms compatible with invasive pneumococcal disease. No changes were made to the methods of data collection during the study. Some isolates were from outpatients, but most were from patients hospitalized with community-acquired invasive pneumococci disease. Only one isolate per patient was considered. Patients over 15 years old were considered to be adults.
Identification and serotyping.
The isolates were sent at −20°C by hospital laboratories to the reference laboratory, ARU, in Trypticase soy broth (TSB; Oxoid, Basingstoke, England) containing 20% glycerol. On reception by the ARU, the purity of the pneumococcal isolate was checked using standard methods, and the isolate was then stored at −80°C in TSB containing 20% glycerol. Isolates were serotyped by dot blotting, the Quellung reaction, or both (11).
Antimicrobial susceptibility testing.
Susceptibility testing was performed by the agar dilution method. MICs of penicillin (Wyeth Lederle Portugal, Algés, Portugal), cefotaxime (Farma-APS Produtos Farmacêuticos, Lisboa, Portugal), ceftriaxone (Roche Farmacêutica Química, Amadora, Portugal), tetracycline (Laboratórios Atral, Carregado, Portugal), chloramphenicol (Edol, Linda-a-Velha, Portugal), erythromycin (Abbott Laboratórios, Amadora, Portugal), clindamycin (Pharmacia Corporation Laboratórios, Carnaxide, Portugal), ofloxacin (Aventis Pharma, Mem-Martins, Portugal), ciprofloxacin (Bayer Portugal, Carnaxide, Portugal), and vancomycin (Lilly Farma, Algés, Portugal) were determined according to the testing conditions and susceptibility interpretation standards proposed by the Clinical and Laboratory Standards Institute (CLSI; formerly NCCLS) (29). Susceptibility to trimethoprim-sulfamethoxazole was performed by a disk diffusion method according to CLSI recommendations (29). Isolates with intermediate- or high-level resistance were classified as nonsusceptible. Isolates that were nonsusceptible to at least three different antibiotic classes were classified as multidrug resistant. Erythromycin-nonsusceptible isolates were classified as having the macrolide (M) or macrolide-lincosamide-streptogramin B (MLSB) phenotype. The M phenotype was scored when the isolate was nonsusceptible only to erythromycin. The MLSB phenotype was scored when the isolate was nonsusceptible to both erythromycin and clindamycin (20). MICs of vancomycin and ciprofloxacin were only determined from 1 January 1999. An isolate with a MIC of ciprofloxacin of ≥4 μg/ml was considered nonsusceptible according to the association with mutations in the genes encoding DNA topoisomerase IV and DNA gyrase A (36).
Statistical analyses.
SPSS software, version 13.0, was used for statistical analysis. The chi-square test or Fisher's exact test was used when appropriate. Two-sided P values of <0.05 were considered to be statistically significant. Correlations between antimicrobial nonsusceptibility rates were assessed using the Spearman correlation coefficient.
RESULTS
A total of 1,331 pneumococcus strains were isolated during an 11-year period in 24 hospitals and public health institutions across Portugal and were included in the study: 73.3% were isolated from blood, 20.4% from CSF, 4.5% from pleural fluid, and 1.9% from both blood and CSF (Table 1). The age of the patient was known for 1,219 (91.6%) of the isolates. Ninety-seven (8.0%) isolates were from patients <1 year old, 156 (12.8%) were from patients 1 to 9 years old, 77 (6.3%) were from patients 10 to 14 years old, 557 (45.7%) were from patients 15 to 64 years old, and 332 (27.2%) were from patients ≥65 years of age. The ages of patients for the remaining 112 isolates were unknown.
TABLE 1.
Penicillin susceptibility and MDR according to biological source
| Perioda | Biological source | PENb | No. (%) of isolates with MDR | Total no. of isolates |
|---|---|---|---|---|
| 1994-1998 | Blood | 22 (10.3/3.0) | 9 (5.7) | 165 |
| CSF | 20 (18.6/4.7) | 5 (5.9) | 86 | |
| CSF + blood | 0 (0) | 0 (0) | 1 | |
| Pleural fluid | 4 (11.8/11.8) | 3 (17.6) | 17 | |
| 1999-2001 | Blood | 78 (18.4/3.7) | 23 (6.6) | 354 |
| CSF | 20 (16.2/2.9) | 5 (4.8) | 105 | |
| CSF + blood | 5 (41.7/0.0) | 0 (0) | 12 | |
| Pleural fluid | 6 (21.1/10.5) | 2 (11.1) | 19 | |
| Pleural fluid + blood | 0 (0) | 0 (0) | 1 | |
| 2002-2004 | Blood | 98 (20.9/0.7) | 57 (12.9) | 455 |
| CSF | 16 (18.8/1.3) | 7 (9.0) | 80 | |
| CSF + blood | 1 (0.0/8.3) | 0 (0) | 12 | |
| Pleural fluid | 4 (16.7/0.0) | 1 (4.3) | 24 |
For the period 1994 to 1998, 12 laboratories participated, and for the periods 1999 to 2001 and 2002 to 2004, 24 laboratories participated. The period 2002 to 2004 was after the introduction of pneumococcal conjugate vaccine.
No. of isolates nonsusceptible to penicillin (% of isolates with intermediate/high-level resistance).
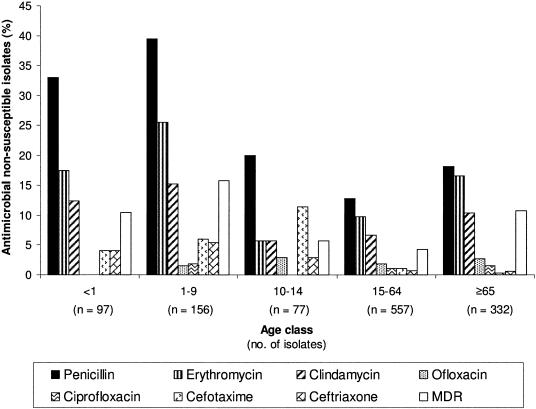
The in vitro susceptibility data for 10 antimicrobial agents are presented in Table 2. The proportion of penicillin-nonsusceptible isolates was 20.6% (18.0% with intermediate resistance [MIC of 0.1 to 1 μg/ml] and 2.6% with high-level resistance [MIC of ≥2 μg/ml]), and the MIC90 was 0.5 μg/ml (Table 2). Isolates with intermediate penicillin resistance became more frequent: from 13.0% in the period 1994 to 1998 and 18.5% in 1999 to 2001 to 20% in 2002 to 2004 (P = 0.047) (Table 3). However, the percentage of isolates with high-level resistance decreased from 4.1% to 3.7% and then to 0.9% (P = 0.003) (Table 3). The percentage of isolates nonsusceptible only to penicillin decreased in the most recent years to 11.7% (2002 to 2004). Penicillin nonsusceptibility was found associated with macrolide nonsusceptibility (P = 0.002), trimethoprim-sulfamethoxazole resistance (P = 0.017), and multidrug resistance (P = 0.001) among adults. The isolates with intermediate penicillin resistance from adults were also associated with isolates nonsusceptible to penicillin plus erythromycin isolates (P = 0.006). Similar proportions of isolates had intermediate and high-level resistance in blood (18.1%, and 2.4%, respectively) and CSF (17.9% and 3.0%, respectively) (P = 0.84) (Table 1). Thus, the proportions of isolates nonsusceptible to penicillin were similar for the blood (20.5%) and CSF (20.9%) isolate subgroups (P = 0.87) (Table 1). Isolates with intermediate resistance were more common in children between 1 and 9 years of age (36.5%) (Fig. 1). The frequencies of both isolates with intermediate resistance (23.0%) and those with high-level resistance (6.0%) were higher in children than in adults (15.8%; 1.0%, respectively) (P < 0.001) (Fig. 1). Serotype 14 was consistently the most frequent of the nonsusceptible (both intermediate and high-level resistance) isolates. Serogroup 19 increased approximately fivefold between the periods 1999 to 2001 and 2002 to 2004. Between the same periods, serogroups 23, 9, and 6 decreased (Table 4).
TABLE 2.
Cumulative MICs of 10 antimicrobial agents for isolates of S. pneumoniae collected in Portugal between 1994-2004
| Antimicrobial agent | Cumulative % of isolates inhibited by MIC (μg/ml) of:
|
% of isolates with resistancea:
|
Total no. of isolates | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | I | R | ||
| Penicillin | 57.7b | 76.8 | 79.4 | 84.7 | 86.2 | 92.3 | 97.4 | 100.0c | 18.0 | 2.6 | 1,331 | |||||
| Cefotaxime | 5.3b | 57.1 | 60.6 | 62.6 | 87.8 | 94.3 | 98.8 | 99.9 | 100.0 | 2.3 | 0.2 | 1,329 | ||||
| Meningeal isolates | 5.1b | 62.8 | 68.9 | 71.6 | 88.5 | 93.6 | 99.3 | 100.0 | 5.7 | 0.7 | 296 | |||||
| Nonmeningeal isolates | 5.3b | 55.5 | 58.3 | 60.0 | 87.6 | 94.5 | 98.6 | 99.9 | 100.0 | 1.2 | 0.1 | 1,033 | ||||
| Ceftriaxone | 4.1b | 56.6 | 60.8 | 62.5 | 87.4 | 94.8 | 99.2 | 99.9 | 100.0 | 1.9 | 0.2 | 1,329 | ||||
| Meningeal isolates | 2.7b | 62.2 | 68.9 | 71.3 | 88.5 | 94.3 | 99.7 | 100.0 | 5.4 | 0.3 | 296 | |||||
| Nonmeningeal isolates | 4.5b | 55.0 | 58.5 | 60.0 | 87.1 | 95.0 | 99.0 | 99.9 | 100.0 | 0.9 | 0.1 | 1,033 | ||||
| Tetracycline | 0.7b | 9.8 | 50.1 | 62.4 | 85.4 | 87.7 | 88.5 | 90.4 | 94.0 | 97.2 | 100.0c | 0.8 | 11.5 | 1,329 | ||
| Erythromycin | 1.3b | 49.1 | 85.2 | 85.6 | 86.2 | 89.5 | 90.4 | 91.6 | 93.4 | 94.1 | 95.7 | 100.0c | 0.5 | 13.8 | 1,329 | |
| Clindamycin | 0.8b | 61.0 | 90.1 | 90.6 | 90.8 | 92.6 | 93.0 | 93.2 | 93.5 | 93.9 | 94.9 | 100.0c | 0.2 | 9.2 | 1,322 | |
| Choramphenicol | 1.2 | 8.5 | 74.1 | 97.3 | 98.4 | 100.0 | NA | 2.7 | 1,322 | |||||||
| Ofloxacin | 0.5 | 29.0 | 98.0 | 99.7 | 99.8 | 99.8 | 100.0 | 1.7 | 0.3 | 1,322 | ||||||
| Ciprofloxacin | 0.1 | 3.4 | 22.6 | 81.4 | 98.6 | 99.7 | 99.8 | 99.8 | 99.8 | 100.0 | NA | 1.4 | 1,104 | |||
| Vancomycin | 0.1b | 6.1 | 57.4 | 100.0 | 0.0 | 0.0 | 1,103 | |||||||||
I, intermediate; R, resistant. NA, not applicable.
MIC equal to or less than the given value.
MIC equal to or greater than the given value.
TABLE 3.
Year-to-year changes in nonsusceptible and multidrug-resistant S. pneumoniae isolates during 11-year study
| Yr | % of nonsusceptible isolates (no. intermediate/resistant)a
|
Total no. of isolates | % (no.) of isolates with MDR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | CRO | CTX | TET | ERY | CLI | CHL | CIP | OFX | |||
| 1994 | 12.0 (2/1) | 4.0 (1/0) | 8.0 (2/0) | 8.0 (1/1) | 4.0 (0/1) | 4.0 (1/0) | 4.0 (NA/1) | ND | 4.0 (0/1) | 25 | 4.0 (1) |
| 1995 | 20.0 (13/1) | 2.9 (1/1) | 2.9 (1/1) | 10.0 (0/7) | 5.7 (0/4) | 2.9 (0/2) | 4.3 (NA/3) | ND | 4.3 (3/0) | 70 | 2.9 (2) |
| 1996 | 14.5 (4/5) | 4.8 (3/0) | 8.1 (5/0) | 11.3 (0/7) | 4.8 (0/3) | 4.8 (0/3) | 6.5 (NA/4) | ND | 4.8 (3/0) | 62 | 8.2 (5) |
| 1997 | 18.2 (8/4) | 4.5 (3/0) | 7.6 (4/1) | 9.1 (1/5) | 7.6 (0/5) | 6.1 (0/4) | 0.0 (NA/0) | ND | 1.5 (1/0) | 66 | 6.3 (4) |
| 1998 | 17.4 (8/0) | 2.2 (1/0) | 0.0 (0/0) | 4.3 (0/2) | 13.0 (0/6) | 6.5 (0/3) | 2.2 (NA/1) | ND | 2.2 (1/0) | 46 | 11.4 (5) |
| 1999 | 16.6 (19/10) | 2.9 (5/0) | 2.3 (5/0) | 8.7 (1/14) | 12.7 (0/22) | 9.2 (0/16) | 1.2 (NA/2) | 1.2 (NA/2) | 1.2 (0/2) | 175 | 4.6 (8) |
| 2000 | 28.5 (35/4) | 2.9 (3/1) | 4.4 (5/1) | 13.1 (2/16) | 11.7 (1/15) | 10.9 (0/15) | 2.2 (NA/3) | 0.0 (NA/0) | 0.0 (0/0) | 137 | 6.6 (9) |
| 2001 | 22.9 (37/4) | 2.2 (4/0) | 2.2 (4/0) | 11.2 (0/20) | 16.8 (0/30) | 11.7 (0/21) | 2.2 (NA/4) | 0.6 (NA/1) | 1.1 (2/0) | 179 | 7.3 (13) |
| 2002 | 21.1 (42/2) | 1.4 (3/0) | 1.4 (3/0) | 10.0 (1/20) | 12.0 (0/25) | 7.7 (0/16) | 2.9 (NA/6) | 0.5 (NA/1) | 0.0 (0/0) | 209 | 7.9 (16) |
| 2003 | 17.7 (28/1) | 0.0 (0/0) | 0.0 (0/0) | 17.7 (3/26) | 20.1 (2/31) | 13.0 (2/19) | 3.1 (NA/5) | 1.8 (NA/3) | 1.2 (2/0) | 164 | 11.8 (19) |
| 2004 | 23.2 (44/2) | 0.5 (1/0) | 0.5 (1/0) | 18.2 (1/35) | 23.2 (4/42) | 11.4 (0/22) | 3.6 (NA/7) | 3.5 (NA/7) | 5.7 (10/1) | 198 | 15.6 (30) |
| Total | 20.6 (240/34) | 2.0 (25/2) | 2.4 (30/3) | 12.3 (10/153) | 14.4 (7/184) | 9.4 (3/121) | 2.7 (NA/36) | 1.4 (NA/15) | 2.0 (22/4) | 1,331 | 8.6 (112) |
PEN, penicillin; CRO, ceftriaxone; CTX, cefotaxime; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; CHL, chloramphenicol; CIP, ciprofloxacin; OFX, ofloxacin. NA, not applicable; ND, not determined.
FIG. 1.
Distribution by age class of S. pneumoniae isolates that were nonsusceptible to antimicrobial agents.
TABLE 4.
Serotype distribution of S. pneumoniae isolates that were nonsusceptible to different antimicrobial agents from 1994 to 2004
| Antimicrobial agent and period | No. (%) of isolates with pneumococcal serogroup/type:
|
Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 7 | 9 | 14 | 15 | 17 | 19 | 23 | 24 | 33 | Othersa | NDb | ||
| Penicillin | |||||||||||||||
| 1994-1998 | 0 | 3 (6.5) | 0 | 7 (15.2) | 20 (43.5) | 1 (2.2) | 0 | 3 (6.5) | 10 (21.7) | 0 | 2 (4.3) | 0 | 46 | ||
| 1999-2001 | 0 | 7 (6.4) | 1 (0.9) | 17 (15.6) | 58 (53.2) | 1 (0.9) | 0 | 5 (4.6) | 19 (17.4) | 0 | 1 (0.9) | 0 | 109 | ||
| 2002-2004 | 1 (0.8) | 4 (3.4) | 0 | 10 (8.4) | 37 (31.1) | 5 (4.2) | 1 (0.8) | 26 (21.8) | 16 (13.4) | 2 (1.7) | 0 | 17 (14.3) | 119 | ||
| Total | 1 (0.4) | 14 (5.1) | 1 (0.4) | 34 (12.4) | 115 (42.0) | 7 (2.6) | 1 (0.4) | 34 (12.4) | 45 (16.4) | 2 (0.7) | 3 (1.1) | 17 (6.2) | 274 | ||
| Cefotaxime | |||||||||||||||
| 1994-1998 | 0 | 4 (28.6) | 6 (42.9) | 0 | 4 (28.6) | 14 | |||||||||
| 1999-2001 | 1 (7.1) | 4 (28.6) | 9 (64.3) | 0 | 0 | 14 | |||||||||
| 2002-2004 | 0 | 0 | 3 (75) | 1 (25) | 0 | 4 | |||||||||
| Total | 1 (3.1) | 8 (25) | 18 (56.3) | 1 (3.1) | 4 (12.5) | 32 | |||||||||
| Ceftriaxone | |||||||||||||||
| 1994-1998 | 0 | 3 (30) | 3 (30) | 1 (10) | 3 (30) | 10 | |||||||||
| 1999-2001 | 1 (7.7) | 4 (30.8) | 8 (61.5) | 0 | 0 | 13 | |||||||||
| 2002-2004 | 0 | 0 | 3 (75) | 1 (25) | 0 | 4 | |||||||||
| Total | 1 (3.7) | 7 (25.9) | 14 (51.9) | 2 (7.4) | 3 (11.1) | 27 | |||||||||
| Erythromycin | |||||||||||||||
| 1994-1998 | 0 | 0 | 7 (36.8) | 0 | 1 (5.3) | 6 (31.6) | 1 (5.3) | 0 | 2 (10.5) | 1 (5.3) | 0 | 0 | 1 (5.3) | 0 | 19 |
| 1999-2001 | 0 | 2 (2.9) | 20 (29.4) | 2 (2.9) | 3 (4.4) | 21 (30.9) | 3 (4.4) | 0 | 12 (17.6) | 3 (4.4) | 0 | 1 (1.5) | 1 (1.5) | 0 | 68 |
| 2002-2004 | 1 (1.0) | 0 | 10 (9.6) | 0 | 3 (2.9) | 21 (20.2) | 5 (4.8) | 1 (1.0) | 28 (26.9) | 2 (1.9) | 2 (1.9) | 4 (3.8) | 1 (1.0) | 26 (25.0) | 104 |
| Total | 1 (0.5) | 2 (1.0) | 37 (19.4) | 2 (1.0) | 7 (3.7) | 48 (25.1) | 9 (3.1) | 1 (0.5) | 42 (22.0) | 6 (3.1) | 2 (1.0) | 5 (2.6) | 3 (1.6) | 26 (13.6) | 191 |
| Clindamycin | |||||||||||||||
| 1994-1998 | 0 | 7 (53.8) | 0 | 0 | 1 (7.7) | 1 (7.7) | 0 | 2 (15.4) | 1 (7.7) | 0 | 0 | 1 (7.7) | 0 | 13 | |
| 1999-2001 | 2 (3.8) | 17 (32.7) | 1 (1.9) | 0 | 14 (26.9) | 2 (3.8) | 0 | 12 (23.1) | 3 (5.8) | 0 | 1 (1.9) | 0 (0.0) | 0 | 52 | |
| 2002-2004 | 0 | 9 (15.3) | 0 | 2 (3.4) | 4 (6.8) | 5 (8.5) | 1 (1.7) | 17 (28.8) | 2 (3.4) | 2 (3.4) | 4 (6.8) | 1 (1.7) | 12 (20.3) | 59 | |
| Total | 2 (1.6) | 33 (26.6) | 1 (1.8) | 2 (1.6) | 19 (15.3) | 8 (6.5) | 1 (0.8) | 31 (25.0) | 6 (4.8) | 2 (1.6) | 5 (4.0) | 2 (1.6) | 12 (9.7) | 124 | |
| Ciprofloxacin | |||||||||||||||
| 1994-1998 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 1 | ||||||||
| 1999-2001 | 1 (33.3) | 0 | 2 (66.7) | 0 | 0 (33.3) | 0 | 3 | ||||||||
| 2002-2004 | 1 (9.1) | 1 (9.1) | 2 (18.2) | 1 (9.1) | 1 (9.1) | 5 (45.5) | 11 | ||||||||
| Total | 2 (13.3) | 1 (6.7) | 4 (26.7) | 1 (6.7) | 2 (13.3) | 5 (33.3) | 15 | ||||||||
Includes serogroups/types 4 (n = 1), 8 (n = 1), 10 (n = 1), 11 (n = 1); 22 (n = 1), and 34 (n = 1).
Not determined.
S. pneumoniae isolates that were nonsusceptible to either cefotaxime (P = 0.001) or ceftriaxone (P = 0.007) became rarer over time, from 5.2% and 3.7% (1994 to 1998), respectively, to 0.7 (2002 to 2004) (Table 3). S. pneumoniae isolates were classified for susceptibility to cephalosporins according to two sets of CLSI criteria: those in use before 2002 and those in use after 2002 (Table 5). Nonsusceptibility to celphalosporins appeared to be less frequent after 2002. According to the 2002 guidelines, isolates nonsusceptible to cefotaxime (P < 0.001) and ceftriaxone (P < 0.001) were more frequently recovered from CSF (6.4% and 5.7%, respectively) than from blood (1.3% and 1.0%, respectively). Using the CLSI guidelines in force before 2002, no such association was found for either cefotaxime (6.4% versus 5.5%; P = 0.57) or ceftriaxone (5.7% versus 5.0%; P = 0.66). Cefotaxime-nonsusceptible isolates were more prevalent in the 10- to 14-year-old age group (11.4%; P = 0.005), whereas isolates that were nonsusceptible to ceftriaxone were most common in the 1- to 9-year-old age group (5.4%; P < 0.001) (Fig. 1). The main serotype and serogroup among these isolates were serotype 14 (51.9% of isolates nonsusceptible to ceftriaxone and 56.3% of those nonsusceptible to cefotaxime) and serogroup 9 (25.9% for ceftriaxone and 25.0% for cefotaxime) (Table 4).
TABLE 5.
Frequencies of susceptibility to cefotaxime and ceftriaxone among 1329 S. pneumoniae isolates according to CLSI guidelines published before and after 2002
| Antimicrobial agent and CLSI guideline | No. (%) of nonsusceptible isolates
|
||||||
|---|---|---|---|---|---|---|---|
| 1994-1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | |
| Cefotaxime | |||||||
| Before 2002 | 20 (7.4) | 16 (9.2) | 11 (8.0) | 10 (5.6) | 7 (3.3) | 6 (3.7) | 6 (3.0) |
| After 2002 | 14 (5.2) | 4 (2.3) | 6 (4.4) | 4 (2.2) | 3 (1.4) | 0 (0.0) | 1 (0.5) |
| Ceftriaxone | |||||||
| Before 2002 | 16 (5.9) | 17 (9.8) | 8 (5.8) | 10 (5.6) | 7 (3.3) | 7 (4.3) | 4 (2.0) |
| After 2002 | 10 (3.7) | 5 (2.9) | 4 (2.9) | 4 (2.2) | 3 (1.4) | 0 (0.0) | 1 (0.5) |
| Total no. of isolates | 269 | 173 | 137 | 179 | 209 | 164 | 198 |
Among the 191 erythromycin-nonsusceptible (MIC, ≥0.5 μg/ml) isolates recovered in this study (14.4% of all isolates; Table 2), 67.4% were also clindamycin nonsusceptible (MLSB) and 32.6% had the M phenotype. Erythromycin nonsusceptibility increased from 7.1% (1994 to 1998) to 18.2% (2002 to 2004) among pneumococci (P < 0.001) (Table 3). The frequency of the MLSB phenotype was 68.4% in the period from 1994 to 1998, increased to 76.5% in 1999 to 2001, and then decreased to 60.8% in 2002 to 2004 (P < 0.001). Isolates nonsusceptible only to macrolides increased from 3.7% (1994 to 1998) to 8.4% (1999 to 2001) and then to 9.1% (2002 to 2004). Erythromycin nonsusceptibility was associated with multidrug resistance (P = 0.017) and with nonsusceptibility to penicillin plus erythromycin (P < 0.001) among children. In isolates from adults, erythromycin nonsusceptibility was also associated with tetracycline (P < 0.001), chloramphenicol (P = 0.017), and ciprofloxacin (P = 0.012) nonsusceptibility. Erythromycin- and clindamycin-nonsusceptible isolates were most frequent among the isolates from children 1 to 9 years of age (25.5% and 15.2% respectively), children <1 year of age (17.5% and 12.4%, respectively), and adults ≥65 years of age (16.6% and 10.4%, respectively) (Fig. 1). Many erythromycin-nonsusceptible isolates were of serogroups 19 and 15, and serotype 33 emerged after the period 1999 to 2001. Overall, however, serotype frequencies did not change through time (P = 0.068) (Table 4).
During the period of the study, the frequencies of ciprofloxacin- and ofloxacin-nonsusceptible isolates reached 1.4% and 2.0%, respectively; the MIC90 of both antibiotics was 2 μg/ml (Table 2). Isolates nonsusceptible to ciprofloxacin increased from 0.6% (1999 to 2001) to 1.8% (2003) and to 3.5% (2004) (P = 0.04) (Table 3). The percentage of pneumococci nonsusceptible to ofloxacin decreased from 3.3% (1994 to 1998) to 0.8% (1999 to 2001) and then increased to 2.3% (2002 to 2004) (P = 0.04) (Table 3). The proportion of isolates from children that were nonsusceptible to ciprofloxacin (1.3%) was not significantly different from that observed for isolates from adults (2.1%) (P = 0.47) (Fig. 1). Ciprofloxacin-nonsusceptible isolates were from serotypes 1, 6, 11, 22, and 33 and were mainly from serotype 14 (26.7%), which appeared only since the period 1999 to 2001 (Table 4).
All isolates were susceptible to vancomycin, with a MIC90 of 0.5 μg/ml (Table 2).
The frequencies of multidrug-resistant pneumococci were 6.5% (1994 to 1998) and 6.2% (1999 to 2001) and then increased to 11.7% in 2002 to 2004 (P = 0.002) (Table 3). The proportions of multidrug-resistant isolates among blood (9.3%) and CSF (6.3%) isolates were similar (P = 0.28) (Table 1). Isolates with multidrug resistance were most frequent in children of the 1- to 9-year-old age group and in adults ≥65 years old (Fig. 1). Overall, the multidrug resistance of pneumococci isolated from children (13.1%) was higher than that from adults (6.7%) (P < 0.001). In children, the rate of multidrug-resistant isolates was mainly associated with macrolides (P = 0.017), and in adults this rate was mainly associated with resistance to penicillin (P = 0.001), tetracycline (P = 0.005), macrolides (P > 0.001), chloramphenicol (P = 0.037), and ciprofloxacin (P = 0.03). Among multidrug-resistant isolates, serogroup 6 and serotype 14 decreased greatly between the periods 1999 to 2001 and 2002 to 2004 and serotype 23 decreased from 1994 to 1998 to 7.7% in the period 2002 to 2004. In contrast, serogroup 19 was not found in 1994 to 1998 but made up 43.1% of the isolates in 2002 to 2004 (P < 0.001) (Table 4). The 29 multidrug-resistant phenotypes observed during the 11 years of the study are shown in Table 6. The predominant phenotype of multidrug resistance was nonsusceptibility to penicillin, tetracycline, erythromycin, and clindamycin. Strains with this phenotype were mostly serotypes 6, 14, 15, 19, and 24. The second most frequent phenotype was nonsusceptibility to penicillin, erythromycin, and trimethoprim-sulfamethoxazole, and all isolates with this phenotype were serotype 14 or serotype 1. The multidrug-resistant phenotype involving nonsusceptibility to penicillin, tetracycline, and erythromycin (the third most frequent) was only found in 2002 to 2004 and only among isolates of serotype 19. Only 6 of the 29 phenotypes did not include isolates with nonsusceptibility to macrolides. Isolates with coresistance to penicillin and erythromycin became increasingly frequent, rising from 3.3% (1994 to 1998) to 5.5% (1999 to 2001) and then 9.1% (2002 to 2004) (P = 0.001).
TABLE 6.
Distribution of 112 MDR S. pneumoniae isolates by serogroup/type in 1994 to 1998, 1999 to 2001, and 2002 to 2004
| MDR phenotypea | No. of MDR isolates/period
|
Total no. of isolates | Serogroup/type (%) | ||
|---|---|---|---|---|---|
| 1994-1998 | 1999-2001 | 2002-2004 | |||
| PEN TET ERY CLI | 1 | 9 | 22 | 32 | 19 (31.3), 14 (25.0), 6 (15.6), 15 (15.6), 24 (6.3), 17 (3.1), 23 (3.1) |
| PEN ERY SXT | 2 | 1 | 7 | 10 | 14 (90.0), 1 (10.0) |
| PEN TET ERY | 9 | 9 | 19 (100) | ||
| TET ERY CLI SXT | 2 | 5 | 1 | 8 | 6 (75.0), 14 (35.0) |
| TET ERY CLI CHL SXT | 2 | 2 | 3 | 7 | 6 (71.4), 19 (28.6) |
| PEN TET ERY CLI SXT | 1 | 2 | 4 | 7 | 6 (57.1), 9 (28.6), 19 (14.3) |
| PEN TET ERY CLI CHL SXT | 2 | 4 | 6 | 23 (50.0), 6 (16.7), 14 (16.7), 19 (16.7) | |
| PEN TET CHL SXT | 1 | 4 | 5 | 23 (80.0), 19 (20.0) | |
| TET ERY CLI CHL | 3 | 3 | 19 (66.7), 6 (33.3) | ||
| PEN TET CHL CTX CRO SXT | 2 | 1 | 3 | 23 (66.7), 19 (33.3) | |
| ERY CLI CHL SXT | 2 | 2 | 6 (100) | ||
| PEN ERY CLI SXT | 1 | 1 | 2 | 14 (50.0), 15 (50.0) | |
| PEN TET ERY CLI CHL CTX CRO SXT | 1 | 1 | 2 | 6 (50.0), 23 (50.0) | |
| TET CHL SXT | 1 | 1 | 6 (100) | ||
| TET ERY CHL | 1 | 1 | 19 (100) | ||
| TET ERY CHL SXT | 1 | 1 | 6 (100) | ||
| PEN OFL CIP SXT | 1 | 1 | 11 (100) | ||
| PEN ERY CTX CRO SXT | 1 | 1 | 14 (100) | ||
| PEN ERY OFL CIP SXT | 1 | 1 | 14 (100) | ||
| PEN TET SXT | 1 | 1 | 14 (100) | ||
| PEN TET CHL CTX SXT | 1 | 1 | 23 (100) | ||
| PEN TET ERY SXT | 1 | 1 | 19 (100) | ||
| PEN TET ERY OFL CIP SXT | 1 | 1 | 14 (100) | ||
| PEN TET ERY CHL SXT | 1 | 1 | 14 (100) | ||
| PEN TET ERY CLI CTX SXT | 1 | 1 | 14 (100) | ||
| PEN TET ERY CLI CTX CRO | 1 | 1 | 14 (100) | ||
| PEN TET ERY CLI OFL CIP | 1 | 1 | 6 (100) | ||
| PEN TET ERY CLI OFL CIP SXT | 1 | 1 | 14 (100) | ||
| PEN TET ERY CLI CHL | 1 | 1 | 23 (100) | ||
| Total | 17 | 30 | 65 | 112 | |
PEN, penicillin; CRO, ceftriaxone; CTX, cefotaxime; TET, tetracycline; ERY, erythromycin; CLI, clindamycin; CHL, chloramphenicol; CIP, ciprofloxacin; OFX, ofloxacin; SXT, trimethoprim-sulfamethoxazole.
DISCUSSION
Between 1994 and 2004 the Antimicrobial Resistance Unit at the National Institute of Health Dr. Ricardo Jorge in Lisbon recovered 1,331 invasive S. pneumoniae strains isolated in 24 hospitals covering several regions of Portugal.
The first pneumococcal isolates nonsusceptible to penicillin to be described in Portugal were isolated in 1989 (4.6%) as part of a collection of strains from invasive and noninvasive diseases (32). Such strains became more widespread over the years, until they made up 9% (1992 to 1994) of isolates collected from invasive disease (6). We report that this trend continued, and these strains accounted for 28.5% of isolates in the year 2000. Then the rate declined to 17.7% in 2003 and in 2004 increased again to 23.2%. Overall, the proportion of isolates that was nonsusceptible to penicillin increased until the period 1999 to 2001 (22.2%) and then stabilized in the period 2001 to 2004 (20.8%).
The frequency of penicillin-nonsusceptible pneumococcal isolates in our collection is lower than that observed among invasive isolates in other countries, such as Spain (35.6% in 2001 to 2003) (30), France (47.5% in 2000 to 2002) (7), and Israel (37.3% in 2004) (9). It was, however, similar to those of several eastern European countries (9) and higher than those in Italy (12.1% in 1999 to 2000) (27), Belgium (17.7% in 2000) (12), Luxembourg (11.2% in 2004), and the majority of the northern European countries (9). Differences in rates of pneumococcal penicillin resistance between countries have been shown to be associated with levels of antimicrobial consumption (3, 5, 13).
We found that penicillin nonsusceptibility was associated with macrolides (P = 0.002), trimethoprim-sulfamethoxazole (P = 0.017), and multidrug resistance (P = 0.001). Similar associations have been reported elsewhere in the world (18, 22, 33). Previous studies in Portugal gave similar findings, except for macrolides (6, 32); however, the resistance to macrolides was very low when these studies were conducted.
Despite nonsusceptibility being relatively prevalent in Portugal, the frequency of isolates with high-level resistance declined consistently, to 0.9% in 2001 to 2004. Work in animal models (1, 37) and humans (25, 41) suggests that high-level penicillin resistance is associated with decreased virulence of pneumococci. Several studies reported that nonmeningeal pneumococcal infections caused by isolates with a penicillin MIC of ≤2 μg/ml can be treated successfully with penicillin (31, 43). However, in severely ill patients with pneumococcal bacteremia, combination antibiotic therapy reduces mortality (2). Several studies have suggested that initial monotherapy with β-lactams for severe pneumococcal bacteremia may be suboptimal, and the use of combined therapy involving a macrolide or quinolone may improve the outcome of the disease (2, 39). Empirical combination antibiotherapy avoids discordant therapy in the context of multidrug-resistant isolates but also causes a selection pressure on those isolates.
Using the 2004 CLSI guidelines (29), we found that ceftriaxone- and cefotaxime-nonsusceptible isolates became less prevalent over time and made up ≤1% of the isolates in 2004. Nevertheless, according to previous CLSI guidelines (28), the frequency of isolates nonsusceptible to cephalosporin was stable until 2001 and then only decreased to 3% in 2002 to 2004. These apparent differences are due to the changes in the CLSI susceptibility interpretation according to the source of isolation. Indeed, the number of isolates recovered from blood or CSF in the study influences the total frequency of nonsusceptible isolates. This fact can cause misleading interpretations of the frequency trends of cephalosporin-nonsusceptible isolates. Earlier studies indicated that cephalosporin-nonsusceptible isolates started to increase after 1993 (6), and our study suggests that their prevalence started to decline after the introduction of the pneumococcal conjugate vaccine in 2001. These findings support the recommendations for the use of cephalosporins, alone or combination with vancomycin, as initial empirical therapy for treatment of bacterial meningitis (16, 42). In Portugal, the antibiotics most used for meningitis have been ceftriaxone (87%) and cefotaxime (10%) (4).
The rates of resistance to macrolides have increased worldwide (19), consistent with our findings in Portugal. This is mainly due to the widespread use of macrolides, mostly azithromycin (8, 19). In Portugal, the predominant macrolide phenotype was MLSB, as shown in this study and among isolates recovered from respiratory tract infections (24). The majority of southern European countries also have a high prevalence of the MLSB phenotype (34). In contrast, the M phenotype is predominant in the United States (23).
The rate of isolates nonsusceptible only to erythromycin (3.7% in 1994 to 1998 to 9.1% in 2002 to 2004) showed the same increasing trend as the rate of isolates with coresistance to penicillin and erythromycin (3.3% in 1994 to 1998 to 9.1% in 2002 to 2004). The increasing use of macrolides could be causing the increase of penicillin and multidrug resistances, due to the coresistance to macrolides. Fatal cases due to macrolide resistance have been described following azithromycin monotherapy (40). The new ketolide, telithromycin, is a potential alternative to the currently used macrolides. However, isolates resistant to telithromycin have already been reported all over the world (15, 35). It seems that telithromycin may be of limited therapeutic value in the long term due to the associated resistance mechanism (14).
We found a significant frequency of isolates resistant to tetracycline and chloramphenicol. These antibiotics are not commonly used in Portugal (13). The high prevalence of such strains can be explained by coresistance, mainly between tetracycline and macrolides. This coresistance is commonly associated with the presence of several transposons which carry the genetic determinants encoding resistance to both antibiotics (21, 26).
Nonsusceptibility to ciprofloxacin increased in recent years from 0.5% in 2002 to 3.5% in 2004. This was associated with multidrug resistance, mainly among adults. In other studies, an association between ciprofloxacin resistance and individuals ≥65 years old has been observed (17, 38). This antibiotic is mostly used in elderly individuals.
Multidrug resistance increased in the last years of our study: from 7.9% in 2002 to 15.6% in 2004. In children, this mainly involved resistance to macrolides; in adults, it was mostly nonsusceptibility to penicillin, macrolides, and ciprofloxacin. The increasing resistance may be associated with the increased use of these antibiotics in recent years (8, 13).
In view of the antibiotic resistance patterns among pneumococci in Portugal, penicillin should be used to treat uncomplicated nonmeningeal pneumococcal infections. The use of cephalosporins to treat meningeal pneumococcal infections and their use combined with a macrolide or quinolone to treat several nonmeningeal pneumococcal infections seem also to be reasonable choices.
Acknowledgments
R.D. was supported by the “NIH Scientific Research Ricardo-Jorge” grant.
The hospitals and principal collaborators who participated in the Antimicrobial Resistance Surveillance Program in Portugal (ARSIP) are as follows: Hospital S. Pedro de Vila Real, Vila Real (A. Paula Castro); Hospital de S. Marcos, Braga (S. Microbiologia); Hospital de Santa Luzia, Viana do Castelo (Adelina Santos); Hospital Eduardo Santos Silva, Vila Nova de Gaia (M. Lourdes Sobral); Instituto Nacional de Saúde Dr. Ricardo Jorge, Porto (L. Bacteriologia); Hospital Especializado de Crianças Maria Pia, Porto (Fernanda Teixeira); Hospital Geral de S. António, Porto (S. Microbiologia); Hospital S. Teotónio, Viseu (José Ribeiro); Centro Hospitalar de Coimbra, Coimbra (João Moreira and Luís Albuquerque); Hospital Universitário de Coimbra, Coimbra (Graça Ribeiro); Hospital Reynaldo dos Santos, Vila Franca de Xira (Margarida Vasconcelos); Hospital Capuchos, Lisboa (M. Teresa Ferreira); Hospital Curry Cabral, Lisboa (M. Helena Peres); Hospital de D. Estefânia, Lisboa (Rosa Barros); Hospital Pulido Valente, Lisboa (S. Microbiologia); Hospital S. Francisco Xavier, Lisboa (Filomena Martins); Hospital de S. José, Lisboa (João Marques and Odete Spencer); Hospital de S. Maria, Lisboa (M. José Salgado); Hospital Fernando Fonseca, Amadora (Luísa Sancho); Hospital Conde de Castro Guimarães, Cascais (Ana Fonseca and Adriana Coutinho); Hospital Garcia de Orta, Almada (Margarida Pinto and José Diogo); Hospital N. Senhora do Rosário, Barreiro (Ana M. Jesus and Teixeira Lopes); Hospital José Joaquim Fernandes, Beja (Rosa Bento); and Centro Hospitalar do Funchal, Madeira (Teresa Afonso).
REFERENCES
- 1.Azoulay-Dupuis, E., V. Rieux, M. Muffat-Joly, J. P. Bédos, E. Valleé, C. Rivier, R. Isturiz, C. Carbon, and P. Moine. 2000. Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob. Agents Chemother. 44:1575-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baddour, L. M., V. L. Yu, K. P. Klugman, C. Feldman, A. Ortqvist, J. Rello, A. J. Morris, C. M. Luna, D. R. Snydman, W. C. Ko, M. B. Chedid, D. S. Hui, A. Andremont, C. C. Chiou, et al. 2004. Combination antibiotic therapy lowers mortality among severely ill patients with pneumococcal bacteremia. Am. J. Respir. Crit. Care Med. 170:440-444. [DOI] [PubMed] [Google Scholar]
- 3.Bronzwaer, S. L., O. Cars, U. Buchholz, S. Molstad, W. Goettsch, I. K. Veldhuijzen, J. L. Kool, M. J. Sprenger, J. E. Degener, et al. 2002. A European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg. Infect. Dis. 8:278-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caniça, M., R. Dias, B. Nunes, L. Carvalho, E. Ferreira, et al. 2004. Invasive culture-confirmed Neisseria meningitidis in Portugal: evaluation of serogroups in relation to different variables and antimicrobial susceptibility (2000-2001). J. Med. Microbiol. 53:921-925. [DOI] [PubMed] [Google Scholar]
- 5.Cars, O., S. Molstad, and A. Melander. 2001. Variation in antibiotic use in the European Union. Lancet 357:1851-1853. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho, C., D. Louro, M. V. Pato, et al. 1996. Estudo multicêntrico de vigilância aos antibióticos em 938 estirpes de Streptococcus pneumoniae. Rev. Port. Doenças Infecciosas 1:29-37. [Google Scholar]
- 7.Decousser, J.-W., P. Pina, F. Viguier, F. Picot, P. Courvalin, P. Allouch, and ColBVH Study Group. 2004. Invasive Streptococcus pneumoniae in France: antimicrobial resistance, serotype, and molecular epidemiology findings from a monthly national study in 2000 to 2002. Antimicrob. Agents Chemother. 48:3636-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias, R., and M. Caniça. 2004. Emergence of invasive erythromycin-resistant Streptococcus pneumoniae strains in Portugal: contribution and phylogenetic relatedness of serotype 14. J. Antimicrob. Chemother. 54:1035-1039. [DOI] [PubMed] [Google Scholar]
- 9.European Antimicrobial Resistance Surveillance System. 26 October 2005, posting date. EARSS interactive database access. [Online.]: http://www.earss.rivm.nl/PAGINA/interwebsite/home_earss.html.
- 10.Felmingham, D., C. Feldman, W. Hryniewicz, K. Klugman, S. Kohno, D. E. Low, C. Mendes, and A. C. Rodloff. 2002. Surveillance of resistance in bacteria causing community-acquired respiratory tract infections. Clin. Microbiol. Infect. 8(Suppl. 2):12-42. [DOI] [PubMed] [Google Scholar]
- 11.Fenoll, A., I. Jado, D. Vicioso, and J. Casal. 1997. Dot blot assay for the serotyping of pneumococci. J. Clin. Microbiol. 35:764-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flamaing, J., J. Verhaegen, and W. E. Peetermans. 2002. Streptococcus pneumoniae bacteraemia in Belgium: differential characteristics in children and the elderly population and implications for vaccine use. J. Antimicrob. Chemother. 50:43-50. [DOI] [PubMed] [Google Scholar]
- 13.Goossens, H., M. Ferech, R. Vander Stichele, M. Elseviers, et al. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 14.Hisanaga, T., D. J. Hoban, and G. G. Zhanel. 2005. Mechanisms of resistance to telithromycin in Streptococcus pneumoniae. J. Antimicrob. Chemother. 56:447-450. [DOI] [PubMed] [Google Scholar]
- 15.Hsueh, P.-R., L.-J. Teng, T.-L. Wu, D. Yang, W.-K. Huang, J.-M. Shyr, Y.-C. Chuang, J.-H. Wan, J.-J. Yan, J.-J. Lu, J.-J. Wu, W.-C. Ko, F.-Y. Chang, Y.-C. Yang, Y.-J. Lau, Y.-C. Liu, C.-M. Lee, H.-S. Leu, C.-Y. Liu, and K.-T. Luh. 2003. Telithromycin- and fluoroquinolone-resistant Streptococcus pneumoniae in Taiwan with high prevalence of resistance to macrolides and β-lactams: SMART Program 2001 data. Antimicrob. Agents Chemother. 47:2145-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Infectious Diseases and Immunization Committee, C.P.S.C. 2001. Therapy of suspected bacterial meningitis in Canadian children six weeks of age and older. Pediatr. Child Health 6:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, R. N., D. J. Biedenbach, and M. L. Beach. 2003. Influence of patient age on the susceptibility patterns of Streptococcus pneumoniae isolates in North America (2000-2001): report from the SENTRY Antimicrobial Surveillance Program. Diagn. Microbiol. Infect. Dis. 46:77-80. [DOI] [PubMed] [Google Scholar]
- 18.Klugman, K. P. 2002. The successful clone: the vector of dissemination of resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 50(Suppl. S2):1-5. [DOI] [PubMed] [Google Scholar]
- 19.Klugman, K. P., and J. R. Lonks. 2005. Hidden epidemic of macrolide-resistant pneumococci. Emerg. Infect. Dis. 11:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclercq, R., and P. Courvalin. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob. Agents Chemother. 35:1267-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerq, R., and P. Courvalin. 2002. Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:2727-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick, A. W., C. G. Whitney, M. M. Farley, R. Lynfield, L. H. Harrison, N. M. Bennett, W. Schaffner, A. Reingold, J. Hadler, P. Cieslak, M. H. Samore, and M. Lipsitch. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 9:424-430. [DOI] [PubMed] [Google Scholar]
- 23.McEllistrem, M. C., J. M. Adams, K. Shutt, L. T. Sanza, R. R. Facklam, C. G. Whitney, J. H. Jorgensen, and L. H. Harrison. 2005. Erythromycin-nonsusceptible Streptococcus pneumoniae in children, 1999-2001. Emerg. Infect. Dis. 11:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melo-Cristino, J., M. Ramirez, N. Serrano, T. Hanscheid, et al. 2003. Macrolide resistance in Streptococcus pneumoniae isolated from patients with community-acquired lower respiratory tract infections in Portugal: results of a 3-year (1999-2001) multicenter surveillance study. Microb. Drug Resist. 9:73-80. [DOI] [PubMed] [Google Scholar]
- 25.Metlay, J. P., J. Hofmann, M. S. Cetron, M. J. Fine, M. M. Farley, C. Whitney, and R. F. Breiman. 2000. Impact of penicillin susceptibility on medical outcomes for adult patients with bacteremic pneumococcal pneumonia. Clin. Infect. Dis. 30:520-528. [DOI] [PubMed] [Google Scholar]
- 26.Montanari, M. P., I. Cochetti, M. Mingoia, and P. E. Varaldo. 2003. Phenotypic and molecular characterization of tetracycline- and erythromycin-resistant strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2236-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moro, M. L., A. Pantosti, D. Boccia, et al. 2002. Antibiotic microbial resistance surveillance in invasive infections caused by Streptococcus pneumoniae and Staphylococcus aureus: the EARSS (European Antimicrobial Resistance Surveillance System) project in Italy (April 1999-April 2000). Ann. Ig. 14:361-371. (In Italian.) [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 29.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 30.Oteo, J., E. Lázaro, F. J. de Abajo, F. Baquero, J. Campos, and Spanish Members of the European Antimicrobial Resistance Surveillance System. 2004. Trends in antimicrobial resistance in 1,968 invasive Streptococcus pneumoniae strains isolated in Spanish hospitals (2001 to 2003): decreasing penicillin resistance in children's isolates. J. Clin. Microbiol. 42:5571-5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallares, R., F. Gudiol, J. Linares, J. Ariza, G. Rufi, L. Murgui, J. Dorca, and P. F. Viladrich. 1987. Risk factors and response to antibiotic therapy in adults with bacteremic pneumonia caused by penicillin-resistant pneumococci. N. Engl. J. Med. 317:18-22. [DOI] [PubMed] [Google Scholar]
- 32.Pato, M. V., C. B. Carvalho, A. Tomasz, and The Multicenter Study Group. 1995. Antibiotic susceptibility of Streptococcus pneumoniae isolates in Portugal. A multicenter study between 1989 and 1993. Microb. Drug Resist. 1:59-69. [DOI] [PubMed] [Google Scholar]
- 33.Powis, J., A. McGeer, K. Green, O. Vanderkooi, K. Weiss, G. Zhanel, T. Mazzulli, M. Kuhn, D. Church, R. Davidson, K. Forward, D. Hoban, A. Simor, Canadian Bacterial Surveillance Network, and D. E. Low. 2004. In vitro antimicrobial susceptibilities of Streptococcus pneumoniae clinical isolates obtained in Canada in 2002. Antimicrob. Agents Chemother. 48:3305-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinert, R. R., A. Ringelstein, M. van der Linden, M. Y. Cil, A. Al-Lahham, and F.-J. Schmitz. 2005. Molecular epidemiology of macrolide-resistant Streptococcus pneumoniae isolates in Europe. J. Clin. Microbiol. 43:1294-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinert, R. R., M. van der Linden, and A. Al-Lahham. 2005. Molecular characterization of the first telithromycin-resistant Streptococcus pneumoniae isolate in Germany. Antimicrob. Agents Chemother. 49:3520-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson, D. C., D. Bast, A. McGeer, and D. E. Low. 2001. Evaluation of susceptibility testing to detect fluoroquinolone resistance mechanisms in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:1911-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieux, V., C. Carbon, and E. Azoulay-Dupuis. 2001. Complex relationship between acquisition of beta-lactam resistance and loss of virulence in Streptococcus pneumoniae. J. Infect. Dis. 184:66-72. [DOI] [PubMed] [Google Scholar]
- 38.Sahm, D. F., D. E. Peterson, I. A. Critchley, and C. Thornsberry. 2000. Analysis of ciprofloxacin activity against Streptococcus pneumoniae after 10 years of use in the United States. Antimicrob. Agents Chemother. 44:2521-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waterer, G. W. 2005. Monotherapy versus combination antimicrobial therapy for pneumococcal pneumonia. Curr. Opin. Infect. Dis. 18:157-163. [DOI] [PubMed] [Google Scholar]
- 40.Waterer, G. W., R. G. Wunderink, and C. B. Jones. 2000. Fatal pneumococcal pneumonia attributed to macrolide resistance and azithromycin monotherapy. Chest 118:1839-1840. [DOI] [PubMed] [Google Scholar]
- 41.Winston, L. G., J. L. Perlman, D. A. Rose, and J. L. Gerberding. 1999. Penicillin-nonsusceptible Streptococcus pneumoniae at San Francisco General Hospital. Clin. Infect. Dis. 29:580-585. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization Emerging and other Communicable Diseases, Surveillance and Control. 3 November 2001, posting date. Antimicrobial and support therapy for bacterial meningitis in children. Report of the meeting of 18-20 June 1997, Geneva, Switzerland. [Online.] http://www.who.int/entity/csr/resources/publications/meningitis/whoemcbac982.pdf.
- 43.Yu, V. L., C. C. Chiou, C. Feldman, A. Ortqvist, J. Rello, A. J. Morris, L. M. Baddour, C. M. Luna, D. R. Snydman, M. Ip, W. C. Ko, M. B. Chedid, A. Andremont, K. P. Klugman, et al. 2003. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin. Infect. Dis. 37:230-237. [DOI] [PubMed] [Google Scholar]