During the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy (Washington, D.C., 16 to 19 December 2005), several roundtable speakers mentioned that the experience in Greece regarding the use of colistin for the treatment of patients with multidrug-resistant gram-negative bacterial infections is encouraging. However, some speakers suggested that the good results reported by investigators in Greece might be related to the “high daily dosage” of the intravenously administered colistin. We would like to clarify the issue of daily dosage of intravenous colistin, trying to help clinicians with the confusion regarding the recommended dosage of colistin on the two sides of the Atlantic Ocean.
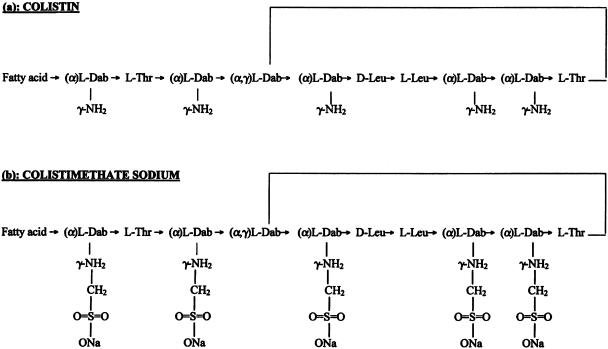
The average and maximum daily doses of intravenous colistin used in our patients at the Henry Dunant Hospital in Athens, Greece, have been 4.5 and 9 million IU (3). In our hospital, we have used the colistin product manufactured and distributed by Norma, Hellas SA, Athens, Greece, as well as the product manufactured by Alpharma A/S, Copenhagen, Denmark (now Actavis Group, Iceland), and distributed by Forest Laboratories, Kent, United Kingdom. The amount of colistin included in a vial of each of these products (80 mg) refers to colistimethate sodium and not to colistin base (2). In contrast, the amount of colistin included in a vial of the product manufactured in the United States by Parkedale Pharmaceuticals, Inc., Rochester, Mich., and distributed by Monarch Pharmaceuticals, Inc., Bristol, Tenn. (150 mg), refers to colistin base (4). One milligram of colistin base is contained in 2.4 mg of colistimethate sodium. Thus, the average and maximum daily dosages of colistin administered in patients in the United States are not less than the respective daily dosages administered in our patients. Figure 1 shows the molecules of colistin and colistimethate sodium.
FIG. 1.
Molecules of colistin and colistimethate sodium.
The above-described data suggest that the best way to avoid confusion related to the dosing of colistin is to base the doses on international units of the drug. Moreover, labeling of the different colistin formulations based on international units would probably help to avoid further confusion when dosing the drug. It should be emphasized that the definition of an international unit of the drug is biological; specifically, 1 IU of colistin is defined as the amount of colistin that inhibits the growth of Escherichia coli 95 I.S.M. in 1 ml broth at pH 7.2 (1). Pure colistin base has been assigned a potency of 30,000 IU per mg, while colistimethate sodium has a potency of 12,500 IU per mg. Thus, recommendations regarding dosing of colistin should clearly refer to either colistin base or colistimethate sodium to avoid possible confusion. In addition, the number of international units of colistin in each vial should be clearly included in the labeling information of the various colistin formulations manufactured and distributed by different pharmaceutical companies around the world.
REFERENCES
- 1.Anonymous. 2002. Colistin: summary report. EMEA/MRL/016/95-Final. Veterinary Medicines and Inspections, European Agency for the Evaluation of Medical Products, London, United Kingdom.
- 2.Forest Laboratories. 2002. Colomycin (package insert). Forest Laboratories, UK Limited, Bexley, Kent, United Kingdom.
- 3.Kasiakou, S. K., A. Michalopoulos, E. S. Soteriades, G. Samonis, G. J. Sermaides, and M. E. Falagas. 2005. Combination therapy with intravenous colistin for management of infections due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Antimicrob. Agents Chemother. 49:3136-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monarch Pharmaceuticals Inc. 2002. Coly-mycin M parenteral (package insert). Monarch Pharmaceuticals, Inc., Bristol, Tenn.