Abstract
The use of indwelling medical devices—pacemakers, prosthetic joints, catheters—is rapidly growing and is often complicated by infections with biofilm-forming microbes that are resistant to antimicrobial agents and host defense mechanisms. We investigated for the first time the use of microbe-specific monoclonal antibodies (MAbs) as delivery vehicles for targeting biofilms with cytocidal radiation. MAb 18B7 (immunoglobulin G1 [IgG1]), which binds to capsular polysaccharides of the human pathogenic fungus Cryptococcus neoformans, penetrated cryptococcal biofilms, as shown by confocal microscopy. When the alpha radiation-emitter 213-Bismuth (213Bi) was attached to MAb 18B7 and the radiolabeled MAb was added to C. neoformans biofilms, there was a 50% reduction in biofilm metabolic activity. In contrast, when the IgM MAb 13F1 labeled with 213Bi was used there was no penetration of the fungal biofilm and no damage. Unlabeled 18B7, 213Bi-labeled nonspecific MAbs, and gamma and beta types of radiation did not have an effect on biofilms. The lack of efficacy of gamma and beta radiation probably reflects the radioprotective properties of polysaccharide biofilm matrix. Our results indicate that C. neoformans biofilms are susceptible to treatment with antibody-targeted alpha radiation, suggesting a novel option for the prevention or treatment of microbial biofilms on indwelling medical devices.
Advances in medical science have produced a wide variety of devices that are implanted into the human body, including pacemakers, prosthetic joints, catheters, and artificial valves. For example, each year urinary catheters are inserted into five million patients in acute-care hospitals and extended-care facilities (17). The rate of infection of these indwelling devices is very high—25% for catheters (17), 7.4% for central nervous system shunts (23), 5% for prosthetic joints (25), and 0.5% for pacemakers (12). Treatment of infectious diseases associated with indwelling medical devices usually requires surgical intervention combined with a prolonged course of antimicrobial therapy (24), with costs of therapy often exceeding $50,000 per patient. There is also significant morbidity and mortality associated with such treatments. To exacerbate the problem, microbial biofilms on indwelling medical devices are often resistant to antimicrobial agents and host defense mechanisms (11). In fact, some antibiotics may contribute to the problem, with aminoglycoside antibiotics actually inducing bacterial biofilm formation (14). Thus, radically new approaches are urgently needed for elimination of microbial biofilms in patients.
Passive antibody therapy is a potentially useful therapeutic and preventive strategy against a variety of infectious diseases (4). The specificity of the antigen-antibody interaction provides an attractive option for delivering microbicidal agents to sites of infection. Radioimmunotherapy (RIT) takes advantage of the specificity of the antigen-antibody interaction to deliver cytotoxic radiation to the vicinity of the target, mediating an antimicrobial effect. Recently, we demonstrated the feasibility of RIT as an anti-infective therapy by treating murine cryptococcosis with a monoclonal antibody (MAb) to the human pathogenic fungus Cryptococcus neoformans capsular glucuronoxylomannan (GXM) labeled with 213-Bismuth (213Bi) or 188-Rhenium (188Re) (6, 7). Subsequently, we showed the applicability of RIT to other fungal and bacterial infections (8, 9). Based on our previous work, we hypothesized that antibody can penetrate the biofilm, bind to microbial cells, and deliver microbicidal radiation. We evaluated the microbicidal properties of two radionuclides—213Bi and 188Re. The radionuclide 213Bi emits highly energetic (E = 5.9 MeV) α particles (helium atoms) capable of killing a cell with one or two hits in close proximity (50 to 80 μm) to its targets, while 188Re emits high-energy (Emax = 2.2 MeV) β particles (electrons) with a much longer range in tissue (several millimeters) and with multiple hits per cell needed for delivery for a lethal effect. As a model for investigating the susceptibility of biofilms to RIT we have chosen the C. neoformans system. C. neoformans can form biofilms on prosthetic medical devices (26) which are resistant to host immune microbicidal mechanisms and drug therapy (19). However, of greater medical importance may be the fact that C. neoformans often forms a slimy layer on the meninges which is effectively a biofilm. Hence, cryptococcal biofilms are probably quite common with and without the presence of prosthetic devices. In our laboratories we have recently developed a system to study cryptococcal biofilms formation in vitro (18); that system was used in this study.
MATERIALS AND METHODS
Antibodies, radioisotopes, and radiolabeling of antibodies.
GXM-binding murine MAbs 18B7 immunoglobulin G1 (IgG1) and 13F1 (IgM) were produced as in described in references 3 and 22. MAb 18B7 has been successfully used for RIT for C. neoformans both in vitro and in vivo (6, 7, 9). Both 18B7 and 13F1 are not protective against C. neoformans infection in the amounts utilized in RIT (10 to 30 μg). As the molecular mass of 13F1 (IgM) is five times higher than that of 18B7 (IgG1), we used 13F1 to evaluate the influence of the size of the molecule on its ability to penetrate the exopolysaccharide matrix of a biofilm. Isotype-matching (IgG1) control MAb MOPC21 was acquired from MP Biochemicals, Germany. Actinium (225Ac) for construction of 225Ac-213Bi generators was produced at the Institute for Transuranium Elements, Karlsruhe, Germany. 188Re was eluted from a 188Re-188W generator (Oak Ridge National Laboratory, Oak Ridge, TN). Radiolabeling of MAbs with 213Bi and 188Re was performed as described previously (6). Radioactivity was measured in a dose calibrator (Capintec, Ramsey, NJ) or in a gamma counter (Wallac, Finland).
Biofilm formation.
C. neoformans B3501 strain was grown in Sabouraud dextrose broth (Difco Laboratories, Detroit, Mich.) for 24 h at 30°C in a rotary shaker at 150 rpm (to early stationary phase). Cells were then collected by centrifugation, washed twice with phosphate-buffered saline (PBS), counted using a hemacytometer, and suspended at 107 cells/ml in minimal medium (20 mg/ml thiamine, 30 mM glucose, 26 mM glycine, 20 mM MgSO4 × 7H2O, and 58.8 mM KH2PO4). Then, 100 μl of the suspension was added into individual wells of polystyrene 96-well plates (Fisher, MA) and incubated at 37°C without shaking. Biofilms formed over a 48-h period. Following the adhesion stage, the wells containing C. neoformans biofilms were washed three times with 0.05% Tween 20 in Tris-buffered saline (TBS) to remove nonadherent cryptococcal cells by use of a microtiter plate washer (Skan Washer 400; Molecular Devices, VA). Fungal cells that remained attached to the plastic surface were considered true biofilms. All assays were carried out in triplicate.
Measurement of biofilm metabolic activity by XTT reduction assay.
A semiquantitative measure of C. neoformans biofilm formation was obtained from the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay. For C. neoformans strains, 50 μl of XTT salt solution (1 mg/ml in PBS) and 4 μl of menadione solution (Sigma) (1 mM in acetone) were added to each well. Microtiter plates were incubated at 37°C for 5 h. The metabolic activity of the yeast cells within the biofilm was measured from mitochondrial dehydrogenase activity that reduced XTT tetrazolium salt to XTT formazan, resulting in colorimetric change. The colorimetric change was measured using a microtiter reader (Multiskan MS; Labsystem, Helsinki, Finland) at 492 nm. Microtiter wells containing either heat-killed C. neoformans or minimal medium without C. neoformans cells were included as negative controls.
C. neoformans planktonic cells.
To determine the density of C. neoformans planktonic cells used for comparison to biofilms we estimated cell numbers from the XTT reduction signal by use of a dose-response curve. Briefly, cells of C. neoformans B3501 were grown in minimal medium for 48 h at 30°C in a rotary shaker at 150 rpm (to stationary phase), collected by centrifugation, washed twice with PBS, counted using a hemacytometer, and suspended at various densities (5 × 106, 1 × 107, and 5 × 107 cells/ml) in minimal medium. Hence, these cells were in stationary-growth phase, which approximates the metabolic state of biofilm cells. Then, 100 μl of each suspension was added into individual wells of polystyrene 96-well plates to final densities of 5 × 105, 1 × 106, and 5 × 106 cells/ml. The viability was measured by XTT reduction.
Confocal microscopy.
C. neoformans biofilms were grown for 48 h in 96-well microtiter plates containing minimal medium. Wells containing mature biofilms were washed three times with PBS and incubated in the presence of 100 μg/ml MAb 18B7 for 2 h at 37°C. After MAb treatment, biofilms were incubated for 45 min at 37°C in 75 μl of PBS containing the fluorescent stains FUN-1 (10 μM), concanavalin A-Alexa Fluor 488 conjugate (ConA; 25 μM) and goat anti-mouse IgG1 or IgM-Alexa Fluor 350 conjugate (GAM-γ1-AF; Molecular Probes, Eugene, OR) (50 μg/ml). FUN-1 (excitation wavelength = 470 nm; emission = 590 nm) is converted to orange-red cylindrical intravacuolar structures by metabolically active cells. ConA (excitation wavelength = 488 nm; emission = 505 nm) binds to glucose and mannose residues of cell wall and capsule polysaccharides and fluoresces green. GAM-γ1-AF (excitation wavelength = 346 nm; emission = 442 nm) reacts with the Fc portion of the heavy chain of mouse IgG1 and fluoresces blue. Microscopic examinations of biofilms formed in microtiter plates were performed with confocal microscopy using an Axiovert 200 M inverted microscope. The objective used was 40× (numerical aperture of 0.6). Depth measurements were taken at regular intervals across the width of the device. To determine the structure of the biofilms, a series of horizontal (xy) optical sections with a thickness of 1.175 μm were taken throughout the full length of the biofilm. Confocal images of green (ConA), red (FUN-1), and blue (GAM-γ1-AF) fluorescence were recorded simultaneously using a multichannel mode. Z-stack images and measurements were corrected utilizing Axio Vision 4.4 software in deconvolution mode (Carl Zeiss MicroImaging, NY).
RESULTS AND DISCUSSION
Biofilms of C. neoformans strain B3501 were grown in the individual wells of polystyrene 96-well plates for 48 h and then washed to remove nonadherent cryptococcal cells. Using the confocal microscopy and a combination of three fluorescent stains—FUN-1, concanavalin A-Alexa Fluor 488 conjugate (ConA), and goat anti-mouse IgG1-Alexa Fluor 350 conjugate (GAM-γ1-AF)—we demonstrated that the capsular polysaccharide-binding MAb 18B7 (IgG1) penetrated the biofilm matrix and bound to metabolically active C. neoformans cells (Fig. 1). Thus, 18B7 MAb was chosen as a “delivery vehicle” to deliver radionuclides to the biofilms.
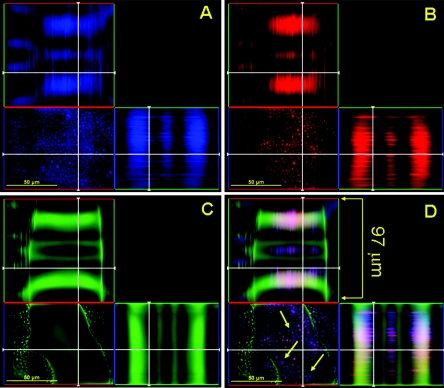
FIG. 1.
Confocal microscopic image of a mature C. neoformans biofilm treated with capsular binding MAb 18B7. Orthogonal images of a mature C. neoformans biofilm show capsular binding MAb 18B7 (blue; GAM-γ1-AF) penetration within internal regions of a biofilm (A); metabolically active (red; FUN-1-stained) C. neoformans cells (B); extracellular polysaccharide material (green; ConA stained) (C); and a superimposition of panels A, B, and C (D). The thickness of mature biofilms is approximately 97 μm. Arrows denote the locations of MAb 18B7 in a mature cryptococcal biofilm. Pictures were taken using a 40× power field. Scale bars, 50 μm.
To determine whether pentameric IgM was able to pass through the polysaccharide matrix of cryptococcal biofilm, a similar experiment was carried out utilizing GXM-binding MAb 13F1. However, 13F1 was excluded by the exopolymeric matrix and did not penetrate the fungal biofilm (Fig. 2).
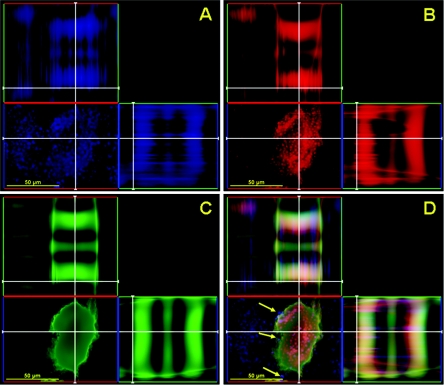
FIG. 2.
Confocal microscopic image of a mature C. neoformans biofilm treated with capsular binding MAb 13F1. Orthogonal images of a mature C. neoformans biofilm show capsular binding MAb 13F1 (blue; GAM-μ-AF) penetration within internal regions of a biofilm (A); metabolically active (red; FUN-1-stained) C. neoformans cells (B); extracellular polysaccharide material (green; ConA-stained) (C); and a superimposition of panels A, B, and C (D). Arrows denote the locations of MAb 13F1 in a mature cryptococcal biofilm. Pictures were taken using a 40× power field. Scale bars, 50 μm.
C. neoformans B3501 biofilms and planktonic cells were incubated with two different doses of 213Bi-18B7 MAb or without it. Addition of 30 μCi 213Bi-18B7 MAb caused a 50% reduction in biofilm metabolic activity (Table 1), which was measured by the XTT reduction assay (21). Planktonic (unattached) cells used as controls throughout the study were more susceptible to 213Bi-18B7 MAb than biofilms, with 15 and 30 μCi 213Bi-18B7 MAb causing 25 and 65% reductions, respectively, in the metabolic activity of planktonic cells. This result correlates with previous studies which suggest that microbial biofilms display more resistance to antimicrobial therapy than planktonic cells (1, 5). The killing of C. neoformans cells with 213Bi-18B7 MAb was antibody specific, as the same activities of control MAb 213Bi-MOPC21 had no effect on either biofilms or planktonic cells (Table 1). Unlabeled MAb 18B7 also had no effect on either biofilms or planktonic cells (results not shown).
TABLE 1.
Susceptibility of C. neoformans B3501 biofilms to 213Bi- and 188Re-labeled MAbsa
| Antibody (radioactivity level [μCi]) | % Metabolic activity
|
|
|---|---|---|
| B3501 biofilm | B3501 planktonic cells | |
| 213Bi-18B7 (15) | 96 (5) | 75 (0.3) |
| 213Bi-18B7 (30) | 47 (5) | 31 (1.5) |
| 213Bi-MOPC21 (15) | 99 (0.8) | 94 (0.3) |
| 213Bi-MOPC21 (30) | 100 (2) | 94 (0.6) |
| 213Bi-13F1 (15) | 100 (1) | 86 (2) |
| 213Bi-13F1 (30) | 98 (1) | 34 (1) |
| 188Re-18B7 (200) | 99 (9) | 74 (1) |
| 188Re-18B7 (400) | 99 (2) | 17 (6) |
The metabolic activity of age-matched biofilms and planktonic cells was measured using a XTT reduction assay. The values represent the averages of three XTT measurements, and standard deviations are given in parentheses.
The dose of 213Bi-18B7 MAb per B3501 cell which resulted in significant damage to the cells in both the planktonic and biofilm states—3 pCi per cell—was in concert with our previous data for C. neoformans 24067 strain (1 pCi per cell) (9), which implies that α particles were able to effectively penetrate the architecture of the biofilms to deliver microbicidal radiation to the cells. It should be noted that the number of cells in a biofilm cannot be known precisely with the techniques used. However, we obtained estimates of cell number from their ability to reduce XTT and used that measure to estimate the dose of radiation per cell in a biofilm. Encouragingly, the dose required for killing C. neoformans B3501 in a biofilm was of the same order of magnitude as the dose required to kill B3501 or 24067 planktonic cells.
To confirm that antibody penetration through the exopolymeric matrix was necessary for cryptococcal biofilm damage, yeast cells were exposed to similar doses of 213Bi conjugated to MAb 13F1. Fungal biofilms were resistant to treatment with alpha radiation when delivered by IgM (Table 1). Conversely, the metabolic activity of planktonic cells was decreased 65% when treated with a dose of 30 μCi.
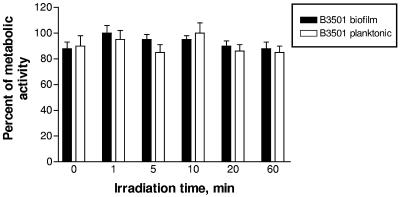
To prove that high-linear-energy-transfer types of ionizing radiation such as α particles are needed for destruction of biofilms, we investigated the effects of other, non-high-linear-energy-transfer types of radiation on cryptococcal biofilms, namely, external gamma radiation and β particles (electrons) delivered to the biofilms by 18B7 MAb. Irradiation of biofilms with a 137Cs source at a dose rate of 14 Gy/min for 0 to 60 min delivered doses of 0 to 840 Gy to the cells. According to the microdosimetry calculations which we reported in reference 9, 30 μCi 213Bi-18B7 MAb would deliver approximately 110 Gy to C. neoformans cells. Immediately after irradiation or 6 to 48 h after, the metabolic activity of the biofilms and planktonic cells was assessed by XTT assay. External radiation had no effect on the metabolic activity of biofilms and planktonic cells (Fig. 3). These results are in concordance with our previous data on extreme radioresistance of C. neoformans planktonic cells, with sublethal doses for this fungus being approximately 6,000 Gy (4). As the lethal whole-body dose for a human is around 5 Gy, external radiation is not an option for medical application for biofilm destruction.
FIG. 3.
Susceptibility of C. neoformans B3501 biofilms to gamma radiation. B3501 biofilms and planktonic cells were irradiated for different times with gamma photons from a 137Cs source at a dose rate of 14 Gy/min. Bars represent the averages of three XTT measurements.
We also investigated whether β emitter 188Re was effective against C. neoformans biofilms when delivered by C. neoformans-specific MAb 18B7. C. neoformans B3501 biofilms and planktonic cells were incubated for 3 h at 37°C with or without two doses of 188Re-18B7 MAb. As the decay half-life of 188Re is 17 h, the microtiter plate was incubated for an additional 24 h at 4°C to allow sufficient time for 188Re-emitted β particles to exert damage on the cells after the initial incubation. Biofilms treated with 200 and 400 μCi of 188Re-18B7 showed no decrease in metabolic activity. However, the metabolic activity of planktonic cells was significantly decreased to 30 and 85% after treatment with 200 and 400 μCi of 188Re-18B7, respectively, as determined by XTT assay (Table 1). Probably, the structural organization of these biofilms provided the yeast cells with a sheltered niche for protection against the β particles emitted by 188Re. Consistent with this notion we have recently shown that C. neoformans capsular polysaccharide possesses significant radioprotective properties (2). Conversely, radioprotectors are not effective for α radiation (13).
RIT was developed for cancer treatment to take advantage of the specificity of the antigen-antibody interaction to deliver radionuclides that emanate lethal doses of cytotoxic radiation close to the target cell (16). RIT has become a successful therapy for certain cancers, as evidenced by the recent approval of MAb-based drugs such as Zevalin and Bexxar (anti-CD20 MAbs labeled with 90-Yttrium and 131-Iodine, respectively) for the treatment of relapsed or refractory B-cell non-Hodgkin's lymphoma. Recent reports on the use of RIT as an initial treatment for follicular lymphoma (15) are encouraging, thus making RIT a first-line therapy choice in treatment of cancer.
Likewise, RIT can kill microorganisms quickly and efficiently, but this treatment has not been exploited clinically as a therapeutic antimicrobial strategy. However, the development of RIT for infectious diseases is potentially easier than its application to tumor therapy given antigenic and tissue perfusion differences between the sites of microbial infection and normal organs (reviewed in reference 10). This is the first report of a study in which the effect of RIT was tested against microbial biofilms. Our results indicate that C. neoformans biofilms are susceptible to RIT with α emitters. Since removing certain types of indwelling devices is difficult, one can imagine situations where it may be possible to treat infected devices in situ with RIT by local administration of radiolabeled MAb in close proximity to the infected device; alternatively, as MAbs may have a role in preventing biofilm formation (18), a prophylactic dose of unlabeled and radiolabeled antibody may be administered immediately after insertion of the device. In this regard, successful clinical experience has been accumulated in oncology in locoregional administration of radiolabeled MAbs (20). Novel therapeutic strategies against biofilm-related microbial infections may also be designed by combining RIT and conventional antimicrobial therapy.
Acknowledgments
We thank M. W. Brechbiel, National Institutes of Health (NIH), who was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, for the generous gift of CHXA" ligand.
The research was supported by National Institute of Allergy and Infectious Diseases grants AI52042 and AI60507 (E.D.) and AI033142, AI033774, and HL059842 (A.C.) and by the European Commission (C.A. and A.M.).
REFERENCES
- 1.Al-Fattani, M. A., and L. J. Douglas. 2004. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 48:3291-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bryan, R. A., O. Zaragoza, T. Zhang, G. Ortiz, A. Casadevall, and E. Dadachova. 2005. Radiological studies reveal radial differences in the architecture of the polysaccharide capsule of Cryptococcus neoformans. Eukaryot. Cell 4:465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. A. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall, A., E. Dadachova, and L. Pirofski. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2:695-703. [DOI] [PubMed] [Google Scholar]
- 5.Chandra, J., P. K. Mukherjee, S. D. Leidich, F. F. Faddoul, L. L. Hoyer, L. J. Douglas, and M. A. Ghannoum. 2001. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 80:903-908. [DOI] [PubMed] [Google Scholar]
- 6.Dadachova, E., A. Nakouzi, R. A. Bryan, and A. Casadevall. 2003. Ionizing radiation delivered by specific antibody is therapeutic against a fungal infection. Proc. Natl. Acad. Sci. USA 100:10942-10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dadachova, E., R. A. Bryan, A. Frenkel, T. Zhang, C. Apostolidis, J. S. Nosanchuk, J. D. Nosanchuk, and A. Casadevall. 2004. Evaluation of acute hematologic and long-term pulmonary toxicities of radioimmunotherapy of Cryptococcus neoformans infection in murine models. Antimicrob. Agents Chemother. 48:1004-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadachova, E., T. Burns, R. A. Bryan, C. Apostolidis, M. W. Brechbiel, J. D. Nosanchuk, A. Casadevall, and L. Pirofski. 2004. Feasibility of radioimmunotherapy of experimental pneumococcal infection. Antimicrob. Agents Chemother. 48:1624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dadachova, E., R. W. Howell, R. A. Bryan, A. Frenkel, J. D. Nosanchuk, and A. Casadevall. 2004. Susceptibility of the human pathogenic fungi Cryptococcus neoformans and Histoplasma capsulatum to gamma-radiation versus radioimmunotherapy with alpha- and beta-emitting radioisotopes. J. Nucl. Med. 45:313-320. [PubMed] [Google Scholar]
- 10.Dadachova, E., and A. Casadevall. 2005. Antibodies as delivery vehicles for radioimmunotherapy of infectious diseases. Expert. Opin. Drug Del. 2:1075-1084. [DOI] [PubMed] [Google Scholar]
- 11.Donlan, R. 2005. New approaches for the characterization of prosthetic joint biofilms. Clin. Orthop. Relat. Res. 437:12-19. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson, T. J., Lindsay, B. D. and J. P. Boineau. 1994. Should surgeons still be implanting pacemakers? Ann. Thorac. Surg. 57:588-596. [DOI] [PubMed] [Google Scholar]
- 13.Goddu, S. M., V. R. Narra, R. S. Harapanhalli, R. W. Howell, and D. V. Rao. 1996. Radioprotection by DMSO against the biological effects of incorporated radionuclides in vivo—comparison with other radioprotectors and evidence for indirect action of Auger electrons. Acta Oncol. 35:901-907. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman, L. R., D. A. D'Argenio, M. J. MacCoss, Z. Zhang, R. A. Jones, and S. I. Miller. 2005. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 436:1171-1174. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski, M. S., J. A. Radford, S. A. Gregory, J. P. Leonard, S. J. Knox, S. Kroll, and R. L. Wahl. 2005. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N. Engl. J. Med. 352:441-449. [DOI] [PubMed] [Google Scholar]
- 16.Milenic, D., E. D. Brady, and M. W. Brechbiel. 2004. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Dis. 3:488-498. [DOI] [PubMed] [Google Scholar]
- 17.Maki, D. G., and P. A. Tambyah. 2001. Engineering out the risk of infection with urinary catheters. Emerg. Infect. Dis. 7:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez, L. R., and A. Casadevall. 2005. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect. Immun. 73:6350-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez, L. R., and A. Casadevall. 2006. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50:1021-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merlo, A., J. Mueller-Brand, and H. R. Maecke. 2003. Comparing monoclonal antibodies and small peptidic hormones for local targeting of malignant gliomas. Acta Neurochir. Suppl. 88:83-91. [DOI] [PubMed] [Google Scholar]
- 21.Meshulam, T. L. S., L. Christin, and R. D. Diamond. 1995. A simplified new assay for assessment of fungal cell damage with the tetrazolium dye, (2,3)-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT). J. Infect. Dis. 172:1153-1156. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee, J., A. Casadevall, and M. D. Scharff. 1993. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J. Exp. Med. 177:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebuck, J., K. R. Murry, D. H. Rhoney, D. B. Michael, and W. M. Coplin. 2000. Infection related to intracranial pressure monitors in adults: analysis of risk factors and antibiotic prophylaxis. J. Neurol. Neurosurg. Psychiatry 69:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trampuz, A., and W. Zimmerli. 2005. New strategies for the treatment of infections associated with prosthetic joints. Curr. Opin. Investig. Drugs 6:185-190. [PubMed] [Google Scholar]
- 25.Virk, A., and D. R. Osmon. 2001. Prosthetic joint infection. Curr. Treatment Options Infect. Dis. 3:287-300. [Google Scholar]
- 26.Walsh, T. J., R. Schlegel, M. M. Moody, J. W. Costerton, and M. Salcman. 1986. Ventriculoatrial shunt infection due to Cryptococcus neoformans: an ultrastructural and quantitative microbiological study. Neurosurgery 18:373-375. [DOI] [PubMed] [Google Scholar]