Abstract
The use of fluoroquinolones has been linked to increasing bacterial resistance and infection and/or colonization with already resistant pathogens both as a risk factor and based on volume of use. Changes in individual fluoroquinolones used in an institution may also be related to these clinical problems. Interrupted time series analysis, which allows for assessment of the associations of an outcome attributable to a specific event in time, was used to study the effect of changes in our hospital's fluoroquinolone formulary on fluoroquinolone susceptibility rates in select gram-negative pathogens and the methicillin-resistant Staphylococcus aureus (MRSA) isolation rate. Susceptibility rates to ciprofloxacin were considered for the period of 1993 through 2004, while the MRSA isolation rate was assessed from 1995 through 2004. Levofloxacin was added to the formulary in 1999, and gatifloxacin was substituted for levofloxacin in 2001. Statistically significant changes in the already declining rates of susceptibility of Pseudomonas aeruginosa (P, 0.042) and Escherichia coli (P, 0.004) to ciprofloxacin and in the already rising MRSA isolation rate (P, 0.001) were associated with the addition of levofloxacin to the formulary. Substitution of gatifloxacin for levofloxacin on the formulary was associated with reversals in the downward trend in E. coli susceptibility to ciprofloxacin and the upward trend in MRSA isolation rate. No associations were detected on susceptibility of Klebsiella pneumoniae or Proteus mirabilis to ciprofloxacin. These findings suggest that potential changes in susceptibility to fluoroquinolones and isolation of MRSA may vary by both drug and organism.
Infections with antibiotic-resistant bacteria have been linked to a variety of negative consequences (9, 21). These include both patient-related and economic outcomes. At the patient level, for instance, infection with methicillin-resistant Staphylococcus aureus (MRSA) has been shown in some studies to be associated with longer length of hospital stay and higher cost to the health care system than for patients infected with drug-susceptible counterparts (1, 7, 13, 46). An increase in mortality associated with infections with MRSA has also been reported (29, 46). Similar associations have also been reported for some gram-negative pathogens such as Pseudomonas aeruginosa (8). Estimates for total U.S. health care dollars spent due to infections caused by antibiotic-resistant bacteria are as high as $5 billion annually (22). Components of the expenditures include the extra time in a hospital and increased number of physician visits when antibiotics are ineffective, the cost of newer antibiotics to replace ineffective antibiotics, lost productivity/workdays, and even death. Curbing the occurrence and spread of bacterial resistance is an important health care initiative and has led such entities as the Centers for Disease Control and Prevention (CDC) to issue recommendations designed to meet this goal. Among the recommendations in the CDC's 12-step program are items addressing inappropriate antibiotic use (http://www.cdc.gov/drugresistance/healthcare/ha/12steps_HA.htm).
It has long been thought that there is a relationship between the extent of a given antibiotic's use and the incidence of bacterial resistance to that antibiotic or antibiotic class (34). This association may also take the form of rising rates of infection caused by already resistant pathogens such as vancomycin-resistant enterococci and MRSA. It is also believed that some antibiotics are more strongly associated with these resistance-associated problems and that even within an antibiotic class, some agents pose a larger risk than others. As a case in point, it has been theorized that some fluoroquinolones have a greater propensity to increase resistance than others and that the use of one might adversely affect susceptibility rates to another (14, 45).
Our objective in the current study was to assess the effect of changes in our formulary fluoroquinolones on relevant resistance rates in the institution, as reflected by resistance rates of various gram-negative pathogens to ciprofloxacin over time and by the institution's isolation rate of MRSA.
(This work was presented in part at the 42nd Annual Meeting of the Infectious Diseases Society of America, Boston, Mass., 2004, abstr. 375 and 507.)
MATERIALS AND METHODS
Facility and data.
The Medical University of South Carolina Medical Center is a 600-bed academic medical center with both adult and pediatric beds. It is designated as a level 1 trauma center. The average daily census rate is 468, and annual admissions are 28,951. Using the institution's infection control and microbiology databases, two markers were identified to assess the effect of introduction of new fluoroquinolone antibiotics onto our formulary. Specifically, isolation rates of hospital-acquired MRSA and susceptibility rates of four gram-negative pathogens to ciprofloxacin were examined. Data on census (hospital-wide, adult only), antibiotic usage, and susceptibility to ciprofloxacin were collected on a quarterly basis from July 1993 through December 2004. The organisms of interest were Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Quarterly isolation rates for hospital-acquired MRSA (infection or colonization) were calculated beginning in October 1995. Hospital-acquired MRSA isolations are those from inpatients who had been in our hospital for at least 3 days at the time of the positive culture or had been readmitted to our institution with 30 days of a previous stay with no intervening admissions of 72 h or more to other health care facilities.
Individual orders for each fluoroquinolone evaluated were electronically transferred from the hospital pharmacy computer system (Cerner, Kansas City, MO) to a spreadsheet (Excel; Microsoft, Redmond, WA) customized for this project. Drug use data reflect doses ordered and dispensed. Orders were deidentified before analysis. The number of grams for each drug order was based upon dose, total number of daily doses, and days of antibiotic therapy. Grams were converted to census-normalized defined daily doses (DDD) (per 1,000 patient days) using World Health Organization definitions (http://www.whocc.no/atcddd/). Prior to the introduction of levofloxacin onto our formulary in 1999, a variety of drugs in this class were used, although ciprofloxacin use comprised the vast majority. In 2001, levofloxacin was replaced with gatifloxacin. Individual and total fluoroquinolone use was quantified for the study period. Only adult fluoroquinolone use was considered in the analysis, as pediatric use of this antibiotic class is negligible in our institution.
The total number of nonurine isolates and their ciprofloxacin susceptibility rates were collected from the hospital's quarterly antibiograms. Only data from hospitalized inpatients were included in the analysis. For each of the four organisms, the percent susceptible was calculated by dividing the number of susceptible isolates by the total number of isolates and multiplying by 100. Duplicate isolates were removed from the database. For our purposes, a duplicate isolate was defined as a like organism, isolated from the same patient, during a given hospitalization.
Data analysis.
Segmented regression analysis for interrupted time series was used to determine the significance of the differences in levels and slopes over time due to two interventions: (i) the addition of levofloxacin to the formulary in 1999 and (ii) a subsequent switch from levofloxacin to gatifloxacin in 2001. Segmented regression analysis of interrupted time series data allows for the assessment of long-term effects on an outcome attributable to a specific event in time, i.e., the implementation of an intervention. In interrupted time series, the level and trend of the preintervention segment serve as the controls for the postintervention segment, providing a methodologically acceptable design for measuring the effect of an intervention (15, 36). Total volume of fluoroquinolone use was measured and controlled for in the analysis, allowing for examination of the effect on resistance observed for both levofloxacin and gatifloxacin independent of changes in overall fluoroquinolone use. Estimations were made of the change in isolation rates of MRSA and susceptibility rates of the gram-negative organisms immediately following the intervention, the difference between the pre- and postintervention slopes of the outcome, and the periodic average intervention effect after the intervention (refer to the Appendix for a more detailed explanation of the model).
Proper estimations of standard errors and significance were made through the detection of and correction for autocorrelation. The Durbin-Watson statistic (11) was used to test for autocorrelation in the residuals. A value of 2.0 for the Durbin-Watson statistic indicated that serial correlation was not detected. If significant autocorrelation was detected, maximum likelihood estimation with first-order differencing was used to correct the problem. Significance was determined at the 0.05 level, and SAS 9.0 (SAS, Cary, NC) was used for the statistical programming.
RESULTS
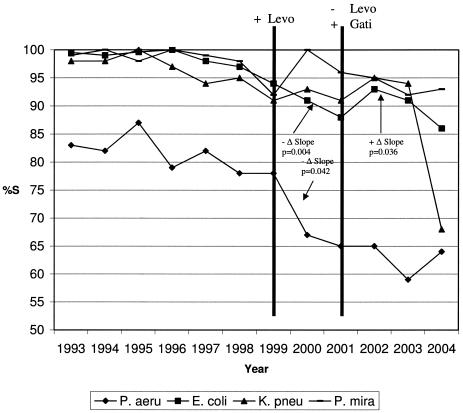
No associations on the susceptibility rates of K. pneumoniae or P. mirabilis to ciprofloxacin were observed related to formulary changes involving levofloxacin or gatifloxacin (Table 1 and Fig. 1). With P. aeruginosa, a significant negative change (P, 0.042) in the already downward trend in ciprofloxacin susceptibility was detected after introduction of levofloxacin but not after the change from levofloxacin to gatifloxacin (P, 0.081) (Table 1 and Fig. 1). With E. coli, a significant negative change (P, 0.004) in the already downward slope of ciprofloxacin susceptibility was detected after introduction of levofloxacin (Table 1 and Fig. 1). After conversion from levofloxacin to gatifloxacin, a significant reversal (P, 0.036) of the downward trend of the E. coli susceptibility to ciprofloxacin was observed (positive slope). In each analysis, the effect of total fluoroquinolone usage was not significant.
TABLE 1.
Relationships with susceptibility rates to ciprofloxacin over timea
| Variable | Sign (P value) for:
|
|||
|---|---|---|---|---|
| P. aeruginosa | E. coli | K. pneumoniae | P. mirabilis | |
| Intercept | + (<0.0001) | + (<0.0001) | + (<0.0001) | + (<0.0001) |
| Time (count variable) | − (0.436) | − (0.039) | − (0.927) | − (0.623) |
| Levofloxacin | + (0.948) | + (0.184) | + (0.829) | − (0.155) |
| Levofloxacin/time (slope variable) | − (0.042) | − (0.004) | − (0.929) | + (0.146) |
| Gatifloxacin | + (0.183) | + (0.0002) | + (0.250) | − (0.291) |
| Gatifloxacin/time (slope variable) | + (0.081) | + (0.036) | − (0.349) | − (0.188) |
| Total fluoroquinolones | − (0.900) | + (0.446) | − (0.351) | + (0.825) |
| Durbin-Watson statistic | 1.90 | 2.37 | 1.76 | 2.35 |
Significance determined at the 0.05 level. Refer to the Appendix for details of the model.
FIG. 1.
Ciprofloxacin susceptibility rates over time. It should be noted that statistical results are based on quarterly data. However, for visual clarity, annual susceptibility rates have been plotted for Fig. 1. %S, percent susceptible; Levo, levofloxacin; Gati, gatifloxacin; P. aeru, P. aeruginosa; K. pneu, K. pneumoniae; P. mira, P. mirabilis.
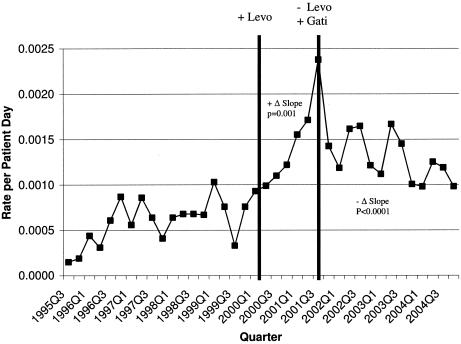
A significant positive change (increased rate) (P, 0.001) in the already upward-slope trend of the MRSA isolation rate was observed related to introduction of levofloxacin (Table 2 and Fig. 2). During the later change from levofloxacin to gatifloxacin, a strong negative change (P, <0.0001) was observed. The effect of the total volume of fluoroquinolones used during these periods was positive and significant (P, 0.017) and therefore also related to changes in MRSA isolation rates.
TABLE 2.
Relationships with MRSA isolation rate over timea
| Variable | Sign (P value) |
|---|---|
| Intercept | + (0.225) |
| Time (count variable) | + (0.904) |
| Levofloxacin | − (0.257) |
| Levofloxacin/time (slope variable) | + (0.001) |
| Gatifloxacin | + (0.343) |
| Gatifloxacin/time (slope variable) | − (<0.0001) |
| Total fluoroquinolones | + (0.017) |
| Durbin-Watson statistic | 1.78 |
Significance determined at the 0.05 level. Refer to the Appendix for details of the model.
FIG. 2.
MRSA isolation rate over time. Levo, levofloxacin; Gati, gatifloxacin.
For the sake of perspective in considering these results, other information regarding fluoroquinolone use and bacterial susceptibility is important. During the time period considered here, there were no significant changes in the amount of ciprofloxacin used from one observation point to another. While the susceptibility of P. aeruginosa and E. coli to ciprofloxacin declined during this period, the percent susceptible of these organisms and P. mirabilis to levofloxacin remained stable. Unlike the case for ciprofloxacin, the percentage of K. pneumoniae isolates susceptible to levofloxacin declined. Lastly, the percentage of MRSA of all S. aureus isolates increased from 16 to 57 from 1995 through 2004.
DISCUSSION
Changes in bacterial susceptibility and isolation rates for resistant bacteria such as MRSA are likely affected by many factors, including changes in infection control practices, the increase of community-acquired infections with antibiotic-resistant pathogens, and usage patterns of antibiotics, among others. The introduction and/or use of certain antibiotics has long been hypothesized to play an important role within an individual institution. These effects may be related to the mix of antibiotics used in a hospital or the total quantity of an antibiotic class used, although they may also be specific antibiotic dependent. The rise of resistance to fluoroquinolones has been accompanied by their apparent association with the selection of already multidrug-resistant bacteria and their associated infections. As recently reviewed by Jacoby, bacteria may become resistant to fluoroquinolones by virtue of mutation or acquisition of plasmids and express resistance modes, including target site (parC or gyrA) alterations, efflux, and production of target site-protective proteins (23). Importantly, fluoroquinolone resistance may coexist in bacteria along with other important forms of resistance such as production of extended-spectrum beta-lactamases (26, 43).
The evolution of fluoroquinolone-resistant gram-positive and gram-negative pathogens has been well documented (20, 24, 30, 50). In both cases, increasing resistance has been linked to increasing quinolone use, although publication bias may also contribute to this overall perception. There is also evidence that some quinolones may be more problematic than others in this regard. With gram-negative organisms, the use of fluoroquinolones in individual patients has been identified as a risk factor for associated resistance, while the general increasing use of these agents has been associated with increasing fluoroquinolone resistance. In a report of a 5-year observational study of E. coli bacteremia from Spain, investigators revealed a statistically significant correlation between ciprofloxacin resistance and an upward trend in ciprofloxacin and norfloxacin use in both the community and the hospital. When 27 cases were matched to 54 controls with ciprofloxacin-susceptible strains, it was found that patients in the cases were more likely to have received a prior fluoroquinolone. The analysis revealed prior fluoroquinolone use as the only independent risk factor (44). Muder et al. also conducted a case-controlled study of risk factors for acquisition of ciprofloxacin-resistant gram-negative isolates. As with the previous study, using multivariate analysis, they found prior receipt of a fluoroquinolone (ciprofloxacin or norfloxacin) to be the single most significant risk factor (40). Of note, length of therapy with ciprofloxacin has also been reported to be an independent risk factor for acquisition of multidrug-resistant P. aeruginosa (42). Many other studies have found a general association between increasing fluoroquinolone use and increasing resistance. In a study of gram-negative isolates from intensive care units throughout the United States collected from 1994 through 2000, a marked decline in ciprofloxacin susceptibility was significantly associated with the increasing national consumption of fluoroquinolones (41). This relationship was also found in another study of 10 hospitals in the United States, especially among gram-negative bacteria (51). In a benchmarking study involving 174 hospitals from 1994 through 1999, the relationship between fluoroquinolone expenditures and ciprofloxacin resistance among isolates of P. aeruginosa was studied. Again, a positive relationship between ofloxacin and levofloxacin expenditures and ciprofloxacin resistance was found (2). Similarly, Mohr et al. found a significant relationship between increasing P. aeruginosa resistance and levofloxacin use (35). Interestingly, these investigators found no other associations with the use of other fluoroquinolones or with overall antibiotic use, raising the possibility that some agents in the class might have greater resistance potential. In a study by Polk and colleagues in which fluoroquinolone use in both hospitals and their surrounding communities was considered, extent of use was found to be predictive of resistance rates in P. aeruginosa. Again, levofloxacin use was associated with resistance but ciprofloxacin use was not (45).
Are some fluoroquinolones more prone to lead to resistance than others? This is suggested in some of these studies, and plausible explanations have been put forward. Both ofloxacin and levofloxacin are less active in vitro against P. aeruginosa. Thus, with traditional dosing of either, antibiotic exposure may be inadequate to prevent emergence of resistance. As noted in the work of Thomas et al., achieving area under the serum concentration curve-to-MIC (AUC/MIC) ratios of ≥100 is associated with decreased emergence of resistance (47). This pharmacodynamic target may not be commonly achieved by typical doses of these two agents. It may also be that some bacteria are more prone to fluoroquinolone resistance than others. In vitro work by Gilbert et al. showed a greater likelihood of resistance selection in P. aeruginosa with levofloxacin as opposed to ciprofloxacin, which has greater in vitro potency against this organism (14). Kern et al. reported high rates of resistance in E. coli isolated from hematology/oncology patients in a high-fluoroquinolone-use hospital but no correlation between fluoroquinolone use and resistance prevalence in P. aeruginosa, coagulase-negative staphylococci, or S. aureus (25). A similar observation was reported by Lautenbach et al., who noted variations between fluoroquinolone use and resistance across various organisms (27). Our own observations support this concept, as we saw strong relationships between changes in our fluoroquinolone formulary and changing resistance rates in P. aeruginosa and E. coli but not in K. pneumoniae or P. mirabilis. Regardless of the exact intricacies of the fluoroquinolone use-resistance relationship among gram-negative pathogens, it is clear that the existence of such resistance is associated with negative consequences in patients (8, 28).
The relationship between fluoroquinolone use and infection or colonization with MRSA has been well documented (37). As with gram-negative pathogens, fluoroquinolone use has been found to be a risk factor for MRSA colonization and infection, and the extent of use of these agents in institutions has been related to rising MRSA isolation rates. The relationship is not surprising, given the activity of some members of the class against methicillin-susceptible S. aureus, which would provide selective pressure favoring MRSA. Further, the effects of fluoroquinolones on the expression of resistance determinants and fibronectin binding proteins may also enhance colonization (3, 4, 48). In case-controlled studies, both Weber et al. and Dziekan et al. found fluoroquinolone use to be an independent risk factor for MRSA colonization (12, 49). Using multivariable Cox proportional hazards modeling, Harbarth et al. also found previous fluoroquinolone use to be an independent risk factor for MRSA carriage (17). A number of other investigators have reported the association between fluoroquinolone use and increasing MRSA isolation rates (6, 10, 16, 19, 33, 39). MacDougall et al. reported that MRSA was more strongly associated with levofloxacin than ciprofloxacin, which might be predicted given the greater in vitro potency of the former against methicillin-susceptible S. aureus (32). This is consistent with the results of the present study. It must be stressed that our analysis considers only hospital-acquired MRSA and that a consideration of the rising rates of community-acquired MRSA is not taken into account.
The use of interrupted time series analysis to study how changes in antibiotic use might affect resistance offers advantages over other methods such as correlation analysis or simple linear regression. Time series methodology allows for the modeling of a series of events or observations over time by dissecting the variation into cyclic changes (such as seasonal variation, trend, and unknown “noise”) while taking into consideration the possible association between successive observations (5, 18, 38). Lopez-Lozano et al. demonstrated the value of time series methodology by analyzing two antimicrobial-microorganism combinations, ceftazidime-resistant gram-negative bacilli and imipenem-P. aeruginosa, in a Spanish hospital over a 90-month period (31). Using this methodology, the authors were able to demonstrate a temporal relationship between antimicrobial use and resistance, quantify the effect of use on resistance, and estimate the delay between variations of use and subsequent variations in resistance. Monnet et al. used similar methodology to determine the effects of antimicrobial drug use on MRSA in a Danish hospital over a 60-month period, allowing for strong temporal relationships to be revealed between antimicrobial use and the varying prevalence of MRSA over time (39).
Potential limitations of our methods should be considered. It should be noted that the use of other antibiotics, which might also affect the resistance patterns assessed, were not considered in our model. Further, we did not evaluate the potential associations of infection control efforts with these apparent relationships. An intensification of the infection control program (active surveillance, etc.) has been implemented at our hospital, but these changes did not begin to go into effect until late in 2001. Thus, the upward trends in resistance and MRSA isolation rates we studied were unlikely to have been affected by changes in infection control practices.
Our results are consistent with previous observations suggesting that changes in antibiotics used in the hospital environment may affect susceptibility patterns among gram-negative pathogens and trends in colonization/infection rates with MRSA. While we found that the total volume of fluoroquinolone usage was not a significant influence on the susceptibility patterns of certain gram-negative organisms to ciprofloxacin, the total volume of all fluoroquinolones used did appear to influence the isolation rates of hospital-acquired MRSA. At the same time, MRSA isolation rates as well as changing resistance rates among some gram-negative bacteria were apparently related to differences among individual fluoroquinolones in use. It is therefore appropriate, when analyzing antibiotic use-resistance relationships, to consider which antibiotics of a given class are in use, as well as the amount of use of both individual antibiotics and the class as a whole over time.
APPENDIX
The date of the intervention serves as an instrument variable. Thus, the interrupted time series analysis can be specified as E(Yt) = α + β1x1 + β2x2 + β3(x1 × x2) + βixi + ηt, where Yt is the dependent variable, x1 is an ascending “count” variable indicating the order (period) that the observations were taken, x2 is a dummy variable representing whether the observation was collected before or during the intervention, and xi represents other covariates in the equation (15). Thus, as an example, if the intervention is a change in the formulary, and the dependent variable is the change in the isolation rates of MRSA, then α represents the intercept of the autoregression function prior to the change in formulary, β1 represents the linear trend (slope) of isolation rates before the intervention, β2 represents the change in intercept of the autoregression function from the preintervention phase to the postintervention phase, β3 represents the change in the isolation rates over time (slope) from the preintervention period to the postintervention period, βi represents the associations from possible covariates (i.e., total fluoroquinolone use), and ηt represents the random error at time t. If the interaction term associated with β3 is negative and significant, it suggests that, on average, the intervention significantly decreases the isolation rate for MRSA.
Acknowledgments
J.A.B. has been a consultant for and received research grant funding from OrthoMcNeil and Bristol-Myers Squibb and is a member of speakers' bureaus for OrthoMcNeil and Schering.
REFERENCES
- 1.Abramson, M. A., and D. J. Sexton. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect. Control Hosp. Epidemiol. 20:408-411. [DOI] [PubMed] [Google Scholar]
- 2.Bhavnani, S. M., W. A. Callen, A. Forrest, K. K. Gilliland, D. A. Collins, J. A. Paladino, and J. J. Schentag. 2003. Effect of fluoroquinolone expenditures on susceptibility of Pseudomonas aeruginosa to ciprofloxacin in U.S. hospitals. Am. J. Health Syst. Pharm. 60:1962-1970. [DOI] [PubMed] [Google Scholar]
- 3.Bisognano, C., P. E. Vaudaux, D. P. Lew, E. Y. Ng, and D. C. Hooper. 1997. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 41:906-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisognano, C., P. Vaudaux, P. Rohner, D. P. Lew, and D. C. Hooper. 2000. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob. Agents Chemother. 44:1428-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Box, G. E. P., and G. Jenkins. 1976. Time series analysis: forecasting and control, revised edition. Holden Day, San Francisco, Calif.
- 6.Campillo, B., C. Dupeyron, and J. P. Richardet. 2001. Epidemiology of hospital-acquired infections in cirrhotic patients: effect on carriage of methicillin-resistant Staphylococcus aureus and influence of previous antibiotic therapy and norfloxacin prophylaxis. Epidemiol. Infect. 127:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbon, C. 1999. Costs of treating infections caused by methicillin-resistant staphylococci and vancomycin-resistant enterococci. J. Antimicrob. Chemother. 44(Suppl. A):31-36. [DOI] [PubMed] [Google Scholar]
- 8.Carmeli, Y., N. Troillet, A. W. Karchmer, and M. H. Samore. 1999. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch. Intern. Med. 159:1127-1132. [DOI] [PubMed] [Google Scholar]
- 9.Cosgrove, S. E., and Y. Carmeli. 2003. The impact of antimicrobial resistance on health and economic outcomes. Clin. Infect. Dis. 36:1433-1437. [DOI] [PubMed] [Google Scholar]
- 10.Crowcroft, N. S., O. Ronveaux, D. L. Monnet, and R. Mertens. 1999. Methicillin-resistant Staphylococcus aureus and antimicrobial use in Belgian hospitals. Infect. Control Hosp. Epidemiol. 20:31-36. [DOI] [PubMed] [Google Scholar]
- 11.Durbin, J., and G. S. Watson. 1951. Testing for serial correlation in least squares regression. II. Biometrika 38:159-178. [PubMed] [Google Scholar]
- 12.Dziekan, G., A. Hahn, K. Thüne, G. Schwarzer, K. Schäfer, F. Daschner, and H. Grundmann. 2000. Methicillin-resistant Staphylococcus aureus in a teaching hospital: investigation of nosocomial transmission using a matched case-control study. J. Hosp. Infect. 46:263-270. [DOI] [PubMed] [Google Scholar]
- 13.Engemann, J. J., Y. Carmeli, S. E. Cosgrove, V. G. Fowler, M. Z. Bronstein, S. L. Trivette, J. P. Briggs, D. J. Sexton, and K. S. Kaye. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 36:592-598. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert, D. N., S. J. Kohlhepp, K. A. Slama, G. Grunkemeier, G. Lewis, R. J. Dworkin, S. E. Slaughter, and J. E. Leggett. 2001. Phenotypic resistance of Staphylococcus aureus, selected Enterobacteriaceae, and Pseudomonas aeruginosa after single and multiple in vitro exposures to ciprofloxacin, levofloxacin, and trovafloxacin. Antimicrob. Agents Chemother. 45:883-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillings, D., D. Makuc, and E. Siegel. 1981. Analysis of interrupted time series mortality trends: an example to evaluate regionalized perinatal care. Am. J. Public Health 71:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graffunder, E. M., and R. A. Venezia. 2002. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J. Antimicrob. Chemother. 49:999-1005. [DOI] [PubMed] [Google Scholar]
- 17.Harbarth, S., N. Liassine, S. Dharan, P. Herrault, R. Auckenthaler, and D. Pittet. 2000. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 31:1380-1385. [DOI] [PubMed] [Google Scholar]
- 18.Helfenstein, U. 1996. Box-Jenkins modelling in medical research. Stat. Methods Med. Res. 5:3-22. [DOI] [PubMed] [Google Scholar]
- 19.Hill, D. A., T. Herford, and D. Parratt. 1998. Antibiotic usage and methicillin-resistant Staphylococcus aureus: an analysis of causality. J. Antimicrob. Chemother. 42:676-677. [DOI] [PubMed] [Google Scholar]
- 20.Hoban, D. J., G. V. Doern, A. C. Fluit, M. Roussel-Delvallez, and R. N. Jones. 2001. Worldwide prevalence of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S81-S93. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg, S. D., S. L. Solomon, and P. A. Blake. 1987. Health and economic impacts of antimicrobial resistance. Rev. Infect. Dis. 9:1065-1078. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. 1998. Antimicrobial resistance: issues and options. Workshop report. National Academy Press, Washington, D.C. [PubMed]
- 23.Jacoby, G. A. 2005. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 41(Suppl. 2):S120-S126. [DOI] [PubMed] [Google Scholar]
- 24.Karlowsky, J. A., D. C. Draghi, M. E. Jones, C. Thornsberry, I. R. Friedland, and D. F. Sahm. 2003. Surveillance for antimicrobial susceptibility among clinical isolates of Pseudomonas aeruginosa and Acinetobacter baumannii from hospitalized patients in the United States, 1998 to 2001. Antimicrob. Agents Chemother. 47:1681-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kern, W. V., M. Steib-Bauert, K. de With, S. Reuter, H. Bertz, U. Frank, and H. von Baum. 2005. Fluoroquinolone consumption and resistance in haematology-oncology patients: ecological analysis in two university hospitals 1999-2002. J. Antimicrob. Chemother. 55:57-60. [DOI] [PubMed] [Google Scholar]
- 26.Lautenbach, E., B. L. Stron, W. B. Bilker, J. B. Patel, P. H. Edelstein, and N. O. Fishman. 2001. Epidemiological investigation of fluoroquinolone resistance in infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Clin. Infect. Dis. 33:1288-1294. [DOI] [PubMed] [Google Scholar]
- 27.Lautenbach, E., B. L. Strom, W. B. Bilker, A. M. Marr, L. A. Larosa, and N. O. Fishman. 2004. Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae isolates from inpatients and outpatients, 1989-2000: differences in the emergence and epidemiology of resistance across organisms. Clin. Infect. Dis. 38:655-662. [DOI] [PubMed] [Google Scholar]
- 28.Lautenbach, E., J. P. Metlay, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2005. Association between fluoroquinolone resistance and mortality in Escherichia coli and Klebsiella pneumoniae infections. The role of inadequate empirical antimicrobial therapy. Clin. Infect. Dis. 41:923-929. [DOI] [PubMed] [Google Scholar]
- 29.Linden, P. K., A. W. Pasculle, R. Manez, D. J. Kramer, J. J. Fung, A. D. Pinna, and S. Kusne. 1996. Differences in outcomes for patients with bacteremia due to vancomycin-resistant Enterococcus faecium or vancomycin-sensitive Enterococcus faecium. Clin. Infect. Dis. 22:663-670. [DOI] [PubMed] [Google Scholar]
- 30.Livermore, D. M., D. James, M. Reacher, C. Graham, T. Nichols, P. Stephens, A. P. Johnson, and R. C. George. 2002. Trends in fluoroquinolone (ciprofloxacin) resistance in Enterobacteriaceae from bacteremias, England and Wales, 1990-1999. Emerg. Infect. Dis. 8:473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Lozano, J. M., D. L. Monnet, A. Yague, A. Burgos, N. Gonzalo, P. Campillos, and M. Saez. 2000. Modelling and forecasting antimicrobial resistance and its dynamic relationship to antimicrobial use: a time series analysis. Int. J. Antimicrob. Agents 14:21-31. [DOI] [PubMed] [Google Scholar]
- 32.MacDougall, C., J. P. Powell, C. K. Johnson, M. B. Edmond, and R. E. Polk. 2005. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin. Infect. Dis. 41:435-440. [DOI] [PubMed] [Google Scholar]
- 33.MacDougall, C., S. E. Harpe, J. P. Powell, C. K. Johnson, M. B. Edmond, and R. E. Polk. 2005. Pseudomonas aeruginosa, Staphylococcus aureus, and fluoroquinolone use. Emerg. Infect. Dis. 11:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGowan, J. E., Jr. 1987. Is antimicrobial resistance in hospital microorganisms related to antibiotic use? Bull. N. Y. Acad. Med. 63:253-268. [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr, J. F., A. Jones, L. Ostrosky-Zeichner, A. Wanger, and G. Tillotson. 2004. Associations between antibiotic use and changes in susceptibility patterns of Pseudomonas aeruginosa in a private, university-affiliated teaching hospital: an 8-year-experience: 1995-2002. Int. J. Antimicrob. Agents 24:346-351. [DOI] [PubMed] [Google Scholar]
- 36.Mol, P. G. M., J. E. Wieringa, P. V. NannanPanday, R. O. B. Gans, J. E. Degener, M. Laseur, and F. M. Haaijer-Ruskamp. 2005. Improving compliance with hospital antibiotic guidelines: a time-series intervention analysis. J. Antimicrob. Chemother. 55:550-557. [DOI] [PubMed] [Google Scholar]
- 37.Monnet, D. L. 1998. Methicillin-resistant Staphylococcus aureus and its relationship to antimicrobial use: possible implications for control. Infect. Control Hosp. Epidemiol. 19:552-559. [DOI] [PubMed] [Google Scholar]
- 38.Monnet, D. L., J. M. Lopez-Lozano, P. Campillos, A. Burgos, A. Yague, and N. Gonzalo. 2001. Making sense of antimicrobial use and resistance surveillance data: application of ARIMA and transfer function models. Clin. Microbiol. Infect. 7(Suppl. 5):29-36. [DOI] [PubMed] [Google Scholar]
- 39.Monnet, D. L., F. M. MacKenzie, J. M. Lopez-Lozano, A. Beyaert, M. Camacho, R. Wilson, D. Stuart, and I. M. Gould. 2004. Antimicrobial drug use and methicillin-resistant Staphylococcus aureus, Aberdeen, 1996-2000. Emerg. Infect. Dis. 10:1432-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muder, R. R., C. Brennen, A. M. Goetz, M. M. Wagener, and J. D. Rihs. 1991. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs Medical Center. Antimicrob. Agents Chemother. 35:256-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neuhauser, M. M., R. A. Weinstein, R. Rydman, L. H. Danziger, G. Karam, and J. P. Quinn. 2003. Antibiotic resistance among gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 289:885-888. [DOI] [PubMed] [Google Scholar]
- 42.Paramythiotou, E., J. C. Lucet, J. F. Timsit, D. Vanjak, C. Paugam-Burtz, J. L. Trouillet, S. Belloc, N. Kassis, A. Karabinis, and A. Andremont. 2004. Acquisition of multidrug-resistant Pseudomonas aeruginosa in patients in intensive care units: roles of antibiotics with antipseudomonal activity. Clin. Infect. Dis. 38:670-677. [DOI] [PubMed] [Google Scholar]
- 43.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum β-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 44.Péna, C., J. M. Albareda, R. Pallares, M. Pujol, F. E. Tubau, and J. Ariza. 1995. Relationship between quinolone use and emergence of ciprofloxacin-resistant Escherichia coli in bloodstream infections. Antimicrob. Agents Chemother. 39:520-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polk, R. E., C. K. Johnson, D. McClish, R. P. Wenzel, and M. B. Edmond. 2004. Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluoroquinolone use in US hospitals and their surrounding communities. Clin. Infect. Dis. 39:497-503. [DOI] [PubMed] [Google Scholar]
- 46.Rello, J., A. Torres, M. Ricart, J. Valles, J. Gonzalez, A. Artigas, and R. Rodriguez-Roisin. 1994. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. Am. J. Respir. Crit. Care Med. 150:1545-1549. [DOI] [PubMed] [Google Scholar]
- 47.Thomas, J. K., A. Forrest, S. M. Bhavnani, J. M. Hyatt, A. Cheng, C. H. Ballow, and J. J. Schentag. 1998. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob. Agents Chemother. 42:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venezia, R. A., B. E. Domaracki, A. M. Evans, K. E. Preston, and E. M. Graffunder. 2001. Selection of high-level oxacillin resistance in heteroresistant Staphylococcus aureus by fluoroquinolone exposure. J. Antimicrob. Chemother. 48:375-381. [DOI] [PubMed] [Google Scholar]
- 49.Weber, S. G., H. S. Gold, D. C. Hooper, A. W. Karchmer, and Y. Carmeli. 2003. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg. Infect. Dis. 9:1415-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenzel, R. P., D. F. Sahm, C. Thornsberry, D. C. Draghi, M. E. Jones, and J. A. Karlowsky. 2003. In vitro susceptibilities of gram-negative bacteria isolated from hospitalized patients in four European countries, Canada, and the United States in 2000-2001 to expanded-spectrum cephalosporins and comparator antimicrobials: implications for therapy. Antimicrob. Agents Chemother. 47:3089-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zervos, M. J., E. Hershberger, D. P. Nicolau, D. J. Ritchie, L. K. Blackner, E. A. Coyle, A. J. Donnelly, S. F. Eckel, R. H. Eng, A. Hiltz, A. G. Kuyumjian, W. Krebs, A. McDaniel, P. Hogan, and T. J. Lubowski. 2003. Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991-2000. Clin. Infect. Dis. 37:1643-1648. [DOI] [PubMed] [Google Scholar]