Abstract
DW-224a showed the most potent in vitro activity among the quinolone compounds tested against clinical isolates of gram-positive bacteria. Against gram-negative bacteria, DW-224a was slightly less active than the other fluoroquinolones. The in vivo activities of DW-224a against gram-positive bacteria were more potent than those of other quinolones.
The classic fluoroquinolones such as ciprofloxacin, norfloxacin, fleroxacin, and ofloxacin have shown strong activity against gram-negative bacteria, but the effectiveness of these compounds against gram-positive bacteria has been debated (1, 4). Recently, the emergence of multidrug-resistant gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase-negative staphylococci, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci, has generated worldwide concern in the medical community (2, 5, 6, 11). Therefore, recent efforts have been directed toward the synthesis of novel quinolone compounds that can provide improved activities against gram-positive organisms yet retain the broad-spectrum activity of ciprofloxacin (1, 4, 7).
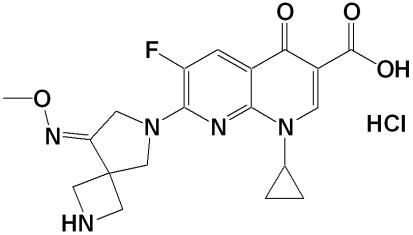
DW-224a is a new group of fluoroquinolone antibiotics with the formula {1-cyclopropyl-6-fluoro-7-[8-(methoxyimino)-2,6-diazaspiro [3,4]oct-6-yl]-4-oxo-1,4-dihydro[1,8]naphthyridine-3-carboxylic acid hydrochloride} (Fig. 1), synthesized by the research laboratory of Dong Wha Pharmaceutical Industry, Ltd. (Anyang City, Republic of Korea).
FIG. 1.
Chemical structure of DW-224a.
In this study, we compared the in vitro activities of DW-224a with those of ciprofloxacin, moxifloxacin, and gemifloxacin against clinical isolates. The comparative in vivo efficacy of DW-224a against systemic bacterial infections in mice was also investigated.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003 [J. H. Kwak et al., abstr. F-415].)
The studied compounds were obtained as follows. DW-224a, ciprofloxacin, moxifloxacin, and gemifloxacin were synthesized at the R & D Center, Dong Wha Pharmaceutical Industry, Ltd., Anyang City, Republic of Korea. Oxacillin was purchased from Sigma Aldrich Korea. Test organisms used in this study were originally isolated from human clinical specimens. Fecal samples were obtained from general hospitals in Seoul, Republic of Korea, between 2001 and 2004. The challenge organisms used in the mouse systemic infections were obtained as follows: Streptococcus pneumoniae ATCC 6305 was obtained from the Korean Culture Center of Microorganisms, Seoul, Republic of Korea; Staphylococcus aureus giorgio, Escherichia coli 851E, and Morganella morganii 1375E were kindly provided by LG Life Sciences Ltd., Daejon, Republic of Korea (10); and Klebsiella pneumoniae P427, Streptococcus pyogenes B144, and MRSA strain P197 were selected through the screening of clinical isolates.
In vitro MICs were determined by an agar dilution method as described by the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) (8). Mueller-Hinton agar medium was used for testing aerobic and facultative organisms. Streptococcus pneumoniae, Streptococcus pyogenes, and Moraxella catarrhalis were grown on Mueller-Hinton agar supplemented with 5% defibrinated sheep blood (Hankuk Media Ltd., Gunpo City, Republic of Korea). Mueller-Hinton agar supplemented with 3% Fildes enrichment (Oxoid Ltd., Basingstoke, Hampshire, England) was used for Haemophilus influenzae. GCII agar (Difco) supplemented with 1% IsoVitaleX (Becton Dickinson) was used for Neisseria gonorrhoeae. Test strains were grown for 18 h and diluted with the same fresh medium to a density of approximately 107 CFU/ml. These test strains were applied with a multipin inoculator to agar plates containing serial dilutions of the antimicrobial agents, yielding 104 CFU per spot. Plates were incubated at 37°C for 18 h and examined for growth. Neisseria gonorrhoeae was incubated under 5% CO2. The MIC was considered to be the lowest concentration that completely inhibited growth on agar plates, disregarding a single colony or a faint haze caused by the inoculum.
The in vivo activity of DW-224a against systemic infections in mice was determined. Four-week-old male ICR mice weighing 18 to 22 g (Daehan Bio Link Co., Ltd., Eum-sung Gun, Republic of Korea) were used for the systemic infection model. They were maintained in animal rooms kept at 23 ± 2°C with 55% ± 20% relative humidity. Test organisms for infection were cultured in tryptic soy agar medium (Difco) at 37°C for 18 h. For S. pneumoniae and S. pyogenes, tryptic soy agar medium was supplemented with 5% defibrinated sheep blood. For use as inocula, bacterial strains were suspended in 0.9% saline solution containing 5% gastric mucin (Difco), except for S. pneumoniae ATCC 6305, which was suspended in 0.9% saline solution. Mice were used in groups of six for each inoculum and were challenged intraperitoneally with a single 0.5-ml portion of the bacterial suspension, corresponding to an inoculum range of 10 to 100 times the minimal lethal dose of bacteria. Four dose levels were used for each antibiotic, depending on the in vitro antimicrobial activity of the compound. Antibiotics at various dose regimens were orally administered to mice twice, at 1 and 4 h postinfection. Mortality was recorded for 7 days, and the median effective dose needed to protect 50% of the mice (ED50) was calculated by the Probit method (3). The challenge inoculum was sufficient to kill 100% of the untreated control mice, which died within 48 h postinfection.
The in vitro efficacy of DW-224a against the clinical isolates is presented in Table 1. DW-224a was slightly superior to gemifloxacin and much more active than moxifloxacin and ciprofloxacin against clinical isolates of gram-positive bacteria. Against methicillin-sensitive S. aureus and MRSA, the antibacterial activity of DW-224a was comparable to that of gemifloxacin but 2- to 16-fold more potent than those of moxifloxacin and ciprofloxacin. The MIC90s of DW-224a against methicillin-sensitive S. aureus and MRSA were 0.03 μg/ml and 4 μg/ml, respectively. DW-224a was also more active than ciprofloxacin and moxifloxacin and was slightly superior to gemifloxacin against Staphylococcus epidermidis (MIC90, 0.125 μg/ml), S. pyogenes (MIC90, 0.06 μg/ml), Enterococcus faecalis (MIC90, 2 μg/ml), and Enterococcus faecium (MIC90, 16 μg/ml). Against S. pneumoniae, the activity of DW-224a (MIC90, 0.03 μg/ml) was 8-fold higher than that of gemifloxacin and at least 16-fold higher than those of moxifloxacin and ciprofloxacin. Among the compounds tested, DW-224a showed the most potent antibacterial activities against gram-positive bacteria. Against most members of the family Enterobacteriaceae, the activity of DW-224a was twofold or fourfold lower than those of ciprofloxacin and gemifloxacin, respectively, but it was comparable to that of moxifloxacin. DW-224a inhibited 90% of the E. coli, K. pneumoniae, Klebsiella oxytoca, Enterobacter cloacae, Enterobacter aerogenes, and Morganella morganii isolates at concentrations of 1, 1, 0.25, 2, 0.25, and 0.5 μg/ml, respectively. Against Acinetobacter baumannii (MIC90, 4 μg/ml), Acinetobacter calcoaceticus (MIC90, <0.008 μg/ml), and Stenotrophomonas maltophilia (MIC90, 1 μg/ml), DW-224a showed an activity comparable to the other compounds. DW-224a was highly active against H. influenzae (MIC90, 0.008 μg/ml) and M. catarrhalis (MIC90, 0.03 μg/ml). Against N. gonorrhoeae, DW-224a (MIC90, 1 μg/ml) was eightfold more active than ciprofloxacin. The activity of DW-224a against Pseudomonas aeruginosa (MIC90, 64 μg/ml) was inferior to those of ciprofloxacin and gemifloxacin, but it was comparable to that of moxifloxacin.
TABLE 1.
In vitro antibacterial activities of DW-224a and quinolones against clinical isolates
| Microorganisma (no. of isolates) | Antimicrobial agent | MIC (μg/ml)
|
Microorganisma (no. of isolates) | Antimicrobial agent | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |||||
| MSSA (62) | DW-224a | 0.008-0.06 | 0.015 | 0.03 | Moxifloxacin | 0.008-1 | 0.125 | 0.125 | ||
| Ciprofloxacin | 0.06-0.5 | 0.25 | 0.5 | Gemifloxacin | 0.015-0.125 | 0.03 | 0.125 | |||
| Moxifloxacin | 0.015-0.25 | 0.06 | 0.06 | |||||||
| Gemifloxacin | 0.008-0.06 | 0.015 | 0.03 | Citrobacter freundii (13) | DW-224a | 0.008-16 | 0.06 | 1 | ||
| Ciprofloxacin | 0.008-2 | 0.015 | 0.25 | |||||||
| MRSA (120) | DW-224a | 0.008-32 | 2 | 4 | Moxifloxacin | 0.015-8 | 0.125 | 2 | ||
| Ciprofloxacin | 0.125->64 | 32 | >64 | Gemifloxacin | 0.008-4 | 0.03 | 0.5 | |||
| Moxifloxacin | 0.03->64 | 4 | 16 | |||||||
| Gemifloxacin | 0.008-64 | 2 | 4 | Morganella morganii (38) | DW-224a | 0.008-4 | 0.25 | 0.5 | ||
| Ciprofloxacin | 0.008-0.5 | 0.015 | 0.03 | |||||||
| MSCNS (14) | DW-224a | 0.008-0.25 | 0.015 | 0.125 | Moxifloxacin | 0.008-4 | 0.25 | 0.25 | ||
| Ciprofloxacin | 0.125-2 | 0.125 | 2 | Gemifloxacin | 0.008-2 | 0.03 | 0.125 | |||
| Moxifloxacin | 0.03-2 | 0.125 | 0.25 | |||||||
| Gemifloxacin | 0.008-0.25 | 0.015 | 0.25 | Proteus mirabilis (21) | DW-224a | 0.125-64 | 0.25 | 2 | ||
| Ciprofloxacin | 0.015-2 | 0.015 | 0.06 | |||||||
| MRCNS (38) | DW-224a | 0.008-4 | 0.25 | 2 | Moxifloxacin | 0.125-16 | 0.25 | 0.5 | ||
| Ciprofloxacin | 0.06-64 | 8 | 32 | Gemifloxacin | 0.125-32 | 0.125 | 0.25 | |||
| Moxifloxacin | 0.06-16 | 2 | 8 | |||||||
| Gemifloxacin | 0.008-8 | 0.25 | 4 | Proteus vulgaris (18) | DW-224a | 0.125-1 | 0.25 | 0.5 | ||
| Ciprofloxacin | 0.008-0.5 | 0.015 | 0.125 | |||||||
| Enterococcus faecalis (47) | DW-224a | 0.008->4 | 0.125 | 2 | Moxifloxacin | 0.125-1 | 0.25 | 0.5 | ||
| Ciprofloxacin | 0.125->64 | 2 | 64 | Gemifloxacin | 0.03-0.25 | 0.06 | 0.125 | |||
| Moxifloxacin | 0.25-64 | 2 | 32 | |||||||
| Gemifloxacin | 0.008->16 | 0.125 | 4 | Serratia marcescens (39) | DW-224a | 0.25-64 | 2 | 32 | ||
| Ciprofloxacin | 0.06-8 | 1 | 4 | |||||||
| Enterococcus faecium (22) | DW-224a | 2-32 | 2 | 16 | Moxifloxacin | 0.008-16 | 1 | 4 | ||
| Ciprofloxacin | 1->64 | 8 | 64 | Gemifloxacin | 0.125-16 | 2 | 8 | |||
| Moxifloxacin | 0.5-64 | 8 | 64 | |||||||
| Gemifloxacin | 0.5->64 | 2 | 32 | Salmonella enterica serovar | DW-224a | 0.06-0.06 | 0.06 | 0.06 | ||
| Typhi (15) | Ciprofloxacin | 0.03-0.125 | 0.125 | 0.125 | ||||||
| VRE (16) | DW-224a | 0.125-4 | 0.5 | 4 | Moxifloxacin | 0.015-0.06 | 0.03 | 0.03 | ||
| Ciprofloxacin | 0.5-4 | 4 | 4 | Gemifloxacin | 0.06-0.125 | 0.06 | 0.125 | |||
| Moxifloxacin | 0.25-4 | 2 | 4 | |||||||
| Gemifloxacin | 0.015-2 | 0.5 | 2 | Acinetobacter baumannii (37) | DW-224a | 0.008-8 | 0.25 | 4 | ||
| Ciprofloxacin | 0.008-64 | 0.125 | 16 | |||||||
| Streptococcus | DW-224a | 0.015-0.06 | 0.015 | 0.03 | Moxifloxacin | 0.015-8 | 0.03 | 4 | ||
| pneumoniae (32) | Ciprofloxacin | 1-8 | 2 | 8 | Gemifloxacin | 0.008-8 | 0.125 | 4 | ||
| Moxifloxacin | 0.125-0.5 | 0.5 | 0.5 | |||||||
| Gemifloxacin | 0.06-0.25 | 0.125 | 0.25 | Acinetobacter calcoaceticus (8) | DW-224a | 0.008-1 | 0.008 | —b | ||
| Ciprofloxacin | 0.015-1 | 0.25 | — | |||||||
| Streptococcus pyogenes (32) | DW-224a | 0.015-0.125 | 0.03 | 0.06 | Moxifloxacin | 0.008-1 | 0.125 | — | ||
| Ciprofloxacin | 0.5-4 | 1 | 2 | Gemifloxacin | 0.008-0.5 | 0.03 | — | |||
| Moxifloxacin | 0.125-0.5 | 0.25 | 0.25 | |||||||
| Gemifloxacin | 0.015-0.125 | 0.03 | 0.125 | Stenotrophomonas | DW-224a | 0.06-64 | 0.5 | 1 | ||
| maltophilia (27) | Ciprofloxacin | 0.015-16 | 0.5 | 2 | ||||||
| Escherichia coli (65) | DW-224a | 0.015-64 | 0.06 | 1 | Moxifloxacin | 0.03-64 | 0.25 | 1 | ||
| Ciprofloxacin | 0.008-64 | 0.015 | 0.5 | Gemifloxacin | 0.015-32 | 0.25 | 1 | |||
| Moxifloxacin | 0.03-32 | 0.06 | 0.5 | |||||||
| Gemifloxacin | 0.008-16 | 0.015 | 0.25 | Haemophilus influenzae (15) | DW-224a | <0.008-0.008 | <0.008 | 0.008 | ||
| Ciprofloxacin | <0.008 | <0.008 | <0.008 | |||||||
| Enterobacter cloacae (48) | DW-224a | 0.008-8 | 0.06 | 2 | Moxifloxacin | 0.008-0.015 | 0.008 | 0.015 | ||
| Ciprofloxacin | 0.008-2 | 0.015 | 0.5 | Gemifloxacin | <0.008 | <0.008 | <0.008 | |||
| Moxifloxacin | 0.008-4 | 0.06 | 1 | |||||||
| Gemifloxacin | 0.008-2 | 0.015 | 0.5 | Moraxella catarrhalis (20) | DW-224a | <0.008-0.03 | 0.015 | 0.03 | ||
| Ciprofloxacin | <0.008-0.06 | 0.03 | 0.06 | |||||||
| Enterobacter aerogenes (36) | DW-224a | 0.03-1 | 0.125 | 0.25 | Moxifloxacin | 0.015-0.06 | 0.06 | 0.06 | ||
| Ciprofloxacin | 0.008-0.25 | 0.015 | 0.06 | Gemifloxacin | <0.008-0.03 | <0.008 | 0.015 | |||
| Moxifloxacin | 0.008-1 | 0.06 | 0.125 | |||||||
| Gemifloxacin | 0.008-0.5 | 0.06 | 0.125 | Neisseria gonorrhoeae (27) | DW-224a | 0.015-2 | 1 | 1 | ||
| Ciprofloxacin | 0.03-16 | 8 | 8 | |||||||
| Klebsiella pneumoniae (53) | DW-224a | 0.06-8 | 0.125 | 1 | Moxifloxacin | 0.03-4 | 2 | 2 | ||
| Ciprofloxacin | 0.015-2 | 0.06 | 0.5 | Gemifloxacin | 0.008-2 | 1 | 1 | |||
| Moxifloxacin | 0.03-4 | 0.25 | 1 | |||||||
| Gemifloxacin | 0.015-2 | 0.06 | 1 | Pseudomonas aeruginosa (22) | DW-224a | <0.008->64 | 8 | >64 | ||
| Ciprofloxacin | 0.03-64 | 2 | 64 | |||||||
| Klebsiella oxytoca (32) | DW-224a | 0.03-1 | 0.125 | 0.25 | Moxifloxacin | 0.03->64 | 64 | >64 | ||
| Ciprofloxacin | 0.015-0.25 | 0.03 | 0.06 | Gemifloxacin | <0.008->64 | 2 | 64 | |||
MSSA, methicillin-susceptible S. aureus; MSCNS, methicillin-susceptible coagulase-negative staphylococci; MRCNS, methicillin-resistant coagulase-negative staphylococci; VRE, vancomycin-resistant enterococci.
—, not calculated.
The protective efficacy of DW-224a against systemic infections in mice was compared with those of ciprofloxacin, moxifloxacin, and gemifloxacin (Table 2). DW-224a exhibited the most potent protective effects against systemic infections caused by gram-positive bacteria. Against infection caused by S. aureus giorgio, the ED50s of DW-224a, ciprofloxacin, moxifloxacin, and gemifloxacin were 1.11, 16.52, 1.78, and 1.37 mg/kg of body weight, respectively. Especially against quinolone-resistant S. aureus P197 (an MRSA), DW-224a (ED50, 15.52 mg/kg) was more effective than ciprofloxacin (ED50, >40 mg/kg), moxifloxacin (ED50, 35.35 mg/kg), and gemifloxacin (ED50, 26.58 mg/kg). DW-224a was also the most effective drug against infections caused by streptococci, including S. pneumoniae and S. pyogenes strains, for which the ED50s were 0.53 and 2.65 mg/kg, respectively. But, DW-224a was shown to be less effective than the test compounds against infections caused by gram-negative organisms. Against E. coli 851E, DW-224a (ED50, 6.08 mg/kg) was less active than ciprofloxacin (ED50, 0.78 mg/kg), moxifloxacin (ED50, 2.17 mg/kg), and gemifloxacin (ED50, 1.16 mg/kg). Against K. pneumoniae P427, the ED50s of DW-224a, ciprofloxacin, moxifloxacin, and gemifloxacin were 9.77, 0.70, 2.64, and 5.36 mg/kg, respectively. DW-224a (ED50, 4.83 mg/kg) was less active than ciprofloxacin (ED50, 1.41 mg/kg) and gemifloxacin (ED50, 3.41 mg/kg) against M. morganii 1375E but more active than moxifloxacin (ED50, 5.28 mg/kg). In general, the ED50s of DW-224a were well correlated with in vitro MICs.
TABLE 2.
Comparative in vivo activities of DW-224a against systemic infections in mice
| Microorganism inoculum (CFU/mouse) | Mucin (%) | Antimicrobial agenta | MIC (μg/ml) | ED50 (mg/kg) (95% confidence limits) |
|---|---|---|---|---|
| S. aureus giorgio (1 × 108) | 5 | DW-224a | <0.008 | 1.11 (0.17-4.64) |
| Ciprofloxacin | 0.125 | 16.52 (1.27-18.99) | ||
| Moxifloxacin | 0.03 | 1.78 (0.33-7.66) | ||
| Gemifloxacin | <0.008 | 1.37 (0.27-7.67) | ||
| MRSA P197 (5 × 109) | 5 | DW-224a | 0.25 | 15.52 (9.02-26.58) |
| Ciprofloxacin | 8 | >40 | ||
| Moxifloxacin | 1 | 35.35 (20.38-61.33) | ||
| Gemifloxacin | 0.25 | 26.58 (15.03-47.28) | ||
| S. pneumoniae ATCC 6305 (1 × 104)b | DW-224a | 0.008 | 0.53 (0.0029-1.67) | |
| Ciprofloxacin | 0.5 | 10.46 (3.56-32.26) | ||
| Moxifloxacin | 0.06 | 2.00 (0.41-6.21) | ||
| Gemifloxacin | 0.015 | 0.76 (0.15-2.43) | ||
| S. pyogenes B144 (5 × 107) | 5 | DW-224a | 0.06 | 2.65 (0.65-13.73) |
| Ciprofloxacin | 4 | >40 | ||
| Moxifloxacin | 0.25 | 9.04 (2.16-73.6) | ||
| Gemifloxacin | 0.06 | 2.83 (0.37-15.24) | ||
| E. coli 851E (1 × 108) | 5 | DW-224a | 0.03 | 6.08 (1.64-38.80) |
| Ciprofloxacin | 0.008 | 0.78 (0.10-3.20) | ||
| Moxifloxacin | 0.06 | 2.17 (0.61-7.73) | ||
| Gemifloxacin | 0.015 | 1.16 (0.25-4.13) | ||
| K. pneumoniae P427 (5 × 105) | 5 | DW-224a | 0.06 | 9.77 (2.77-36.20) |
| Ciprofloxacin | 0.06 | 0.70 (0.00-2.59) | ||
| Moxifloxacin | 0.25 | 2.64 (0.36-10.68) | ||
| Gemifloxacin | 0.03 | 5.36 (1.42-20.20) | ||
| M. morganii 1375E (2 × 106) | 5 | DW-224a | 0.125 | 4.83 (0.49-26.77) |
| Ciprofloxacin | 0.008 | 1.41 (0.17-5.91) | ||
| Moxifloxacin | 0.125 | 5.28 (1.50-18.45) | ||
| Gemifloxacin | 0.06 | 3.41 (0.77-12.48) |
Antimicrobial agents were orally administered at 1 and 4 h after infection.
Bacterial strains were suspended in 0.9% saline solution containing 5% gastric mucin, except for S. pneumoniae ATCC 6305, which was suspended in 0.9% saline solution.
DW-224a demonstrated potent in vitro and in vivo antibacterial activities against both gram-positive and gram-negative bacteria. The overall antibacterial activities of DW-224a against gram-positive pathogens, such as MRSA, methicillin-resistant coagulase-negative staphylococci, S. pneumoniae, S. pyogenes, and E. faecalis, were more potent than those of the reference compounds, such as ciprofloxacin, moxifloxacin, and gemifloxacin. DW-224a especially showed increased potency against S. pneumoniae, which is the bacterial strain most frequently isolated from patients with community-acquired pneumonia and continues to be a significant cause of mortality. However, DW-224a was slightly less active than other fluoroquinolones against members of the family Enterobacteriaceae. It is a general trend that the improving antibacterial activities of the fluoroquinolones against gram-positive bacteria is likely associated with a relative decrease in activity against gram-negative bacteria, but DW-224a was very active against gram-negative respiratory pathogens such as strains of H. influenzae and M. catarrhalis, known as clinically significant pathogens. These findings suggest that DW-224a, with its expanded antipneumococcal activity, may be very useful for the treatment of community-acquired respiratory tract infections, because β-lactam resistance in H. influenzae and M. catarrhalis is a documented problem in the community (9).
DW-224a at two times the MIC had very rapid bactericidal activity against S. pneumoniae and S. aureus, regrowth after 24 h of incubation was not detected, and the minimal bactericidal concentrations of DW-224a were twofold higher than the MICs for all test strains (J. Kwak, M. J. Seol, H. Kim, H. Park, D. Choi, and Y. Jung, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1947, 2004).
In view of its improved antibacterial activities against gram-positive bacteria and good pharmacokinetic profiles in animals, the clinical usefulness of DW-224a should be established by further studies.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health and Welfare, Republic of Korea (01-PJ1-PG4-01PT01-0013).
REFERENCES
- 1.Ball, P. 2003. Adverse drug reactions: implications for the development of fluoroquinolones. J. Antimicrob. Chemother. 51(Suppl. S1):21-27. [DOI] [PubMed] [Google Scholar]
- 2.Bell, J. M., J. D. Turnidge, and SENTRY Antimicrobial Surveillance Participants. 2002. High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY Antimicrobial Surveillance Program, 1998-1999. Antimicrob. Agents Chemother. 46:879-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bliss, C. I. 1985. Statistics in bioassay. Academic Press, Inc., New York, N.Y.
- 4.Emmerson, A. M., and A. M. Jones. 2003. The quinolones: decades of development and use. J. Antimicrob. Chemother. 51(Suppl. S1):13-20. [DOI] [PubMed] [Google Scholar]
- 5.Endtz, H. P., N. van den Braak, H. A. Verbrugh, and A. van Belkum. 1999. Vancomycin resistance: status quo and quo vadis. Eur. J. Clin. Microbiol. Infect. Dis. 18:683-690. [DOI] [PubMed] [Google Scholar]
- 6.Levy, S. B., and B. Marshall. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:s122-s129. [DOI] [PubMed] [Google Scholar]
- 7.Lode, H., and M. Allewelt. 2002. Role of newer fluoroquinolones in lower respiratory tract infections. J. Antimicrob. Chemother. 49:709-712. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6, 6th ed., vol. 23. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Nilius, A. M., L. L. Shen, D. Hensey-Rudloff, L. S. Almer, J. M. Beyer, D. J. Balli, Y. Cai, and R. K. Flamm. 2003. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob. Agents Chemother. 47:3260-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh, J.-I., K.-S. Paek, M.-J. Ahn, M.-Y. Kim, C. Y. Hong, I.-C. Kim, and J.-H. Kwak. 1996. In vitro and in vivo evaluations of LB20304, a new fluoronaphthyridone. Antimicrob. Agents Chemother. 40:1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song, J.-H., S.-I. Jung, K. S. Ko, N. Y. Kim, J. S. Son, H.-H. Chang, H. K. Ki, W. S. Oh, J. Y. Suh, K. R. Peck, N. Y. Lee, Y. Yang, Q. Lu, A. Chongthaleong, C.-H. Chiu, M. K. Lalitha, J. Perera, T. T. Yee, G. Kumarasinghe, F. Jamal, A. Kamarulzaman, N. Parasakthi, P. H. Van, C. Carlos, T. So, T. K. Ng, and A. Shibl. 2004. High prevalence of antimicrobial resistance among clinical Staphylococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob. Agents Chemother. 48:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]