Abstract
The diffusion of metallo-β-lactamases (MBLs) among clinically important human pathogens represents a therapeutic issue of increasing importance. However, the origin of these resistance determinants is largely unknown, although an important number of proteins belonging to the MBL superfamily have been identified in microbial genomes. In this work, we analyzed the distribution and function of genes encoding MBL-like proteins in the class Rhizobiales. Among 12 released complete genomes of members of the class Rhizobiales, a total of 57 open reading frames (ORFs) were found to have the MBL conserved motif and identity scores with MBLs ranging from 8 to 40%. On the basis of the best identity scores with known MBLs, four ORFs were cloned into Escherichia coli for heterologous expression. Among their products, one (blr6230) encoded by the Bradyrhizobium japonicum USDA110 genome, named BJP-1, hydrolyzed β-lactams when expressed in E. coli. BJP-1 enzyme is most closely related to the CAU-1 enzyme from Caulobacter vibrioides (40% amino acid sequence identity), a member of subclass B3 MBLs. A kinetic analysis revealed that BJP-1 efficiently hydrolyzed most β-lactam substrates, except aztreonam, ticarcillin, and temocillin, with the highest catalytic efficiency measured with meropenem. Compared to other MBLs, BJP-1 was less sensitive to inactivation by chelating agents.
The production of β-lactamases is the most common cause of bacterial resistance to β-lactam antibiotics, particularly among gram-negative pathogens (7, 19). The recent emergence of acquired metallo-β-lactamases (MBLs), mainly of the IMP and VIM types, in important human pathogens (such as Pseudomonas spp., Acinetobacter spp., and members of the family Enterobacteriaceae) has been regarded as extremely worrisome, since these enzymes exhibit a very broad substrate profile, including carbapenems and expanded-spectrum cephalosporins, and are not susceptible to mechanism-based β-lactamase inactivators (6, 26, 31). Due to this, MBLs have become a subject of increasing interest in recent years. However, while a growing knowledge of their structure, catalytic mechanisms, and epidemiology in clinical settings has been achieved (31), the evolutionary history and natural distribution of these enzymes remain largely unclear.
The recent progress in large-scale genome sequencing revealed that genes encoding proteins sharing structural similarity with MBLs are widely distributed in prokaryotic and eukaryotic genomes (Pfam PF00753, http://www.sanger.ac.uk/Software/Pfam/). These proteins are part of a large superfamily of metallohydrolases that includes enzymes of different functions (e.g., glyoxalase II, aryl- and alkyl-sulfatase, cyclase, DNA repair, N-acyl homoserine lactone hydrolase) as well as several hypothetical proteins of unknown function (1, 8, 10). A postgenomic approach has been successful in identifying MBL orthologs encoded by microbial genomes (e.g., CAU-1 from Caulobacter vibrioides [formerly Caulobacter crescentus]) (13), and can be a powerful means to identify new MBLs and bacterial species that could play a role as reservoirs of similar resistance genes.
In this work, we analyzed the presence of putative proteins belonging to the MBL superfamily in the available complete genomes of various species in the order Rhizobiales. We also report on the identification and detailed biochemical characterization of a new subclass B3 MBL from Bradyrhizobium japonicum, named BJP-1.
MATERIALS AND METHODS
Bacterial strains and genetic vectors.
B. japonicum USDA110 was kindly donated by the USDA Rhizobium Germplasm Resource Center. Agrobacterium tumefaciens C85, Mesorhizobium loti ATCC 33669, and Sinorhizobium meliloti ATCC 9930 were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). Escherichia coli DH5α (Gibco Life Technologies, Gaithersburg, Md.) was used as the host for genetic vectors and recombinant plasmids. E. coli BL21(DE3) (Stratagene, Inc., La Jolla, Calif.) was used as a host for T7 promoter-based expression vectors for enzyme production. Plasmid pBC-SK (Stratagene, Inc.) was used for cloning selected genes under the transcriptional control of the Plac promoter to investigate the putative protein function and for in vitro susceptibility testing. Plasmid pET-9a (Novagen, Madison, Wis.) was used for high-level gene expression and recombinant enzyme production (Table 1).
TABLE 1.
Recombinant plasmids and primers used in this study
| Plasmid | Genetic vector | Primer pair | Sequence | Target | Product size (bp) |
|---|---|---|---|---|---|
| pMS-AGR | pBC-SK | Agrob/fwd | 5′-GGTCTAGACATATGATAGGGCTTGGCGATTGTG | Agrobacterium tumefaciens strain C58 AGR_C_1482 | 981 |
| Agrob/rev | 5′-CCGGATCCTCAGACCGGAAAATACTCGCC | ||||
| pET-AGR | pET-9a | Agrob/fwd | |||
| Agrob/rev | |||||
| pMS-LOT | pBC-SK | Mes_loti/fwd | 5′-GCTCTAGACATATGGGATCTCTTGCGGTCAT | Mesorhizobium loti MAFF303099 mll0231 | 762 |
| Mes_loti/rev | 5′-CCGGATCCTCAGGGCCGCTGCCGCA | ||||
| pET-LOT | pET-9a | Mes_loti/fwd | |||
| Mes_loti/rev | |||||
| pMS-SIN | pBC-SK | Sin/fwd | 5′-CGGGAGCTCCATATGCAAGCTCCGGAATTCGAC | Sinorhizobium meliloti 1021 SMc00087 | 1,023 |
| Sin/rev | 5′-CCGAGCTCTCACGCCAGCCGGTACGCG | ||||
| pET-SIN | pET-24a | Sin/fwd | |||
| Sin/rev | |||||
| pLBII-BJP | pBC-SK | Brad/fwd | 5′-GGTCTAGACATATGAGAAGGCTGACGGCC | Bradyrhizobium japonicum USDA 110 blr6230 | 885 |
| Brad/rev | 5′-CCGGATCCCTATTTCTTCTCCAGCGCCG | ||||
| pET-BJP | pET-9a | Brad/fwd | |||
| Brad/rev |
Media and culture conditions.
B. japonicum, S. meliloti, and M. loti were grown aerobically at 30°C in YM medium (yeast extract [3 g/liter], malt extract [3 g/liter], peptone [5 g/liter], glucose [10 g/liter]). A. tumefaciens was grown aerobically at 30°C in nutrient broth (peptone [5 g/liter], meat extract [3 g/liter]). Luria-Bertani (LB) medium was routinely used for propagation of E. coli DH5α derivatives. With BL21(DE3) derivatives, P-0.8G medium was used for routine propagation, while ZYP-0.8G and ZYP-5052 media were used for recombinant protein production (29).
Database screening and sequence analysis.
Database searches were performed using BLAST 2.2.11 running at the National Center for Biological Information (NCBI) (http://www.ncbi.nlm.nih.gov/). Multiple-sequence alignments were performed using ClustalX 1.83 (9) followed by manual adjustments using the BioEdit 7.0.5 package (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Phylogenetic trees were generated using ClustalX 1.83 and TreeView 1.6.6 applications (9, 27). Theoretical calculations of protein molecular mass and pI were carried out using the software available at the ExPASy proteomic server (http://ca.expasy.org/), and leader peptide cleavage site was predicted using SignalP 3.0 (4).
Recombinant DNA methodologies and DNA analysis techniques.
Basic recombinant DNA procedures were performed as described by Sambrook and Russell (28). Briefly, the selected putative open reading frames (ORFs) were amplified by PCR using specific custom primers (Table 1) with the Expand High Fidelity PCR system (Roche Biochemicals, Mannheim, Germany) as described previously (13) using the following cycling conditions: an initial denaturation step at 95°C for 3 min; 30 cycles, with 1 cycle consisting of denaturation at 95°C for 40 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min (10 s added to the extension step to each cycle in cycles 11 to 30); a final extension step at 72°C for 20 min. The amplification products were cloned into pBC-SK vector using XbaI and BamHI restriction sites (or SacI and XhoI for S. meliloti SMc00087 ORF). The NdeI-BamHI fragments from the various plasmids were subcloned into the pET-9a expression vector, except the NdeI-XhoI fragment of pBC-SIN, which was subcloned in pET-24a. All recombinant plasmids were sequenced to exclude the presence of any unwanted PCR-generated mutations.
Antimicrobial susceptibility testing.
The in vitro susceptibility of E. coli strains carrying different plasmids and of B. japonicum USDA 110 was determined by the macrodilution broth method as recommended by the Clinical Laboratory Standards Institute (formerly the National Committee for Clinical Laboratory Standards) (25) using supplemented Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) and YM broth, respectively, with an inoculum size of 105 CFU per tube. Results were recorded after incubation for 24 h at 37°C for E. coli and after incubation for 4 days at 30°C for B. japonicum.
Detection of β-lactamase activity and preparation of crude extracts.
β-Lactamase activity in crude cell extracts and during the purification procedure was assayed spectrophotometrically by monitoring the hydrolysis of the following β-lactam substrates: 200 μM cephalothin at 260 nm (Δɛ = −6,300 M−1 · cm−1), 150 μM imipenem at 300 nm (Δɛ = −9,000 M−1 · cm−1), and 100 μM nitrocefin at 482 nm (Δɛ = 15,000 M−1 · cm−1). Reactions were performed in 50 mM HEPES-NaOH buffer (pH 7.5) containing 50 μM ZnSO4 (HZN buffer) at 30°C in a total volume of 500 μl. Inhibition of enzymatic activity by EDTA was assayed by measuring the residual activity after incubation of the crude extract for 20 min at 25°C in the presence of 5 mM EDTA. Crude cell extracts were prepared from a culture grown aerobically in LB broth at 37°C. Cells were collected by centrifugation, resuspended in HZN buffer, and lysed by sonication (six times, for 15 s each time, at 50 W). The supernatant obtained after centrifugation at 10,000 × g for 10 min to remove cell debris provided the crude extract.
Purification of BJP-1.
The BJP-1 enzyme was purified from a culture of E. coli BL21(DE3)/[pET-BJP] grown in 3 liters of ZYP-5052 medium for 24 h at 37°C. Cells were harvested by centrifugation (10,000 × g, 30 min, 4°C), resuspended in 100 ml of 20 mM Tris-HCl buffer (pH 8.0) containing 50 μM ZnSO4 (TZN buffer) and lysed using a cell disruption system (Constant Systems Ltd., Daventry, United Kingdom). Cellular debris was removed by centrifugation (12,000 × g, 40 min, 4°C), and the clarified supernatant was loaded on a column (1.6 × 40 cm) packed with 70 ml of DEAE-Sepharose FF (flow rate, 3 ml/min) previously equilibrated with TZN buffer. Under these conditions, the β-lactamase did not bind to the column but was eluted using 100 ml of TZN buffer. The active fractions were pooled, and the buffer exchanged to 20 mM ethanolamine (pH 9.2) containing 50 μM ZnSO4 (EZN buffer) using a HiPrep 26/10 desalting column (Amersham Biosciences). The resulting sample was loaded (flow rate, 1 ml/min) on an HR 16/5 column packed with 10 ml of Source Q gel (Amersham Biosciences) previously equilibrated with EZN buffer. Again, the β-lactamase was only slightly retained by the column, and the enzyme was eluted using 40 ml of EZN buffer. All chromatography steps were performed using an Äkta purifier platform (Amersham Biosciences). Enzyme purity and authenticity were assayed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and electrospray mass spectrometry (see below). The pure enzyme (4.4 mg/ml) was stored at −20°C until use.
Protein analysis techniques.
Analytical isoelectric focusing and subsequent zymographic detection of β-lactamase were performed as described previously (23). SDS-PAGE was performed as described by Laemmli (21) using 12% and 5% for resolving and stacking gels, respectively. After electrophoresis, proteins were stained with SimplyBlue SafeStain (Invitrogen). The molecular mass of the native BJP-1 enzyme was estimated by size exclusion chromatography using a Superdex 75 HR 10/30 column (Amersham Biosciences) and HZN buffer supplemented with 150 mM NaCl. The enzyme was eluted in the same buffer at a flow rate of 0.4 ml/min. The column was calibrated with the low-range gel filtration calibration kit (Amersham Biosciences), and apparent partition coefficients were calculated as described previously (11). Protein concentration in solution was determined using a commercial kit (Bio-Rad [Richmond, Calif.] protein assay) with bovine serum albumin as the standard. The molecular mass of the enzymatic preparation of BJP-1 was measured by electrospray mass spectrometry as described previously (13), using a Finnigan LTQ mass spectrometer equipped with an ion spray source (Thermo Electron Co., Shaumberg, Ill.). The data were analyzed with the software delivered with the instrument.
Determination of steady-state kinetic parameters and inactivation by metal chelators.
Substrate hydrolysis by the purified enzyme preparation was monitored at 30°C by measuring the absorbance variation using a Cary 100 UV-visible light spectrophotometer (Varian, Walnut Creek, Calif.). The wavelengths and changes in the extinction coefficients used were as described previously (22). Enzyme concentrations in the assays were 1.3 to 850 nM. The steady-state kinetic parameters (kcat and Km) were calculated after direct fit of initial rates versus substrate concentrations using the Henri-Michaelis equation. Inhibition of enzymatic activity by divalent metal chelators was assayed as previously described (18) and by measuring the residual activity against 100 μM meropenem in 50 mM HEPES-NaOH buffer (pH 7.5) after incubation of the enzyme for 20 min at 25°C in the presence of different concentrations of inhibitors.
RESULTS AND DISCUSSION
MBL homologues in the Rhizobiales genomes.
Microbial species belonging to the order Rhizobiales (class α-Proteobacteria) are the focus of great interest, representing possible human pathogens (e.g., Bartonella henselae and Brucella spp.), plant biotechnology tools (e.g., Agrobacterium tumefaciens), or important environmental factors in agriculture and ecology (e.g., nitrogen-fixing bacteria, such as Bradyrhizobium japonicum). Their importance is reflected by the 31 genome sequence projects currently listed at the NCBI, among which 12 have been completed (Table 2).
TABLE 2.
Distribution of MBL homologues among sequences of the genomes of Rhizobiales and investigation of carbapenem-hydrolyzing enzymes
| Species and strain | Accession no. | Highest identity score with subclass B3 MBLs (%) | Closest enzyme | Code |
|---|---|---|---|---|
| Bradyrhizobium japonicum USDA 110 | NP_772870a | 39.5 | CAU-1 | BJP-1 |
| NP_766862 | 14.9 | GOB-1 | BJ1 | |
| NP_766991 | 17.9 | L1 | BJ2 | |
| NP_767540 | 18.3 | FEZ-1 | BJ3 | |
| NP_769257 | 16.1 | THIN-B | BJ4 | |
| NP_770826 | 14.5 | CAU-1 | BJ5 | |
| NP_771428 | 17.6 | L1 | BJ6 | |
| NP_771689 | 17.4 | GOB-1 | BJ7 | |
| NP_773001 | 16.3 | L1 | BJ8 | |
| NP_773606 | 17.0 | THIN-B | BJ9 | |
| NP_774530 | 14.8 | L1 | BJ10 | |
| NP_774533 | 15.6 | THIN-B | BJ11 | |
| Agrobacterium tumefaciens C58 | NP_353832a | 17.5 | CAU-1 | AT1 |
| NP_396022 | 13.8 | GOB-1 | AT2 | |
| NP_532874 | 15.5 | L1 | AT3 | |
| NP_533962 | 14.5 | THIN-B | AT4 | |
| Bartonella henselae strain Houston-1 | YP_033938 | 16.7 | FEZ-1 | BH1 |
| YP_034354 | 14.6 | GOB-1 | BH2 | |
| Brucella melitensis 16M | NP_539047 | 15.3 | FEZ-1 | BM1 |
| NP_539413 | 16.6 | CAU-1 | BM2 | |
| NP_540349 | 17.2 | CAU-1 | BM3 | |
| Mesorhizobium loti MAFF 303099 | NP_102074a | 17.3 | GOB-1 | ML1 |
| NP_102956 | 15.6 | L1 | ML2 | |
| NP_103423 | 17.2 | L1 | ML3 | |
| NP_103568 | 12.9 | L1 | ML4 | |
| NP_103950 | 14.2 | FEZ-1 | ML5 | |
| NP_104628 | 13.1 | THIN-B | ML6 | |
| NP_104783 | 16.3 | GOB-1 | ML7 | |
| NP_105758 | 15.5 | GOB-1 | ML8 | |
| NP_107838 | 16.7 | THIN-B | ML9 | |
| Nitrobacter winogradskyi Nb-255 | YP_316733 | 17.5 | GOB-1 | NH1 |
| YP_316992 | 16.4 | THIN-B | NH2 | |
| YP_317080 | 13.8 | THIN-B | NH3 | |
| YP_317232 | 18.0 | THIN-B | NH4 | |
| YP_317506 | 15.7 | FEZ-1 | NH5 | |
| YP_319497 | 14.8 | THIN-B | ||
| Rhodopseudomonas palustris CGA009 | NP_945718 | 15.3 | FEZ-1 | RP1 |
| NP_945921 | 18.2 | GOB-1 | RP2 | |
| NP_945963 | 17.5 | L1 | RP3 | |
| NP_946208 | 15.6 | THIN-B | RP4 | |
| NP_946446 | 16.4 | L1 | RP5 | |
| NP_947830 | 15.8 | CAU-1 | RP6 | |
| NP_949760 | 15.7 | L1 | RP7 | |
| Sinorhizobium meliloti 1021 | NP_384865 | 15.1 | L1 | SM1 |
| NP_385042a | 16.6 | THIN-B | SM2 | |
| NP_385810 | 15.9 | L1 | SM3 | |
| NP_386438 | 16.5 | FEZ-1 | SM4 | |
| NP_386770 | 14.1 | THIN-B | SM5 | |
| NP_435818 | 12.8 | L1 | SM6 | |
| NP_436244 | 15.1 | CAU-1 | SM7 |
Homologues cloned in Escherichia coli expression vectors (pBC-SK and pET derivatives, see Materials and Methods for details).
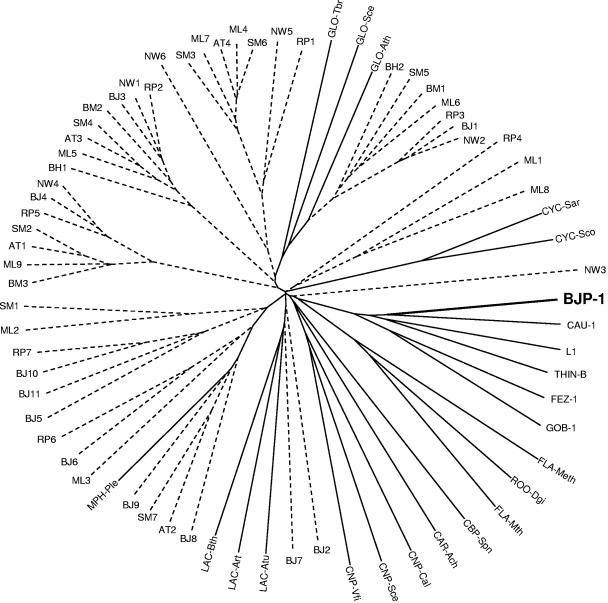
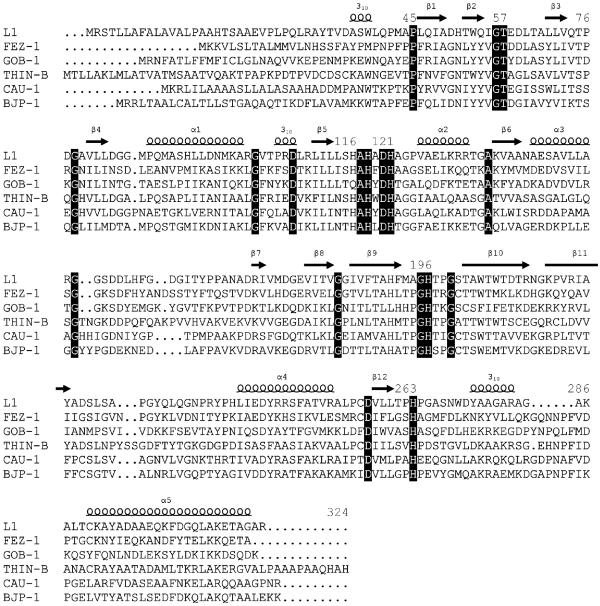
The presence of MBL homologues in these 12 genomes was investigated, and 57 proteins were found to belong to the MBL superfamily and exhibit amino acid sequence identities with members of subclass B3 MBLs ranging from 8% to 40% (Table 2 and Fig. 1). Among these, four homologues were selected for further expression analysis on the basis of their overall sequence similarity with subclass B3 MBLs and the presence of conserved zinc-binding motifs typical of MBLs, in particular the residues flanking His118, His121, and His196 (Fig. 2).
FIG. 1.
Unrooted tree showing the relationship between members belonging to the superfamily of Zn-dependent hydrolases of the β-lactamase fold (metallo-β-lactamase superfamily, Pfam PF00753). This figure includes proteins of unknown function identified in the 12 complete genomes of members of the order Rhizobiales (dashed lines) and proteins of the MBL superfamily for which function has been demonstrated on a biochemical basis (solid lines). The proteins of the MBL superfamily are as follows: L1, FEZ-1, GOB-1, THIN-B, and CAU-1, metallo-β-lactamases of Stenotrophomonas maltophilia, Legionella gormanii, Chryseobacterium meningosepticum, Janthinobacterium lividum, and Caulobacter vibrioides (formerly crescentus), respectively; LAC-Art, -Atu, and -Bth, N-acyl-homoserine lactone hydrolases of Arthrobacter sp. strain IBN 110, Agrobacterium tumefaciens, and Bacillus thuringiensis, respectively; CYC-Sar and -Sco, cyclases involved in the polyketide biosynthetic pathway of Streptomyces arenae and Streptomyces coelicolor, respectively; GLO-Ath, -Sce, and -Tbr, glyoxalase II of Arabidopsis thaliana, Saccharomyces cerevisiae, and Trypanosoma brucei, respectively; CNP-Cal, -Sce, and -Vfi, 3′-5′ cyclic nucleotide phosphodiesterase of Candida albicans, S. cerevisiae, and Vibrio fischeri, respectively; FLA-Meth and -Mth, flavoproteins of Methanothermobacter thermautotrophicus and Moorella thermoacetica, respectively; ROO-Dgi, rubredoxin-oxygen oxidoreductase of Desulfovibrio gigas; CBP-Spn, choline-binding protein of Streptococcus pneumoniae; CAR-Ach, carbofuran hydrolase of Achromobacter sp.; MPH-Ple, methyl parathion hydrolase of Plesiomonas sp. strain M6.
FIG. 2.
Amino acid sequence alignment of BJP-1 in comparison with other enzymes of subclass B3. Residues identical in all sequences are shown in white type on a black background. Secondary structure elements (S, strands; 310 and H, helices; L, loops) of the L1 enzyme are indicated above the sequences (30). Numbering is in accordance with the BBL scheme (16). Gaps introduced to maximize alignment are indicated by periods.
The open reading frames encoding the four homologues were cloned and expressed in two different E. coli-based systems, using two different promoters for the expression of cloned genes. The clones carrying the genes from A. tumefaciens, Mesorhizobium loti, and Sinorhizobium meliloti did not show any β-lactamase activity with either system (Table 3 and data not shown). However, the expression in E. coli of the gene encoding protein blr6230 from B. japonicum (accession no. NP_772870) allowed the measurement of an EDTA-inhibited β-lactam-hydrolyzing activity (Table 3). This functional β-lactamase was named BJP-1 (after B. japonicum).
TABLE 3.
Comparison of the specific activities of crude extracts prepared from different E. coli systems carrying different MBL homologuesa
| Plasmid carried by E. coli strain DH5α | Sp act (μmol/min · mg protein)
|
|
|---|---|---|
| Cephalothin | Imipenem | |
| pMS-AGR | <0.01 | <0.005 |
| pMS-LOT | <0.01 | <0.005 |
| pMS-SIN | <0.01 | <0.005 |
| pLBII-BJP | 5.4 | 0.3 |
| pBC-SK | <0.01 | <0.005 |
Reported values are the means of three measurements, and the standard deviations were below 10%. E. coli DH5α(pBC-SK) harboring the empty vector is shown for comparison.
Contribution of BJP-1 to β-lactam resistance.
No carbapenem-hydrolyzing activity was detected in crude extracts of B. japonicum USDA110 grown in liquid medium. The same result was obtained with samples prepared after growing the strain in medium containing subinhibitory concentrations of ampicillin or imipenem, used as potential β-lactamase inducers (data not shown). Despite this finding, B. japonicum exhibited rather high MICs for several β-lactams, including carbapenems (Table 4), suggesting that other resistance mechanisms might be involved in the overall poor susceptibility to β-lactams (e.g., low affinity of penicillin-binding proteins, permeability barriers or efflux, and/or the production of additional β-lactamases). In particular, the contribution of another putative class A β-lactamase (bll0941, accession no. NP_767581) encoded by the chromosome of B. japonicum and similar to the L2 β-lactamase of Stenotrophomonas maltophilia (38% identity) (accession no. CAA69869) could not be excluded, although this point was not specifically investigated.
TABLE 4.
β-Lactam susceptibility profile of B. japonicum USDA 110 and E. coli DH5α(pLBII-BJP) carrying the cloned blaBJP-1 genea
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| B. japonicum USDA 110 | E. coli DH5α(pLBII-BJP) | E. coli DH5α(pBC-SK) | |
| Ampicillin | 4 | 2 | 2 |
| Piperacillin | >128 | 2 | 2 |
| Cephalothin | 4 | 32 | 4 |
| Cefuroxime | 1 | 16 | 4 |
| Cefotaxime | 1 | 0.12 | 0.12 |
| Ceftazidime | >16 | 0.5 | 0.5 |
| Cefepime | NDb | 0.06 | 0.06 |
| Imipenem | 0.5 | 0.5 | 0.12 |
| Meropenem | 16 | 0.5 | 0.03 |
The E. coli strain containing the empty vector (pBC-SK) is shown for comparison.
ND, not determined.
E. coli DH5α carrying the recombinant plasmid for the expression of blaBJP-1 (pLBII-BJP) showed decreased susceptibility to cephalothin, cefuroxime, imipenem, and meropenem, but not to penicillins or expanded-spectrum cephalosporins (Table 4).
BJP-1 purification and biophysical characterization.
BJP-1 enzyme was purified from a cell extract of E. coli BL21(DE3)[pET-BJP] obtained from a 3-liter culture in autoinducing medium ZYP-5052 grown at 37°C for 24 h. Purification was carried out in two steps of anion-exchange chromatography at pH 8.0 and pH 9.2, with a final yield of approximately 15 mg/liter of culture. The purification process is summarized in Table 5. The purity of the protein preparation was estimated to be >98% according to SDS-PAGE analysis (data not shown) with a protein size of approximately 30 kDa. Size exclusion chromatography yielded a molecular mass of 31 ± 3 kDa for BJP-1, indicating that, under the experimental conditions, the native enzyme is monomeric like most other subclass B3 MBLs (3, 12, 13, 24) except L1 (5, 30). Electrospray mass spectrometry yielded a mass value of 29,893 ± 8 Da, in good agreement with the theoretical mass of the mature protein (29,898.29 Da) obtained after the cleavage of a 20-residue N-terminal signal peptide, as predicted by the SignalP 3.0 algorithm (4). Analytical isoelectric focusing carried out on the purified protein and developed with the chromogenic substrate nitrocefin revealed the presence of a single β-lactamase band of pI 6.9 (data not shown), a value being slightly above the predicted pI (5.9).
TABLE 5.
Summary of the typical procedures of purification of BJP-1 metallo-β-lactamase produced in E. coli BL21(DE3)[pET-BJP]
| Product of purification step | Vol (ml) | Total amt of protein (mg) | Total activity (U)a | Sp act (U/mg protein) | Recovery (%) | Purification (fold) |
|---|---|---|---|---|---|---|
| Cell extract | 90 | 2,000 | 40,860 | 21 | 100 | 1 |
| DEAE Sepharose FTb | 160 | 109 | 27,500 | 252 | 67 | 12 |
| Source Q FT | 55 | 50 | 25,355 | 506 | 61 | 24 |
One unit of activity is defined as the amount of enzyme hydrolyzing 1 μmol of cephalothin per min under the conditions described in Materials and Methods.
FT, flowthrough.
Structural features of the BJP-1 enzyme.
BJP-1 could be aligned over the entire sequence with other subclass B3 MBLs without introducing major gaps (Fig. 2). The closest similarity (pairwise amino acid sequence identity, 39.5%) was observed with the C. vibrioides (C. crescentus) CAU-1 enzyme. BJP-1 is slightly more divergent from the Legionella gormanii FEZ-1 (33% identity), Janthinobacterium lividum THIN-B (30.3% identity), S. maltophilia L1, and Chryseobacterium meningosepticum GOB-1 (28% identity) enzymes.
Although most conserved residues in enzymes of subclass B3 are also present in BJP-1 enzyme, a notable difference is represented by a threonine-to-serine substitution at position 197, just after one of the Zn-binding histidine residues (Fig. 2). Although the nature of these residues is similar, it might be interesting to investigate the role of this conserved residue in the structure and/or function of the enzyme. Similarly, at position 119 (sandwiched between three Zn-binding residues, His118, Asp120, and His121) there is usually an aromatic residue, except in the L1 and BJP-1 enzymes, where an alanine and a leucine are found, respectively.
The monomeric nature of the BJP-1 enzyme correlates with the replacement of the Met175 residue in the L1 enzyme by a lysine in BJP-1. Met175 is responsible for determinant intersubunit interactions with an hydrophobic pocket formed by residues Leu154, Pro198, and Tyr236 in the tetrameric L1 enzyme (17, 30). Interestingly, the two cysteine residues (at positions 256 and 290) involved in the disulfide bridge present in L1 (30), FEZ-1 (17), and probably also in THIN-B (12), are not conserved in BJP-1. In the latter and as in CAU-1, two cysteine residues found in positions 200 and 220 might form a disulfide bridge between the loop preceding strand S10 and that following strand S11, which could be located very close to the active site (Fig. 2) (13).
Kinetic properties of the BJP-1 β-lactamase.
The kinetic parameters were determined for a representative set of β-lactam antibiotics, including penicillins, narrow- and expanded-spectrum cephalosporins, carbapenems, and aztreonam (Table 6). The results showed that BJP-1 exhibits a broad substrate profile, since most tested compounds were hydrolyzed, although with very different catalytic efficiencies (kcat/Km values ranging from 2 × 102 to 6 × 106 M−1 · s−1). Ticarcillin, temocillin, and aztreonam were not recognized by the enzyme. The best substrate of BJP-1 is CENTA, a chromogenic cephalosporin (2), for which the lowest Km value (19 μM) and the highest turnover rate (kcat, 114 s−1) were measured, resulting in a catalytic efficiency at least 1 order of magnitude higher than those of other substrates. BJP-1 shows a higher activity against penicillin G, narrow-spectrum cephalosporins, cefotaxime, and meropenem, while ceftazidime, cefepime, and faropenem were poorly hydrolyzed (kcat/Km, <104 M−1 · s−1). BJP-1 exhibits overall high Km values (Km, >100 μM for most substrates, except CENTA), indicating a poor interaction with β-lactams, in particular with most penicillins, ceftazidime, and cefepime.
TABLE 6.
Kinetic parameters of the purified BJP-1a
| Substrate | kcat (s−1) | Km (μM) |
kcat/Km (M−1 · s−1)
|
|||||
|---|---|---|---|---|---|---|---|---|
| BJP-1 | L1 | GOB-1 | FEZ-1 | CAU-1 | THIN-B | |||
| Ampicillin | 13 | 670 | 1.9 × 104 | 4.4 × 106 | -b | 1.1 × 104 | 5.0 × 105 | 3.7 × 105 |
| Penicillin G | 18 | 130 | 1.3 × 105 | 2.2 × 107 | 1.9 × 106 | 1.1 × 105 | 4.5 × 105 | - |
| Piperacillin | 47 | 700 | 6.7 × 104 | 7.0 × 106 | 1.7 × 106 | 1.2 × 104 | 5.7 × 105 | 2.0 × 105 |
| Temocillin | NHc | NDd | ND | 1.2 × 106 | - | 1.3 × 104 | - | - |
| Ticarcillin | NH | ND | ND | 2.7 × 106 | 5.2 × 105 | 1.3 × 104 | - | - |
| Cephalothin | 133 | 230 | 5.8 × 105 | - | 6.7 × 105 | 2.5 × 106 | 4.3 × 105 | - |
| Cefoxitin | 10 | 140 | 7.1 × 104 | 5.5 × 105 | 2.5 × 105 | 2.7 × 105 | - | - |
| Cefuroxime | 58 | 115 | 5 × 105 | 2.7 × 106 | 9.8 × 105 | 6.6 × 106 | 1.4 × 104 | 2.8 × 106 |
| Cefotaxime | 41 | 300 | 1.4 × 105 | 2.6 × 106 | 8.5 × 105 | 2.4 × 106 | - | 2.0 × 106 |
| Ceftazidime | >3 | >700 | 4.3 × 103 | 1.8 × 105 | 7.6 × 105 | 4.0 × 103 | 2.0 × 103 | 1.4 × 105 |
| Ceftriaxone | >8 | >80 | 9.6 × 104 | - | - | - | - | - |
| Cefepime | >0.08 | >400 | 2 × 102 | 1.9 × 104 | 2.0 × 105 | 6.0 × 103 | - | 7.9 × 103 |
| CENTA | 114 | 19 | 6 × 106 | - | - | - | - | - |
| Imipenem | 15 | 260 | 6 × 104 | 7.3 × 105 | 6.6 × 105 | 2.0 × 105 | 2.0 × 105 | 1.5 × 106 |
| Meropenem | 156 | 190 | 8.3 × 105 | 4.5 × 106 | 5.3 × 106 | 5.0 × 105 | 2.6 × 105 | 5.0 × 106 |
| Faropenem | 2 | 245 | 6.8 × 103 | - | - | - | - | - |
| Aztreonam | NH | ND | ND | ND | ND | ND | ND | ND |
Kinetic parameters of the purified BJP-1 enzyme (standard deviations were below 10%). kcat/Km values of other subclass B3 enzymes are reported for comparison and are from the following references: L1 (14, 15), GOB-1 (3), FEZ-1 (24), CAU-1 (13), and THIN-B (12).
-, data not available.
NH, no hydrolysis detected with enzyme concentrations up to 850 nM.
ND, data could not be determined.
Overall, the functional properties of BJP-1 are very similar to those of some other subclass B3 enzymes, especially FEZ-1, CAU-1, and THIN-B. All these enzymes have a preference for meropenem over imipenem, narrow-spectrum cephalosporins over penicillins, and exhibit poor recognition of cefepime (a representative of zwitterionic expanded-spectrum cephalosporins).
Kinetic data are consistent with the limited effect of BJP-1 expression on E. coli susceptibility to some substrates (e.g., ceftazidime and cefepime, which are poor substrates for the enzyme), while this was less obvious with other β-lactams (e.g., piperacillin and cefotaxime, which are good substrates).
Inactivation of BJP-1 by metal chelators.
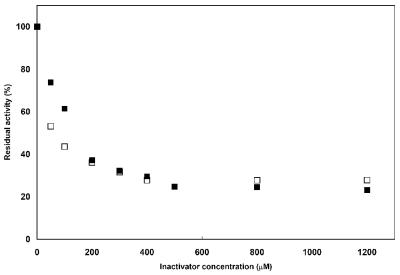
In inactivation experiments where the enzyme activity was recorded in the presence of various concentrations of the metal chelators (up to 50 mM with EDTA) and using meropenem as the reporter substrate, complete enzyme inactivation was not achieved within 30 min, suggesting low inactivation efficiencies (k+2/K) and, thus, a rather low susceptibility to these agents. In other experiments, where the enzyme activity was measured after preincubation with the metal chelators for 20 min, complete enzyme inactivation could not be observed, even at high inactivator concentrations. The most effective agent, o-phenanthroline, inhibited 80% of BJP-1 activity at a concentration of 500 μM, and no further inactivation was observed up to a concentration of 1.2 mM (Fig. 3). A similar behavior was observed for dipicolinic acid with a residual activity of 30% at 300 μM and no further changes up to 1.2 mM (Fig. 3). EDTA was the least efficient inactivator, and no more than 60% of the enzyme activity could be inhibited at concentrations as high as 50 mM (data not shown). This apparent poor sensitivity to chelating agents is a striking peculiarity of BJP-1, since all other enzymes of subclass B3 are readily inactivated by these agents (12, 13, 24). This behavior is apparently dependent on the protein structure and not on the presence of a metal cofactor other than zinc. A preliminary three-dimensional structure of the enzyme, solved using the anomalous signal present in the diffracted intensities of a data set collected with X-rays with a 1.2813-Å wavelength (corresponding to the zinc X-ray absorption edge peak), revealed the presence of the anomalous signal in the data, which clearly indicated that Zn ions were the metal cofactors in the enzyme. In addition, the anomalous difference electron density map computed from those data unambiguously showed that the enzyme possesses two zinc ions in the active site (S. Mangani, personal communication).
FIG. 3.
Inactivation of BJP-1 with dipicolinic acid (empty squares) and o-phenanthroline (full squares).
The structural basis for the slow and incomplete inactivation of BJP-1 by metal chelators remains unclear. With most subclass B3 enzymes, the inactivation by metal chelators was explained by a direct scavenging of the metal ions (the inactivation rate was independent of the inactivator concentration). One exception was represented by THIN-B, whose inactivation followed the formation of a enzyme-metal-inactivator ternary complex, as observed with subclass B1 and B2 enzymes (12). Considering this model, the behavior of BJP-1 might be explained by a rather high velocity constant for the formation of the ternary complex from the apoenzyme and the metal-inactivator complex (k−2), with subsequent release of the active form of the enzyme. This would result in the establishment of an equilibrium between the apoenzyme, the ternary complex, and the active metalloenzyme, explaining the significant amount of residual activity observed.
Concluding remarks.
Among 57 proteins encoded by the 12 complete genomes of Rhizobiales, four proteins were retained for analysis and only one was found to be a functional MBL, which also showed the highest similarity with subclass B3 enzymes. The other enzymes investigated (AT1, ML1, and SM2) did not exhibit β-lactamase activity when produced in E. coli. This suggests that functional MBLs might be present in a cluster of species but that this presence is not a conserved feature that might be extended to microorganisms belonging to the same order or family. The phylogenetic analysis presented in this study can give additional clues of the putative functions of some other MBL homologues, due to their relationships with enzymes for which biochemical function has been ascertained (e.g., BJ1 is related to glyoxalases II, BJ7 to N-acyl-homoserine lactone hydrolases, BJ9 to methyl parathion hydrolases) (Fig. 1).
From the functional standpoint, BJP-1 and CAU-1 shared overall low affinities for β-lactam substrates. It has been hypothesized that CAU-1 might be involved in another metabolic process due to its peculiar genetic context characterized by overlapping ORFs, clearly showing its association with genes encoding proteins involved in methionine biosynthesis. However, the structure of the genetic locus flanking blaBJP-1 is not suggestive of a similar situation. Upstream of the blaBJP-1 gene, there are two ORFs that encode proteins of unknown function which do not exhibit any significant similarity with other known bacterial proteins, while a gene encoding a putative transmembrane signal transduction protein is found downstream. Moreover, it does not seem associated with any of the numerous insertion sequences found in the B. japonicum genome (20). The presence of a functional β-lactamase in a plant symbiont remains enigmatic and we cannot exclude the possibility that it might have another function.
In conclusion, BJP-1 is a new member of the growing subclass B3 of MBLs which exhibits peculiar functional features and represents an interesting model to further investigate the structure-function relationships among subclass B3 enzymes. This comparative analysis might lead to critical insights potentially useful for the design of inhibitors, and more generally, this approach could help to identify the structural features ruling the substrate specificity in the MBL superfamily, whose members evolved very different functions from a conserved structural topology.
Acknowledgments
This work was supported by grants of the European Union (contract no. HPRN-CT-2002-00264 and LSHN-CT-2003-503335). M.S. is supported by the European Research Network on Metallo-β-lactamases (MEBEL) (contract no. HPRN-CT-2002-00264). J.-D.D. is a postdoctoral fellow of the Belgian Fonds National de la Recherche Scientifique.
Thanks are due to Patrick Elia for kindly providing the B. japonicum USDA110 strain.
REFERENCES
- 1.Aravind, L. 1999. An evolutionary classification of the metallo-β-lactamase fold proteins. In Silico Biol. 1:69-91. [PubMed] [Google Scholar]
- 2.Bebrone, C., C. Moali, F. Mahy, S. Rival, J. D. Docquier, G. M. Rossolini, J. Fastrez, R. F. Pratt, J. M. Frère, and M. Galleni. 2001. CENTA as a chromogenic substrate for studying β-lactamases. Antimicrob. Agents Chemother. 45:1868-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellais, S., D. Aubert, T. Naas, and P. Nordmann. 2000. Molecular and biochemical heterogeneity of class B carbapenem-hydrolyzing β-lactamases in Chryseobacterium meningosepticum. Antimicrob. Agents Chemother. 44:1878-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 5.Bicknell, R., E. L. Emanuel, J. Gagnon, and S. G. Waley. 1985. The production and molecular properties of the zinc β-lactamase of Pseudomonas maltophilia IID 1275. Biochem. J. 229:791-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush, K. 1999. β-Lactamases of increasing clinical importance. Curr. Pharm. Des. 5:839-845. [PubMed] [Google Scholar]
- 7.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callebaut, I., D. Moshous, J. P. Mornon, and J. P. De Villartay. 2002. Metallo-β-lactamase fold within nucleic acids processing enzymes: the β-CASP family. Nucleic Acids Res. 30:3592-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31:3497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daiyasu, H., K. Osaka, Y. Ishino, and H. Toh. 2001. Expansion of the zinc metallo-hydrolase family of the β-lactamase fold. FEBS Lett. 503:1-6. [DOI] [PubMed] [Google Scholar]
- 11.Docquier, J. D., J. Lamotte-Brasseur, M. Galleni, G. Amicosante, J. M. Frère, and G. M. Rossolini. 2003. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 51:257-266. [DOI] [PubMed] [Google Scholar]
- 12.Docquier, J. D., T. Lopizzo, S. Liberatori, M. Prenna, M. C. Thaller, J. M. Frère, and G. M. Rossolini. 2004. Biochemical characterization of the THIN-B metallo-β-lactamase of Janthinobacterium lividum. Antimicrob. Agents Chemother. 48:4778-4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Docquier, J. D., F. Pantanella, F. Giuliani, M. C. Thaller, G. Amicosante, M. Galleni, J. M. Frère, K. Bush, and G. M. Rossolini. 2002. CAU-1, a subclass B3 metallo-β-lactamase of low substrate affinity encoded by an ortholog present in the Caulobacter crescentus chromosome. Antimicrob. Agents Chemother. 46:1823-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felici, A., and G. Amicosante. 1995. Kinetic analysis of extension of substrate specificity with Xanthomonas maltophilia, Aeromonas hydrophila, and Bacillus cereus metallo-β-lactamases. Antimicrob. Agents Chemother. 39:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felici, A., G. Amicosante, A. Oratore, R. Strom, P. Ledent, B. Joris, L. Fanuel, and J. M. Frère. 1993. An overview of the kinetic parameters of class B β-lactamases. Biochem. J. 291:151-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J. M. Frère. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Saez, I., P. S. Mercuri, C. Papamicael, R. Kahn, J. M. Frère, M. Galleni, G. M. Rossolini, and O. Dideberg. 2003. Three-dimensional structure of FEZ-1, a monomeric subclass B3 metallo-β-lactamase from Fluoribacter gormanii, in native form and in complex with D-captopril. J. Mol. Biol. 325:651-660. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Valladeres, M., A. Felici, G. Weber, H. W. Adolph, M. Zeppezauer, G. M. Rossolini, G. Amicosante, J. M. Frère, and M. Galleni. 1997. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36:11534-11541. [DOI] [PubMed] [Google Scholar]
- 19.Jacoby, G. A., and L. S. Munoz-Price. 2005. The new β-lactamases. N. Engl. J. Med. 352:380-391. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1979. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frère, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauretti, L., M. L. Riccio, A. Mazzariol, G. Cornaglia, G. Amicosante, R. Fontana, and G. M. Rossolini. 1999. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 43:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercuri, P. S., F. Bouillenne, L. Boschi, J. Lamotte-Brasseur, G. Amicosante, B. Devreese, J. Van Beeumen, J. M. Frère, G. M. Rossolini, and M. Galleni. 2001. Biochemical characterization of the FEZ-1 metallo-β-lactamase of Legionella gormanii ATCC 33297T produced in Escherichia coli. Antimicrob. Agents Chemother. 45:1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing; 14th informational supplement. NCCLS document M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 27.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 29.Studier, F. W. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41:207-234. [DOI] [PubMed] [Google Scholar]
- 30.Ullah, J. H., T. R. Walsh, I. A. Taylor, D. C. Emery, C. S. Verma, S. J. Gamblin, and J. Spencer. 1998. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 A resolution. J. Mol. Biol. 284:125-136. [DOI] [PubMed] [Google Scholar]
- 31.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]