Abstract
Therapies for microsporidiosis in humans are limited, and fumagillin, which appears to be the most broadly effective antimicrosporidial drug, is considered to be moderately toxic. The purpose of this study was to apply an in vitro drug screening assay for Encephalitozoon intestinalis and Vittaforma corneae and an in vivo athymic mouse model of V. corneae infection to assess the efficacy of TNP-470 (a semisynthetic analogue of fumagillin), ovalicin, and eight ovalicin derivatives. TNP-470, ovalicin, and three of the ovalicin derivatives inhibited both E. intestinalis and V. corneae replication by more than 70% in vitro. Another three of the ovalicin derivatives inhibited one of the two microsporidian species by more than 70%. None of the treated athymic mice survived the V. corneae infection, but they did survive statistically significantly longer than the untreated controls after daily treatment with fumagillin administered at 5, 10, and 20 mg/kg of body weight subcutaneously (s.c.), TNP-470 administered at 20 mg/kg intraperitoneally (i.p.), or ovalicin administered at 5 mg/kg s.c. Of two ovalicin derivatives that were assessed in vivo, NSC 9665 given at 10 mg/kg i.p. daily also statistically significantly prolonged survival of the mice. No lesions associated with drug toxicity were observed in the kidneys or livers of uninfected mice treated with these drugs at the highest dose of 20 mg/kg daily. These results thus support continued studies to identify more effective fumagillin-related drugs for treating microsporidiosis.
Microsporidia are single-celled, obligately intracellular parasites that were long classified as protozoa but are now considered to be atypical fungi (7, 27, 45, 48). More than one thousand species of microsporidia have been identified as causing infections in invertebrates and vertebrates, and at least 14 species have been reported to infect humans (14, 18, 47, 48). The most common species infecting humans include Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon cuniculi, and Encephalitozoon hellem. In addition to causing opportunistic infections in persons with AIDS, infections due to these microsporidia are increasingly being reported in organ transplant recipients, children, travelers, contact lens wearers, and the elderly (10, 14, 18, 47). Clinical symptoms associated with intestinal microsporidiosis typically include persistent diarrhea, malabsorption, abdominal pain, and weight loss, and symptoms associated with disseminated infections may include conjunctivitis, sinusitis, myositis, pneumonia, peritonitis, hepatitis, or nephritis (31, 32, 47).
Current therapies for microsporidiosis are variably effective. Albendazole, a benzimidazole that inhibits microtubule assembly, is effective against several microsporidia, including Encephalitozoon species, but it is less effective against E. bieneusi (3, 8, 16, 19). Fumagillin, a product of Aspergillus fumigatus, was used in the 1950s to treat human amebiasis and is still being used to treat honeybees with Nosema apis microsporidial infections (26, 28, 35, 40). Fumagillin also inhibited replication of E. cuniculi in tissue culture (42) and has been applied topically to treat ocular microsporidial infections (8, 16, 21). Fumagillin appears to be effective against Encephalitozoon spp. and E. bieneusi infections, but there are concerns about relapses and toxicity with systemic administration of fumagillin (8, 24, 38).
Since fumagillin appeared to be a broadly effective antimicrosporidian agent, this study was performed to evaluate the in vitro and in vivo antimicrosporidial activities of fumagillin, in comparison with those of TNP-470 (a semisynthetic fumagillin analogue), ovalicin (a structurally related antibiotic that is synthesized by Pseudorotium ovalis), and several ovalicin derivatives. E. bieneusi is the most common microsporidian species that infects humans, but it cannot be grown in long-term culture and does not infect small rodents in the laboratory (13, 17). Therefore, two human microsporidian isolates were used in the in vitro screening. E. intestinalis was used because it is the second-most common microsporidian species infecting humans, and it can be grown in long-term tissue culture. The human microsporidian isolate, Vittaforma corneae, was used in both the in vitro and in vivo models, because it can be grown in tissue culture and causes lethal infections in athymic mice. V. corneae also was included as a surrogate for E. bieneusi in these drug test models, because it is phylogenetically most closely related to E. bieneusi among the microsporidian species that infect humans (2, 46). Both V. corneae and E. bieneusi were observed to replicate in direct contact with the host cell cytoplasm, whereas E. intestinalis was found to replicate within a membrane-bound parasitophorous vacuole (6, 11). In addition, V. corneae and E. bieneusi organisms were both relatively less sensitive to albendazole than Encephalitozoon species (8, 13, 16). Therapies are lacking for E. bieneusi and V. corneae, whereas albendazole is currently used to effectively treat Encephalitozoon infections in humans. Therefore, only V. corneae, as a surrogate for E. bieneusi, was employed in the in vivo murine model to assess the efficacy of fumagillin, TNP-470, ovalicin, and ovalicin derivatives.
MATERIALS AND METHODS
Microsporidia.
E. intestinalis and V. corneae were grown in RK-13 rabbit kidney cells (50506, 50505, and CCL-37, respectively; American Type Culture Collection, Manassas, VA), and supernatants containing microsporidia were washed successively in sterile distilled water, Tris-buffered saline (TBS) containing 0.3% Tween 20, and TBS, followed by density centrifugation in 50% Percoll (Pharmacia, Piscataway, NJ) as previously described (13).
Drugs.
Fumagillin bicyclohexylamine was purchased from Merial Canada, Inc. (Victoriaville, Quebec City, Canada), and TNP-470 was synthesized from fumagillin by Starks Associates, Inc. (Buffalo, NY). Ovalicin was generously provided by Barbara Willi of Novartis Pharma AG (Basel, Switzerland), and ovalicin derivatives were kindly provided through the Developmental Therapeutics Program of the National Cancer Institute (NCI) repository (Rockville, MD). The derivatives were selected from the NCI chemical database utilizing computerized substructure searches of ovalicin. Stock dilutions of drugs were prepared in dimethyl sulfoxide and further diluted in medium for testing in the in vitro screening assays or in saline for treating mice.
In vitro drug assay.
RK-13 cells were plated onto 96-well tissue culture plates at 5 × 104 cells per well in RPMI 1640 medium containing 2 mM l-glutamine and 5% fetal bovine serum overnight at 37°C under 5% CO2 to allow host cells to reach confluence. The next day, medium was replaced with 100 μl of fresh medium containing microsporidia (1.5 × 105 organisms resulting in a 3:1 ratio of parasites to cells) and 100 μl of freshly diluted drugs in medium. Controls were treated with proportionately diluted dimethyl sulfoxide that was used to initially dissolve the drugs. Fresh medium containing drugs or diluent was replaced in each well on days 3 and 7 without disturbing the parasites and cells. On day 10, 10 μl of 10% (wt/vol) sodium dodecyl sulfate was added to all wells to release organisms from the host cells, and the number of microsporidia were counted on hemacytometers. Each treatment was assayed in quadruplicate, and the percent inhibition of microsporidian replication was calculated as follows: 100 − [(number of microsporidia in each treatment well/mean number of microsporidia in the nontreated wells) × 100].
Measurement of drug toxicity in vitro.
Quadruplicate sets of RK-13 host cells not infected with microsporidia were treated with drug only as described above, and host cell viability was measured using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, St. Louis, MO] assay to measure drug toxicity as described previously (13, 39). On day 10 of the assay, 50 μl of MTT (5-mg/ml stock) was added to each well, and the cultures were incubated an additional 4 h at 37°C. The supernatants were then removed, and the formazan was dissolved by the addition of 200 μl acidified isopropanol. Absorbance values were measured using an enzyme-linked immunosorbent assay spectrophotometer at a test wavelength of 570 nm and a reference wavelength of 630 nm. The percentage of host cell viability was calculated as follows: (absorbance of drug-treated host cells/mean absorbance of nontreated host cells) × 100. Drugs causing host cell viability to fall below 85% of controls were considered toxic.
Mice.
Seven-week-old athymic CRL:CD-1nuBR male mice were purchased from Charles Rivers Laboratories (Wilmington, MA) and housed in groups of eight in large-sized sterile filter-topped cages on HEPA-filtered shelf racks with sterile water and food provided ad libitum. Mice were allowed to acclimate for 1 week prior to initiation of the experiments. All protocols and procedures were approved by the Tulane National Primate Research Center Institutional Animal Care and Use Committee.
Microsporidia infection and treatment of mice.
Mice were inoculated intraperitoneally (i.p.) with 1 ml saline containing 1 × 107 V. corneae unless otherwise noted. Drug or diluent inoculations were initiated 24 h later using doses and routes as described for each experiment. Each treatment group for the dose-response experiment and the drug treatment experiments was comprised of eight mice each. Mice were monitored for changes in activity, general appearance, body weight, and survival. Mice were euthanized by carbon dioxide overdose if animals became moribund due to infection or at the completion of the experiment. At necropsy, gross abnormalities were recorded, and major organs, including the brain, heart, liver, spleen, kidney, lymph nodes, and adrenal glands were fixed by immersion in 10% formalin for histopathology. The lungs were perfused with fixative via the trachea prior to immersion. The intestinal tract was flushed and fixed in its entirety. Tissues were embedded in paraffin for routine histologic processing, and multiple sections were stained with hematoxylin and eosin and Gram stain. Tissues were evaluated and scored without prior knowledge of treatment by enumeration of lesions with gram-positive microsporidia in an entire cross section of tissue, including a section through the entire length of the intestine. The number of gram-stained organisms for an entire section of each tissue was determined by counting clusters of organisms visible at ×10 magnification.
Statistical analyses.
Means were compared by Student's t test when comparing two groups or by analysis of variance when comparing more than two groups, using Graphpad Instat, and graphs were prepared using Graphpad Prism software (San Diego, CA).
RESULTS
In vitro screening for antimicrosporidial activity.
Fumagillin, TNP-470, and ovalicin were observed to inhibit both E. intestinalis and V. corneae in vitro by more than 70% at concentrations not toxic to the RK-13 host cells (Table 1). Five of the eight ovalicin derivatives also inhibited E. intestinalis and/or V. corneae by more than 70%. In particular, the derivatives NSC 9168, NSC 9665, and NSC 141539 displayed 50% inhibitory concentration (IC50) values most similar to the IC50 values expressed by fumagillin, TNP-470, and ovalicin.
TABLE 1.
Antimicrosporidial activity of drugs in vitro
| Test compound | Mol wt | Highest nontoxic dosea (μM) | % Inhibition (mean ± SD)b
|
Approximate IC50c (μM)
|
||
|---|---|---|---|---|---|---|
| E. intestinalis | V. corneae | E. intestinalis | V. corneae | |||
| Fumagillin | 458.60 | 4 | 72.1 ± 2.8 | 72.8 ± 3.1 | 0.0025 | 0.002 |
| TNP-470 | 401.88 | 50 | 92.1 ± 1.6 | 90.4 ± 4.3 | 0.0035 | 0.002 |
| Ovalicin | 296.36 | 10 | 73.0 ± 14.9 | 78.9 ± 8.5 | 0.004 | 0.002 |
| 9168d | 458.54 | >100e | 71.7 ± 13.0 | 78.2 ± 19.1 | 0.02 | 0.42 |
| 9665d | 282.38 | >100e | 74.3 ± 30.1 | 93.4 ± 28.6 | 0.001 | 0.066 |
| 56407d | 192.17 | >100e | 70.6 ± 12.3 | 54.9 ± 5.0 | 8.90 | 57.00 |
| 58368d | 639.86 | 10 | 58.7 ± 10.5 | 75.5 ± 10.7 | 6.25 | 0.20 |
| 141538d | 296.36 | 10 | 58.0 ± 9.9 | 54.1 ± 8.9 | 0.75 | 8.20 |
| 141539d | 298.38 | 10 | 83.3 ± 8.9 | 66.8 ± 17.7 | 0.07 | 0.08 |
| 676019d | 431.00 | 10 | 29.5 ± 6.1 | 28.0 ± 14.0 | 50.4 | 97.0 |
| 174554d | 241.28 | >100e | 80.8 ± 11.6 | 91.2 ± 3.3 | 47.1 | 52.9 |
Concentrations of compounds that caused the viability of host cells (RK-13 cells) to fall below 85% of medium-treated host cells were considered to be toxic.
Inhibition values were presented for the highest nontoxic dose of compound.
IC50 values were estimated by interpolation using Graphpad Prism software.
The numbers for these compounds refer to National Cancer Institute database NSC entry numbers, and additional information can be accessed at the website http://dtp.nci.nih.gov/dtpstandard/ChemData/index.jsp.
The highest concentration tested for each compound was 100 μM, and these compounds were not toxic at this dose.
Survival time of V. corneae-infected athymic mice.
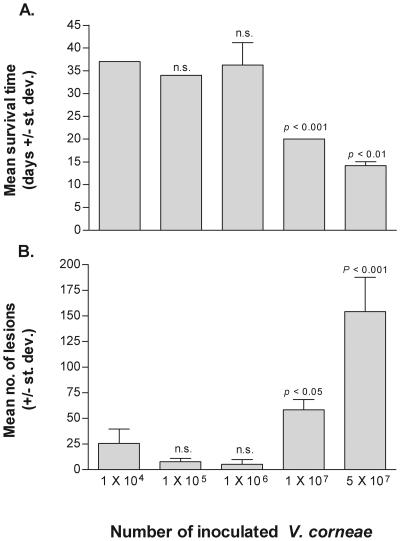
To establish a model that would be used for measuring the efficacy of test drugs, various numbers of V. corneae were injected i.p. into athymic mice. The findings presented in Fig. 1A demonstrated that athymic mice inoculated with 1 × 104, 1 × 105, or 1 × 106 organisms survived between 34 and 37 days, and no statistically significant differences were measured between these groups. Mice inoculated with 1 × 107 V. corneae survived 20 (± 0.0) days, which was statistically significantly less than mice inoculated with 1 × 106 organisms (P < 0.001), and mice inoculated with 5 × 107 organisms survived 14.2 (± 0.84) days, which was significantly less than the mean survival time of mice inoculated with 1 × 107 organisms (P < 0.01).
FIG. 1.
Dose-response experiment. V. corneae microsporidian spores were inoculated i.p. into athymic mice in groups of eight each. Mean survival times and numbers of parasite-associated lesions were compared by Student's t test. Levels of statistically significant differences were indicated for each group in comparison with results from the preceding group of mice receiving the next lower dose of microsporidia. st. dev., standard deviation; n.s., not statistically significant.
Microsporidia-associated lesions in athymic mice.
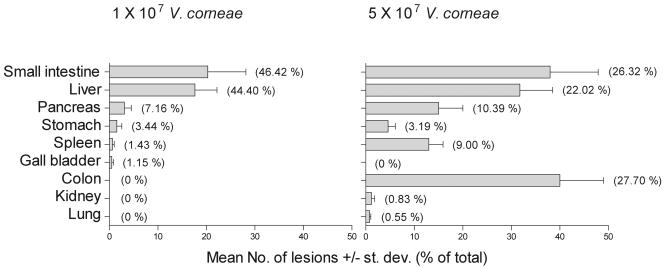
Parasite-associated lesions were counted on Gram-stained tissue sections of each athymic mouse. The results in Fig. 1B showed that the mean numbers of parasite-associated lesions ranged from 50 to 75 in 10 randomly selected fields in each of 16 tissues per mouse inoculated with 1 × 107 organisms, which were significantly higher than the mean numbers of parasite-associated lesions in the groups of mice inoculated with fewer organisms. The distribution of parasite-associated lesions in these two groups of mice is presented in Fig. 2. Tissues most frequently observed with parasite-associated lesions included the small intestine, liver, and pancreas and variably included the colon, lung, spleen, stomach, and kidney, depending on the initial inoculum dose of V. corneae. In mice inoculated with less than 1 × 107 V. corneae, granulomatous lesions were observed, often in the absence of apparent microsporidia, presumably due to a longer infection time and degradation of organisms during the inflammatory responses. These inflammatory lesions in which gram-positive microsporidia could not be discerned were not counted as parasite-associated lesions.
FIG. 2.
Distribution of V. corneae-associated lesions in athymic mice inoculated i.p. with different numbers of organisms. Mice were euthanized after becoming moribund and necropsied for histopathology. The number of lesions with microsporidia were counted at ×10 magnification per tissue section from each athymic mouse, and the means ± standard deviations (st. dev.) of lesions for eight mice per group were plotted. Values presented within parentheses represent the percentage of lesions in each organ compared to the total number of lesions observed in all organs.
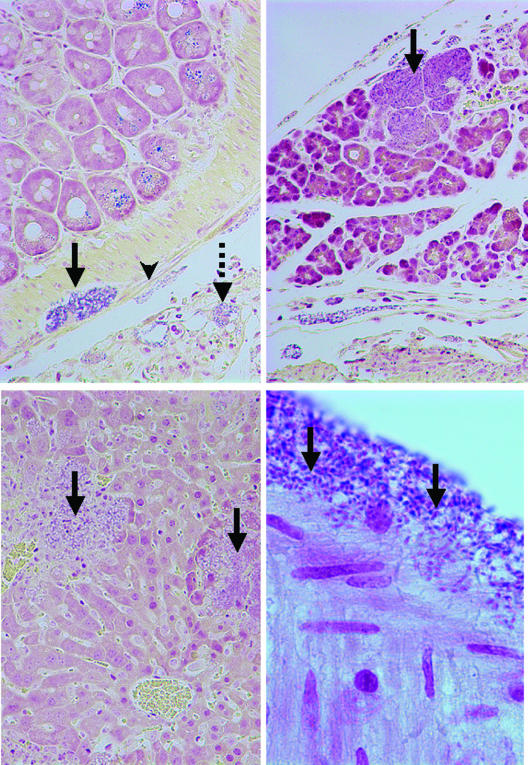
In athymic mice inoculated with the higher doses of ten and fifty million microsporidia, V. corneae infected and replicated in serosal cells and neural cells of the mesenteric plexus and stromal cells in the serosa (Fig. 3). Some infected foci, particularly in the mesentery, were characterized by dense accumulations of macrophages and neutrophils, while other sites contained infected cells without inflammatory cells. Infected foci in the pancreas and liver were dominated by necrosis with minimal inflammatory responses. In the kidney, widely scattered proximal convoluted tubule epithelial cells were rarely associated with inflammation. Infection and inflammation were not observed in the brain, heart, and mucosa of the intestine.
FIG. 3.
Lesions associated with experimental V. corneae infection in athymic mice (Brown Brenn Gram stain). (Top left panel) Small intestine was observed with V. corneae organisms infecting serosal cells (arrowhead), nerve cells of the mesenteric plexus (solid arrow), and cells of the mesentery (dashed arrow) (magnification, ×190). Note that the large zymogen granules in the epithelial cells also stained dark. (Top right panel) Swollen acinar cells of the pancreas were filled with V. corneae organisms (arrow). Inflammation was not usually observed until host cells ruptured (magnification, ×190). (Lower left panel) V. corneae organisms were observed in the liver and lysed random groups of hepatocytes (arrows). Necrotic foci contained debris, neutrophils, and V. corneae organisms (magnification, ×190). (Lower right panel) The light-staining posterior vacuoles of dark-staining V. corneae organisms in intestinal serosa can be observed (arrows) (magnification, ×950).
A statistically significant decrease in mean survival time and a statistically significant increase in the mean number of parasite-associated lesions were measured in an experiment comparing the dose transition of athymic mice inoculated with 1 × 106 V. corneae versus athymic mice inoculated with 1 × 107 V. corneae. Therefore, a parasite inoculum of 1 × 107 V. corneae was used in the subsequent experiments for testing drug efficacy in the athymic mice, which would allow sufficient time for a drug to act if it were capable of inhibiting microsporidia and prolonging survival of the mice.
Effect of fumagillin treatment on V. corneae-infected athymic mice.
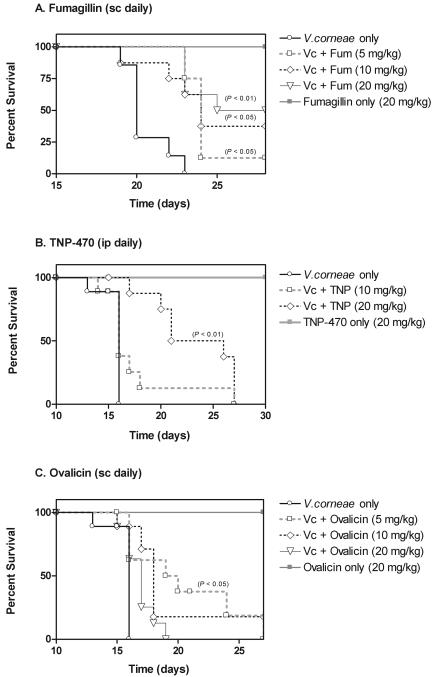
Athymic mice were inoculated with 1 × 107 V. corneae and beginning 24 h later, were inoculated subcutaneously (s.c.) with fumagillin on a daily basis (Fig. 4A). The mean survival times of mice treated with fumagillin at a dose of 5 mg/kg of body weight (24.3 ± 1.4 days; P < 0.05), 10 mg/kg (24.5 ± 3.3 days; P < 0.05), and 20 mg/kg (25.7 ± 2.5 days; P < 0.01) were statistically significantly longer than the mean survival time of the nontreated V. corneae-infected athymic mice (20.6 ± 1.4 days). Statistically significantly fewer parasite-associated lesions were detected in the small intestine and pancreas of mice treated with fumagillin s.c. at 5, 10, and 20 mg/kg daily (Table 2). No statistically significant differences were detected in the number of lesions detected in the liver sections of fumagillin-treated versus nontreated V. corneae-infected athymic mice. The athymic mice that were not infected but were administered fumagillin at a dose of 20 mg/kg s.c. daily survived the length of this study and did not develop drug-associated lesions, suggesting that fumagillin was not toxic at this dose. Conversely, infected athymic mice treated with fumagillin at these doses by i.p. inoculation instead of by the s.c. route failed to survive statistically significantly longer than the untreated control mice (data not shown).
FIG. 4.
Survival curves of V. corneae-infected athymic mice treated with fumagillin (Fum), TNP-470 (TNP), and ovalicin. Ten million microsporidia were inoculated i.p. into athymic mice in groups of eight that were treated daily with drugs beginning 24 h later. Statistically significant differences in survival times between treated and untreated infected mice were measured by Student's t test. Vc, V. corneae.
TABLE 2.
Microsporidium-associated lesions in treated V. corneae-infected athymic mice
| Treatment | Mean no. of lesions (± SD)a
|
||
|---|---|---|---|
| Small intestine | Liver | Pancreas | |
| V. corneae only | 74.0 ± 56.7 | 39.9 ± 20.3 | 18.1 ± 19.8 |
| V. corneae + fumagillin (5 mg/kg s.c.) | 18.3 ± 15.6 (P < 0.01)b | 21.8 ± 30.0 | 4.6 ± 6.3 (P < 0.05) |
| V. corneae + fumagillin (10 mg/kg s.c.) | 8.6 ± 10.7 (P < 0.01) | 13.5 ± 18.9 | 1.6 ± 2.0 (P < 0.05) |
| V. corneae + fumagillin (20 mg/kg s.c.) | 9.5 ± 13.2 (P < 0.01) | 27.3 ± 22.0 | 1.4 ± 2.8 (P < 0.05) |
| V. corneae only | 53.5 ± 49.1 | 25.0 ± 21.2 | 12.4 ± 15.2 |
| V. corneae + TNP-470 (10 mg/kg i.p.) | 19.1 ± 11.9 | 29.9 ± 36.7 | 13.3 ± 13.4 |
| V. cornae + TNP-470 (20 mg/kg i.p.) | 6.3 ± 8.5 (P < 0.05) | 17.9 ± 18.8 | 1.6 ± 3.3 |
| V. corneae only | 53.5 ± 49.1 | 25.0 ± 21.2 | 12.4 ± 15.2 |
| V. corneae + ovalicin (5 mg/kg s.c.) | 11.6 ± 9.5 (P < 0.05) | 17.9 ± 16.7 | 6.8 ± 6.3 |
| V. corneae + ovalicin (10 mg/kg s.c.) | 17.3 ± 14.2 | 12.8 ± 7.3 | 10.0 ± 7.9 |
| V. corneae + ovalicin (20 mg/kg s.c.) | 24.9 ± 25.1 | 22.3 ± 15.7 | 15.9 ± 12.5 |
| V. corneae only | 30.4 ± 16.9 | 14.6 ± 14.8 | 31.0 ± 30.3 |
| V. corneae + NSC 9665 (5 mg/kg i.p.) | 15.3 ± 13.3 | 9.6 ± 4.9 | 43.0 ± 18.4 |
| V. corneae + NSC 9665 (10 mg/kg i.p.) | 8.3 ± 7.2 (P < 0.01) | 15.7 ± 17.8 | 18.9 ± 25.2 |
| V. corneae + NSC 9665 (20 mg/kg i.p.) | 3.5 ± 5.0 (P < 0.001) | 20.8 ± 12.7 | 19.5 ± 15.0 |
| V. corneae only | 25.3 ± 12.8 | 25.0 ± 14.0 | 13.0 ± 7.4 |
| V. corneae + NSC 141539 (5 mg/kg i.p.) | 5.5 ± 5.7 (P < 0.05) | 36.7 ± 29.6 | 5.3 ± 5.6 |
| V. corneae + NSC 141539 (10 mg/kg i.p.) | 22.0 ± 12.5 | 18.9 ± 12.8 | 46.9 ± 22.6 |
| V. corneae + NSC 141539 (20 mg/kg i.p.) | 14.8 ± 9.2 | 25.3 ± 14.8 | 32.4 ± 23.8 |
Groups of eight mice each were inoculated i.p. with 1 × 107 V. corneae microsporidia, and drug treatments were administered daily beginning 24 h later. Microsporidium-associated lesions were counted in one cross-section of each tissue per mouse as described in Materials and Methods.
Values of each drug treatment group and the untreated group of that experiment were compared. Statistically significant differences were determined by Student's t test.
Effect of TNP-470 treatment on V. corneae-infected athymic mice.
Athymic mice infected with V. corneae and treated with TNP-470 at 20 mg/kg i.p. daily survived statistically significantly longer (22.0 ± 4.5 days; P < 0.01) than nontreated athymic mice that were infected with V. corneae (16. 6 ± 1.1 days), as shown in Fig. 4B. Infected mice treated with TNP-470 at 10 mg/kg i.p. daily survived an average of 17.5 ± 4.0 days, which was not significantly longer than that for nontreated control mice. The mean number of microsporidia-associated lesions detected in the small intestines of athymic mice treated with TNP-470 at 20 mg/kg i.p. daily was significantly less than the number of lesions in the small intestines of nontreated infected athymic mice (Table 2). Although the mean number of lesions in the small intestines of mice treated with TNP-470 at 10 mg/kg i.p. daily was less than half that of controls, this difference was not statistically significant due to high variation between individual mice in each treatment group. The mean numbers of microsporidia-associated lesions in the liver and pancreas of TNP-470-treated mice were also highly variable and not statistically significantly less than those of nontreated control mice. The toxicity control athymic mice given TNP-470 at a dose of 20 mg/kg i.p. daily survived this experiment, and no drug-induced lesions were noted. V. corneae-infected athymic mice treated with TNP-470 at these doses by s.c. inoculation instead of i.p. inoculation, however, failed to survive longer than the untreated control mice (not shown).
Effect of ovalicin treatment on V. corneae-infected athymic mice.
As shown in Fig. 4C, athymic mice infected with V. corneae and treated with ovalicin at 5 mg/kg s.c. daily survived significantly longer (19.9 ± 4.0 days; P < 0.05) than nontreated infected control mice (16.6 ± 1.1 days). Infected athymic mice treated with ovalicin at 10 mg/kg or 20 mg/kg s.c. daily, however, did not survive significantly longer than the nontreated controls (18.1 ± 3.8 days and 16.9 ± 1.2 days, respectively). The mean number of microsporidia-associated lesions in the small intestines of the athymic mice treated with ovalicin at 5 mg/kg, but not 10 mg/kg and 20 mg/kg, s.c. daily was significantly lower than that of controls (Table 2). The mean numbers of lesions in the liver and pancreas were not significantly different between treated and nontreated infected athymic mice. As also noted for the fumagillin and TNP-470 toxicity controls, athymic mice administered ovalicin at a dose of 20 mg/kg s.c. daily survived the experiment and developed no lesions.
Effects of treatments with two ovalicin derivatives on V. corneae-infected athymic mice.
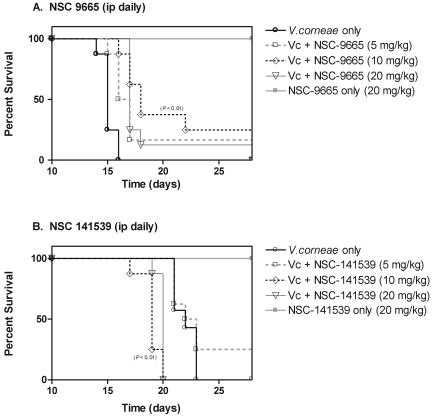
Athymic mice infected with V. corneae and treated with the ovalicin derivative NSC 9665 at 10 mg/kg i.p. daily (Fig. 5A) survived significantly longer (20.5 ± 4.9 days; P < 0.01) than untreated controls did (15.13 ± 0.64 days). The mean numbers of parasite-associated lesions were also significantly lower in the serosal surface of the small intestines of infected mice treated with NSC 9665 at 10 mg/kg and 20 mg/kg i.p. daily (Table 2). Ovalicin derivative NSC 141539, however, did not significantly prolong survival of the infected athymic mice at any of the treatment doses (Fig. 5B), although a statistically significant lower number of mean lesions on the small intestines was observed at 5 mg/kg i.p. daily (Table 2). Neither of these compounds resulted in significantly lower mean numbers of parasite-associated lesions in the liver or pancreas. Toxicity control groups of mice treated only with each compound at 20 mg/kg i.p. daily survived the length of the experiment, and no drug-associated lesions were observed by histopathology.
FIG. 5.
Survival curves of V. corneae-infected athymic mice treated with ovalicin derivatives, NSC 9665 and NSC 141539. Ten million microsporidia were inoculated i.p. into athymic mice in groups of eight that were treated daily with drugs beginning 24 h later. Statistically significant differences in survival times between treated and untreated infected mice were measured by Student's t test. Vc, V. corneae.
DISCUSSION
There is an absence of therapies that effectively treat infections due to the various species of microsporidia affecting humans and animals. Albendazole is effective for treating infections due to Encephalitozoon species but not E. bieneusi (8, 16, 47). A wide range of drugs have been evaluated for efficacy against microsporidia in vitro and in vivo, and fumagillin continues to be among the most effective. Fumagillin was used to treat Nosema apis in honeybees (26, 28, 35, 40), a Cystosporogenes sp. in a colony of eastern spruce budworms (Chorisoneura fumiferana) (44), and Octosporea bayeri in the aquatic invertebrate Daphnia magna (52). Clinically, fumagillin has been applied in humans to topically treat ocular microsporidial infections (8, 21) and was administered orally to treat enteric infections due to E. bieneusi in AIDS patients, although side effects in the latter included neutropenia and leukopenia (38). Relapses and incomplete clearance of E. bieneusi infections occurred if fumagillin was administered at lower doses to reduce toxic side effects. An analogue of fumagillin, TNP-470, was also effective for treating Nucleospora salmonis infections in chinook salmon (Oncorhynchus tshawytscha) (23) and prolonged survival of athymic mice infected with Encephalitozoon cuniculi (9).
E. bieneusi is the most common microsporidian species reported to infect humans, but difficulties exist for testing and identifying effective drugs, because in vitro culture methods and small-animal models are lacking for this microsporidian species. Therefore, the in vitro assay used in this study utilized the human microsporidian isolate V. corneae as a surrogate for E. bieneusi as well as E. intestinalis, which is the second-most common microsporidian species reported to infect humans. The rationale for testing drugs against both microsporidian species was that compounds found to inhibit both species of microsporidia in vitro, and V. corneae in vivo, would be more likely to inhibit E. bieneusi as well.
In the studies presented here, the total numbers of organisms still present after drug treatment for 10 days in culture were counted on hemacytometers to account for both inhibition of host cell infection and inhibition of microsporidian replication within the host cells. This approach is similar to drug testing systems that measure copies of microsporidial rRNA genes by real-time PCR for quantitating drug effects (36, 37). Assays that relied on counting the numbers of infectious foci (i.e., infected host cells) measured inhibition of infection rates but may not have accounted for reduced parasite replication rates (i.e., by not discerning between large and small infectious foci). Hemacytometer counting uses less equipment and costs less, while real-time PCR is probably considered more objective. On comparing the hemacytometer counting method and the real-time PCR method, however, Menotti et al. (37) reported that both methods appeared to be equally reliable for evaluating drug efficacy and for determining the 50% inhibitory concentrations of test compounds.
The results of the in vitro screening assay were reported at drug concentrations that were not toxic to the host cells, since microsporidia can replicate only within intact and viable host cells. At doses that are toxic to the host cells, it would not be possible to determine whether parasite replication decreased due to loss of host cell viability or due to a direct effect on the microsporidia. Another limitation to the in vitro screening studies was that drug toxicity in vitro did not necessarily predict drug toxicity in vivo, so the concentration of a drug found to be toxic in vitro might be more effective at higher serum concentrations without necessarily being toxic in vivo, as appeared to be the case for studies on albendazole (3, 8, 13, 19).
The studies presented here extend the results reported previously for fumagillin and TNP-470 against the Encephalitozoon species of microsporidia (9, 13) to demonstrate that fumagillin and TNP-470 also affected V. corneae in vitro and in vivo. In addition, the structurally related compound ovalicin was shown to inhibit replication of E. intestinalis and V. corneae by more than 70% at concentrations not toxic to host cells in vitro, with IC50 values of 0.004 and 0.002 μM, respectively. Furthermore, several ovalicin derivatives exhibited antimicrosporidial activities with IC50 values ranging from 0.001 μM to 97 μM. Three of these compounds in particular, NSC 9168, NSC 9665, and NSC 141539, exhibited strong activity against both microsporidian species in vitro with IC50 values near those of fumagillin, TNP-470, and ovalicin.
The V. corneae-infected mouse model that was developed and applied in these studies further supported the promise of fumagillin-related compounds for treating microsporidiosis. Although natural infections with microsporidia are believed to occur most commonly through ingestion or inhalation (15, 17, 31, 32, 47), microsporidia were inoculated i.p. in this model system to control for consistent microsporidial dosing of mice. The results of the dose-response experiment led to the selection of a dose of ten million organisms based on the statistically significant decrease in survival time and increase in parasite-associated lesions in comparison to results in animals inoculated with the next lowest dose of one million organisms. At the inoculation dose of 1 × 107 organisms, the mean survival time of the athymic mice was approximately 21 days, which was long enough to allow a test compound to affect the outcome but not so long as to require large amounts of a test compound. The principal sites of parasite-associated lesions were observed in the serosal aspect of the small intestine and in the liver and pancreas. Of note was the dramatic shift in the increased mean number of lesions in the colon after i.p. inoculation with 5 × 107 V. corneae. Although unclear, one explanation for this shift may be the possibility that at the higher inoculum dose, the numbers of spores being shed with urine or feces during the early stages of infection may have been sufficiently high to be ingested, which then caused these lesions in the colon.
Fumagillin, TNP-470, and ovalicin each statistically significantly prolonged survival of the V. corneae-infected athymic mice, but none of these compounds prevented lethal infection at the doses or routes tested. Two of the most effective ovalicin derivatives in vitro were also evaluated in vivo. Although derivative NSC 141539 failed to prolong survival of the infected mice, derivative NSC 9665, administered at a dose of 10 mg/kg i.p. daily, statistically significantly prolonged survival of the V. corneae-infected athymic mice by approximately 4 days. Interestingly, while fumagillin treatment by the s.c. inoculation route prolonged survival, i.p. inoculations of this drug failed to prolong survival. Conversely, TNP-470 significantly prolonged survival when administered i.p., but not when administered s.c. One possible explanation for these different effective routes of drug administration may relate to the short half-life of TNP-470, which was reported in humans to range between 0.10 and 0.88 h after intravenous administration (12, 20). Administration of TNP-470 by i.p. inoculation, the same route used for V. corneae inoculation, thus may have affected the organisms more readily than s.c. drug inoculation. In addition, the formulation used in these studies did not include dextran for improving TNP-470 solubility, and this may have somewhat limited these results as well. Ovalicin was administered only by s.c. inoculation, so no comparisons were made for assessing route of administration for this compound.
The highest dose of the compounds tested in the athymic mouse model was 20 mg/kg daily due to concerns for toxicity that were reported for mice given fumagillin at higher doses (30, 50, 51). At the doses and routes used in the present study, no toxicity was noted other than mild hemorrhage at the i.p. inoculation site in mice administered fumagillin. Of note was the observation that there was no statistically significant reduction in the mean numbers of parasite-associated lesions in the livers of treated mice. Much variability was observed between the mice within any given treatment group, and it was also possible that necrosis (and liver enzyme release) induced by the infection may have contributed to preventing or inhibiting drug activity locally.
In humans administered fumagillin, toxicity has been a concern. For example, neutropenia and leukopenia were observed in patients with microsporidiosis due to E. bieneusi who were treated with fumagillin at a daily dose of 60 mg orally (38). TNP-470 was the most effective and least toxic semisynthetic fumagillin derivative selected for evaluation in antitumor trials (24, 51), but adverse responses were reported in some of the patients being treated for Kaposi's sarcoma (12). To our knowledge, no reports have been published about TNP-470 having been applied for treating microsporidiosis in humans.
The putative target of fumagillin, TNP-470, and ovalicin appears to be methionine aminopeptidase 2 (MetAP2) initially based on reports that these compounds also inhibit angiogenesis through covalent binding between the heterocyclic ring (C-3) epoxide ring with His231 of the human endothelial cell MetAP2 (22, 43). All of the ovalicin-derivatives carried a closed epoxide ring at the C-3 site, yet some of the derivatives were less effective than others, suggesting that the other side groups also influenced the activity of these compounds. Methionine aminopeptidases are ubiquitous proteases that cleave the N-terminal methionine to facilitate protein maturation (1, 34, 41). Two isoforms of MetAP exist in most eukaryotes, but only MetAP2 was found to exist in the genome of the microsporidian Encephalitozoon cuniculi (25, 48, 49, 53). Of particular interest is the observation that fumagillin and its related compounds appear to be highly selective for MetAP2 over MetAP1 (29). Saccharomyces cerevisiae deletion mutants deficient in both MetAP1 and MetAP2 failed to survive, while those lacking either MetAP1 or MetAP2 survived. Those strains that were deficient in MetAP1 and depended on MetAP2 were unable to survive in the presence of ovalicin, while the strains that depended on MetAP1 survived in the presence of ovalicin (22, 43). This specificity for MetAP2 over MetAP1 appeared to depend on a single amino acid residue of Thr362 in the human MetAP1 on the basis of the observation that mutation of this residue to Ala362 in the human MetAP1 conferred ovalicin sensitivity (5). Other residues, however, also contributed to the stability of these drugs in the human MetAP2 active site to affect the degree of sensitivity of MetAP2 to fumagillin, ovalicin, and TNP-470 (5, 33). The key residues associated with the active site and metal-coordinating site are conserved in E. cuniculi MetAP2 and the human MetAP2, and analysis of a structural model of the E. cuniculi MetAP2 active site demonstrated that it could be superimposed on the human MetAP2 model (4, 53). Differences between the human and microsporidian MetAP2s in the surface residues and the absence of the N-terminal polylysine region in the microsporidian MetAP2 suggested that more selective compounds could be designed (48, 49, 53). Fumagillin and TNP-470 also inhibited replication of Plasmodium falciparum and Leishmania donovani in vitro, and it has been suggested that differences between the MetAP2s of these parasites and human MetAP2 could be exploited for more selective derivatives of fumagillin (54).
The results demonstrating that fumagillin, TNP-470, ovalicin, and several ovalicin derivatives inhibited two disparate microsporidian species, E. intestinalis and V. corneae, in vitro and inhibited V. corneae as a surrogate for E. bieneusi in vitro support continued studies to identify more effective fumagillin-related compounds. Such efforts will likely focus on the cloning, expression, and characterization of microsporidial MetAP2 for developing high-throughput screening methods. As this research moves forward, it will also become important to develop animal models of E. bieneusi for comparing efficacy with drug toxicity.
Acknowledgments
We gratefully acknowledge the support and thoughtful advice of Susan Brobst.
This work was supported in part by funding from the National Institutes of Health (NO1-AI75327 and RR00164).
REFERENCES
- 1.Arfin, S. M., R. L. Kendall, L. Hall, L. H. Weaver, and A. E. Stewart. 1995. Eukaryotic methionyl aminopeptidases: two classes of cobalt-dependent enzymes. Proc. Natl. Acad. Sci. 92:7714-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, M. D., C. R. Vossbrinck, E. S. Didier, J. V. Maddox, and J. A. Shadduck. 1995. Small subunit ribosomal DNA phylogeny of various microsporidia with emphasis on AIDS related forms. J. Eukaryot. Microbiol. 42:564-570. [DOI] [PubMed] [Google Scholar]
- 3.Blanshard, C., D. S. Ellis, D. G. Tovey, S. Dowel, and B. G. Gazzard. 1992. Treatment of intestinal microsporidiosis with albendazole in patients with AIDS. AIDS 6:311-313. [DOI] [PubMed] [Google Scholar]
- 4.Bontems, F., P. le Floch, F. Duffieux, C. Biderre, P. Peyret, and J. Y. Lallemand. 2003. Homology modeling and calculation of the cobalt cluster charges of the Encephalitozoon cuniculi methionine aminopeptidase, a potential target for drug design. Biophys. Chem. 105:29-43. [DOI] [PubMed] [Google Scholar]
- 5.Brdlik, C. M., and C. M. Crews. 2004. A single amino acid residue defines the difference in ovalicin sensitivity between type I and type II methionine aminopeptidases. J. Biol. Chem. 279:9475-9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cali, A., and P. Takvorian. 1999. Developmental morphology and life cycles of the microsporidia, p. 85-128. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 7.Cavalier-Smith, T. 1998. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 73:203-266. [DOI] [PubMed] [Google Scholar]
- 8.Conteas, C. N., O. G. Berlin, L. R. Ash, and J. S. Pruthi. 2000. Therapy for human gastrointestinal microsporidiosis. Am. J. Trop. Med. Hyg. 63:121-127. [DOI] [PubMed] [Google Scholar]
- 9.Coyle, C., M. Kent, H. B. Tanowitz, M. Wittner, and L. M. Weiss. 1998. TNP-470 is an effective antimicrosporidial agent. J. Infect. Dis. 177:515-518. [DOI] [PubMed] [Google Scholar]
- 10.Deplazes, P., A. Mathis, and R. Weber. 2000. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib. Microbiol. 6:236-260. [DOI] [PubMed] [Google Scholar]
- 11.Desportes-Livage, I. 2000. Biology of microsporidia. Contrib. Microbiol. 6:140-165. [DOI] [PubMed] [Google Scholar]
- 12.Dezube, B. J., J. H. Von Roenn, J. Holden-Wiltse, T. W. Cheung, S. C. Remick, T. P. Cooley, J. Moore, J. P. Sommadossi, S. L. Shriver, C. W. Suckow, and P. S. Gill. 2000. Phase I dose escalation pharmacokinetics of O-(chloroacetylcarbamoyl) fumagillol (TNP-470) and its metabolites in AIDS patients with Kaposi's sarcoma. Cancer Chemother. Pharmacol. 46:173-179. [DOI] [PubMed] [Google Scholar]
- 13.Didier, E. S. 1997. Effects of albendazole, fumagillin, and TNP-470 on microsporidial replication in vitro. Antimicrob. Agents Chemother. 41:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Didier, E. S. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 126:145-166. [DOI] [PubMed] [Google Scholar]
- 15.Didier, E. S., P. J. Didier, K. F. Snowden, and J. A. Shadduck. 2000. Microsporidiosis in mammals. Microbes Infect. 2:709-720. [DOI] [PubMed] [Google Scholar]
- 16.Didier, E. S., J. A. Maddry, P. J. Brindley, M. E. Stovall, and P. J. Didier. 2005. Therapeutic strategies for human microsporidia infections. Expert Rev. Anti-Infect. Ther. 3:419-434. [DOI] [PubMed] [Google Scholar]
- 17.Didier, E. S., K. F. Snowden, and J. A. Shadduck. 1998. Biology of microsporidian species infecting mammals. Adv. Parasitol. 40:283-320. [DOI] [PubMed] [Google Scholar]
- 18.Didier, E. S., M. E. Stovall, L. C. Green, P. J. Brindley, K. Sestak, and P. J. Didier. 2004. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol. 94:61-76. [DOI] [PubMed] [Google Scholar]
- 19.Dieterich, D. T., E. A. Lew, D. P. Kotler, M. A. Poles, and J. M. Orenstein. 1994. Treatment with albendazole for intestinal disease due to Enterocytozoon bieneusi in patients with AIDS. J. Infect. Dis. 169:178-183. [DOI] [PubMed] [Google Scholar]
- 20.Figg, W. D., J. M. Pluda, R. M. Lush, M. W. Saville, K. Wyvill, E. Reed, and R. Yarchoan. 1997. The pharmacokinetics of TNP-470, a new angiogenesis inhibitor. Pharmacotherapy 17:91-97. [PubMed] [Google Scholar]
- 21.Friedberg, D. N., and D. C. Ritterband. 1999. Ocular microsporidiosis, p. 293-314. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 22.Griffith, E. C., Z. Su, S. Niwayama, C. A. Ramsay, Y. H. Chang, and J. O. Liu. 1998. Molecular recognition of angiogenesis inhibitors fumagillin and ovalicin by methionine aminopeptidase 2. Proc. Natl. Acad. Sci. USA 95:15183-15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins, M. J., M. L. Kent, J. D. Moran, L. M. Weiss, and S. C. Dawe. 1998. Efficacy of the fumagillin analog TNP-470 for Nucleospora salmonis and Loma salmonae infection in chinook salmon Oncorhynchus tshawytscha. Dis. Aquat. Organ. 34:46-49. [DOI] [PubMed] [Google Scholar]
- 24.Ingber, D., T. Fujita, S. Kishimoto, K. Sudo, T. Kanamaru, H. Brem, and J. S. Folkman. 1990. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 348:555-557. [DOI] [PubMed] [Google Scholar]
- 25.Katinka, M. D., S. Duprat, E. Cornillot, G. Méténier, F. Thoarat, G. Prensier, V. Bare, E. Peyretaillade, P. Brottier, P. Wincker, F. Delbac, H. El Alaoui, P. Peyret, W. Saurin, M. Gouy, J. Weissenbach, and C. P. Vivarès. 2001. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 414:450-453. [DOI] [PubMed] [Google Scholar]
- 26.Katznelson, H., and C. A. Jamieson. 1952. Control of nosema disease of honeybees with fumagillin. Science 115:70-71. [DOI] [PubMed] [Google Scholar]
- 27.Keeling, P. J., and N. M. Fast. 2002. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 56:93-116. [DOI] [PubMed] [Google Scholar]
- 28.Killough, J. H., G. B. Magill, and R. C. Smith. 1952. The treatment of amebiasis with fumagillin. Science 115:71-72. [DOI] [PubMed] [Google Scholar]
- 29.Klein, C. D. P., and G. Folkers. 2003. Understanding the selectivity of fumagillin for the methionine aminopeptidase type II. Oncol. Res. 13:513-520. [DOI] [PubMed] [Google Scholar]
- 30.Konno, H., T. Tanaka, T. Kanai, K. Maruyama, S. Nakamura, and S. Baba. 1996. Efficacy of an angiogenesis inhibitor, TNP-470, in xenotransplanted human colorectal cancer with high metastatic potential. Cancer 77:1736-1740. [DOI] [PubMed] [Google Scholar]
- 31.Kotler, D. P., and J. M. Orenstein. 1998. Clinical syndromes associated with microsporidiosis. Adv. Parasitol. 40:321-349. [DOI] [PubMed] [Google Scholar]
- 32.Kotler, D. P., and J. M. Orenstein. 1999. Clinical syndromes associated with microsporidiosis, p. 258-292. In M. Wittner and L. M. Weiss (ed.), The microsporidia and microsporidiosis. ASM Press, Washington, D.C.
- 33.Liu, S., J. Widom, C. W. Kemp, C. M. Crews, and J. Clardy. 1998. Structure of human methionine aminopeptidase-2 complexed with fumagillin. Science 282:1324-1327. [DOI] [PubMed] [Google Scholar]
- 34.Lowther, W. T., and B. W. Matthews. 2000. Structure and function of the methionine aminopeptidases. Biochim. Biophys. Acta 1477:157-167. [DOI] [PubMed] [Google Scholar]
- 35.McCowen, M. C., M. E. Callender, and J. F. Lawlis, Jr. 1951. Fumagillin (H-3), a new antibiotic with amebicidal properties. Science 113:202-203. [DOI] [PubMed] [Google Scholar]
- 36.Menotti, J., B. Cassinat, C. Sarfati, O. Liguory, F. Derouin, and J. M. Molina. 2003. Development of a real-time PCR assay for quantitative detection of Encephalitozoon intestinalis DNA. J. Clin. Microbiol. 41:1410-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menotti, J., M. Santillana-Hayat, B. Cassinat, C. Sarfati, F. Derouin, and J. M. Molina. 2005. Inhibitory activity of human immunodeficiency virus aspartyl protease inhibitors against Encephalitozoon intestinalis evaluated by cell culture-quantitative PCR assay. Antimicrob. Agents Chemother. 49:2362-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molina, J. M., M. Tourneur, C. Sarfati, S. Chevret, A. de Gouvello, J. G. Gobert, S. Balkan, F. Derouin, and Agence Nationale de Recherches sur le SIDA 090 Study Group. 2002. Fumagillin treatment of intestinal microsporidiosis. N. Engl. J. Med. 346:1963-1969. [DOI] [PubMed] [Google Scholar]
- 39.Mossman, T. R., and T. A. T. Fong. 1989. Specific assays for cytokine production by T cells. J. Immunol. Methods 116:151-158. [DOI] [PubMed] [Google Scholar]
- 40.Mussen, E. C. 2. April 2002, posting date. Diagnosing and treating nosema disease. [Online.] http://entomology.ucdavis.edu/faculty/Mussen/beebriefs/Nosema_Disease.pdf.
- 41.Sato, Y. 2004. Aminopeptidases in health and disease: role of aminopeptidases in angiogenesis. Biol. Pharm. Bull. 270:772-776. [DOI] [PubMed] [Google Scholar]
- 42.Shadduck, J. A. 1980. Effect of fumagillin on in vitro multiplication of Encephalitozoon cuniculi. J. Protozool. 27:202-208. [DOI] [PubMed] [Google Scholar]
- 43.Sin, N., L. Meng, M. Q. Wang, J. J. Wen, W. G. Bornmann, and C. M. Crews. 1997. The anti-angiogenic agent fumagillin covalently binds and inhibits the methionine aminopeptidase, MetAP-2. Proc. Natl. Acad. Sci. USA 94:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Frankenhuyzen, K., P. Ebling, B. McCron, T. Ladd, D. Gauthier, and C. Vossbrinck. 2004. Occurrence of Cytosporogenes sp. (Protozoa, Microsporidia) in a multi-species insect production facility and its elimination from a colony of the eastern spruce budworm, Chorisoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). J. Invert. Pathol. 87:16-28. [DOI] [PubMed] [Google Scholar]
- 45.Vossbrinck, C. R., T. G. Andreadis, and L. M. Weiss. 2004. Phylogenetics: taxonomy and the microsporidia as derived fungi. p. 189-213. In D. S. Lindsay and L. M. Weiss (ed.), Opportunistic infections: Toxoplasma, Sarcocystis, and microsporidia. Kluwer Academic Publishers, Boston, Mass.
- 46.Vossbrinck, C. R., M. D. Baker, and E. S. Didier. 1996. Comparative rDNA analysis of microsporidia including AIDS-related species. J. Eukaryot. Microbiol. 43:110S. [DOI] [PubMed] [Google Scholar]
- 47.Weber, R., P. Deplazes, and D. Schwartz. 2000. Diagnosis and clinical aspects of human microsporidiosis. Contrib. Microbiol. 6:166-192. [DOI] [PubMed] [Google Scholar]
- 48.Weiss, L. M., T. D. Edlind, C. R. Vossbrinck, and T. Hashimoto. 1999. Microsporidian molecular phylogeny: the fungal connection. J. Eukaryot. Microbiol. 46:17S-18S. [PubMed] [Google Scholar]
- 49.Weiss, L. M., G. C. Zhou, and H. Zhang. 2003. Characterization of recombinant microsporidian methionine aminopeptidase type 2. J. Eukaryot. Microbiol. 50(Suppl.):597-599. [DOI] [PubMed] [Google Scholar]
- 50.Yamaoka, M., T. Yamamoto, T. Masaki, S. Ikeyama, K. Sudo, and T. Fugita. 1993. Inhibition of tumor growth and metastasis of rodent tumors by the angiogenesis inhibitor O-(chloroacetyl-carbamoyl)fumagillol (TNP-470; AGM-1470). Cancer Res. 53:4262-4267. [PubMed] [Google Scholar]
- 51.Yanase, T., M. Tamura, K. Fujita, S. Kodama, and K. Tanaka. 1993. Inhibitory effect of angiogenesis inhibitor TNP-470 on tumor growth and metastasis of human cell lines in vitro and in vivo. Cancer Res. 53:2566-2570. [PubMed] [Google Scholar]
- 52.Zbinden, M., S. Lass, D. Refardt, J. Hottinger, and D. Ebert. 2005. Octosporea bayer: fumidil B inhibits vertical transmission in Daphnia magna. Exp. Parasitol. 109:58-61. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, H., H. Huang, A. Cali, P. M. Takvorian, C. Feng, G. Zhou, and L. M. Weiss. 2005. Investigations into microsporidian methionine aminopeptidase type 2: a therapeutic target for microsporidiosis. Folia Parasitol. 52:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, P., D. E. Nicholson, J. M. Bujnicki, X. Su, J. J. Brendle, M. Ferdig, D. E. Kyle, W. K. Milhous, and P. K. Chiang. 2002. Angiogenesis inhibitors specific for methionine aminopeptidase 2 as drugs for malaria and leishmaniasis. J. Biomed. Sci. 9:34-40. [DOI] [PubMed] [Google Scholar]