Abstract
The aromatic diamidine pentamidine has long been used to treat early-stage human African trypanosomiasis (HAT). Two analogs of pentamidine, DB75 and DB820, have been shown to be more potent and less toxic than pentamidine in murine models of trypanosomiasis. The diphenyl furan diamidine, DB75, is the active metabolite of the prodrug DB289, which is currently in phase III clinical trials as a new orally active candidate drug to treat first-stage HAT. The new aza analog, DB820, is the active diamidine of the prodrug DB844, currently undergoing preclinical evaluation as a new candidate to treat HAT of the central nervous system. The exact mechanisms of antitrypanosomal activity of aromatic dications remain poorly understood, with multiple mechanisms hypothesized. Pentamidine is known to be actively transported into trypanosomes and binds to DNA within the nucleus and kinetoplast. A long-hypothesized mechanism of action has been that DNA binding ultimately leads to interference with DNA-associated enzymes. Both DB75 and DB820 are intensely fluorescent, which provides an important tool for determining the kinetics of accumulation and intracellular distribution in trypanosomes. We show in the current study that DB75 and DB820 rapidly accumulate and strongly concentrate within trypanosomes, with intracellular concentrations over 15,000-fold higher than mouse plasma concentrations. Both compounds initially accumulate in the DNA-containing nucleus and kinetoplast, but at later time points, they concentrate in non-DNA-containing cytoplasmic organelles. Analyses of the kinetics of uptake and intracellular distribution are necessary to begin to define antitrypanosomal mechanisms of action of DB75, DB820, and other aromatic diamidines.
Human African trypanosomiasis (HAT), a devastating disease caused by Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense, has the potential to affect 60 million people in sub-Saharan Africa (27). HAT has two stages: an early stage in which trypanosomes are confined to the bloodstream and other extracellular fluids and a late stage in which trypanosomes are able to cross the blood-brain barrier and invade the central nervous system (CNS). The symptoms of early-stage HAT are general and include intermittent fever, malaise, and lymphadenopathy. Infections caused by T. b. gambiense and T. b. rhodesiense differ primarily in the manifestations of the disease. The Gambian form of the disease is a chronic disease which takes years to progress, while Rhodesian trypanosomiasis is an acute infection in which the late stage develops only a few weeks or months following infection (26).
Four drugs are currently available for the treatment of HAT, all of which are associated with problems, including toxicity, increasing incidences of treatment failure, and route of administration (14, 15). Pentamidine and suramin are used for the treatment of early-stage trypanosomiasis. Pentamidine is used as a first-line treatment for infections caused by T. b. gambiense, and suramin is used to treat T. b. rhodesiense infections (11, 15). Melarsoprol and eflornithine are used for CNS or second-stage infections. Melarsoprol is the drug most often used to treat second-stage CNS infections caused by both parasites. However, melarsoprol is associated with increasing reports of treatment failure (up to 30% in certain areas of central Africa), and it causes a reactive encephalopathy which can be fatal in up to 10% of treated patients (15). Eflornithine, also known as DFMO, is used against only T. b. gambiense CNS infections (15). Additionally, all of the treatments currently used must be administered by parenteral injection, which is difficult in the rural areas affected by trypanosomiasis (14). Thus, due to the problems associated with current therapy, the need for new treatments is very pressing.
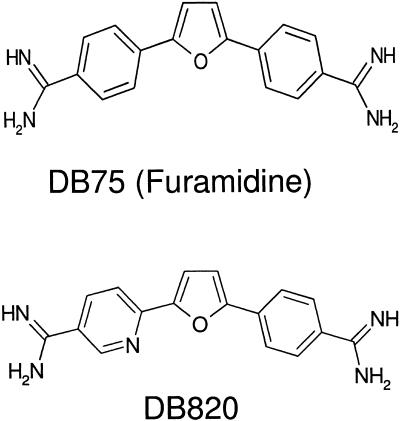
DB75 [2,5-bis(4-amidinophenyl)furan], also known as furamidine (Fig. 1), is the active metabolite of DB289 [2,5-bis(4-amidinophenyl)furan-bis-O-methylamidoxime], a prodrug designed to orally treat HAT (T. b. gambiense is the parasite causing the current epidemic). DB289 recently began phase III clinical trials in the Democratic Republic of Congo and Angola, and a trial has started recruiting patients in southern Sudan. DB75 is a diamidine analog of pentamidine in which the alkoxy chain linking the phenyl rings has been replaced with a furan ring. DB75 has shown excellent in vitro and in vivo activity in mouse and monkey models of first-stage infection (4, 7, 11, 13). DB820 {6-[5-(4-phenylamidinophenyl)-furanyl-2-yl]-nicotinamide}, is an aza analog of DB75. DB820 (Fig. 1) is more potent than DB75 in the T. b. rhodesiense STIB 900 model of first-stage infection, despite having similar in vitro activity against that strain (13). DB844, the O-methylamidoxime prodrug of DB820, has excellent activity in mouse and monkey models of CNS trypanosomiasis. DB844 is a leading candidate for the development of a new oral treatment for CNS trypanosomiasis.
FIG. 1.
Chemical structures of DB75, also known as furamidine, and DB820, antitrypanosomal diamidine derivatives of pentamidine.
Previous studies have shown that pentamidine is actively transported into trypanosomes via the P2, HAPT1, and LAPT1 transporters, with very high concentrations accumulating within trypanosomes (6, 8). Another diamidine, diminazene, has been shown to be transported into trypanosomes primarily by the P2 transporter (9). Little is known, however, about the intracellular distribution of pentamidine, particularly over the time periods required for this slow-acting compound to kill trypanosomes. The lack of fluorescence of pentamidine prevents microscopy studies which could determine localization. The structural modifications of DB75 and DB820 have increased the fluorescence emission of these compounds upon exposure to UV light. Therefore, we are currently able to combine studies of accumulation with studies of distribution in trypanosomes.
The mechanisms of action of DB75 and DB820 as well as those of many other diamidines are not known, and using fluorescence microscopy, we can evaluate distributions, to identify sites of action or potential sites of loss for the compounds in trypanosomes. In this study, T. b. brucei strain S427 was used to investigate the intracellular accumulation and distribution of the UV fluorescent DB75 and DB820 using high-pressure liquid chromatography (HPLC) analysis and fluorescence microscopy.
This study determined that both DB75 and DB820 achieve millimolar concentrations in trypanosomes at each respective time at which the maximum concentration is achieved. DB75 and DB820 also demonstrate a progression in their intracellular distribution. These compounds first accumulate in the nucleus and kinetoplast, which are the DNA-containing organelles of trypanosomes. By later time points, both are also found in organelles that are believed to be the acidocalcisomes as well as other non-DNA-containing organelles. Accumulation at multiple sites in trypanosomes may be crucial to understanding the mechanisms of action of DB75, DB820, and other diamidines.
MATERIALS AND METHODS
Materials.
Complete Baltz's modified essential medium (CBMEM) was prepared from minimal essential medium supplemented with 100 mM hypoxanthine, 2 mM adenosine, 10 mM thymidine, 2 mM sodium pyruvate, 2 mM l-glutamine, 2-mercaptoethanol, and 10% vol/vol heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA). HPLC-grade water and acetonitrile were obtained from Fisher Scientific (Pittsburgh, PA). All chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO) unless otherwise specified.
Parasites.
T. b. brucei strain S427 was cultivated in CBMEM medium as described previously (2). Trypanosomes were maintained in Corning 24-well plates (Fisher Scientific) and were subpassaged every 3 to 5 days when trypanosomes reached a density of 105 to 106 trypanosomes per milliliter.
Antitrypanosomal agents.
DB75 dihydrochloride salt was synthesized by Medichem (Chicago, IL) using previously described methods (5, 7). DB820 trihydrochloride salt was synthesized in the laboratory of David Boykin as previously described (13). For in vitro testing, DB75 and DB820 were prepared as 1-mM stocks in sterile water and, for in vivo testing, the compounds were prepared in sterile saline (0.9% weight/vol).
In vitro antitrypanosomal activity.
In order to determine 50% inhibitory concentration (IC50) values, S427 trypanosomes (2 × 103 per ml) were cultured with serial dilutions of DB75 or DB820 for 70 h using a method described by Raz et al. (20). Alamar Blue (10% vol/vol) (Biogen International, Camarillo, CA) was then added, and trypanosomes were further incubated for 2 h. The fluorescence of Alamar Blue was measured using a PolarStar fluorescence plate reader (BMG, Durham, NC) using an excitation wavelength of 544 nm and an emission wavelength of 588 nm. Fluorescence emission at this wavelength is an indication of cell viability (20). The fluorescence emission derived from each drug concentration was normalized to the fluorescence of the untreated control trypanosomes. The background fluorescence (determined in wells in which 10% Alamar Blue was added to CBMEM medium) was subtracted from total fluorescence values. Dose-response curves were generated from the Alamar Blue fluorescence shift using Prism (GraphPad Software, San Diego, CA). Experiments were performed in triplicate. IC50 values were determined using the Hill equation.
In vitro accumulation in trypanosomes.
To determine in vitro accumulation, 106 trypanosomes were incubated with either 7.5 μM DB75 or 7.5 μM DB820 for time periods ranging from 1 to 24 h. At each time point, cells were washed twice in fresh drug-free medium, lysed, and extracted in 4 volumes of methanol-0.1 N HCl (8:1 vol/vol). Half of each sample was evaporated in a Zymark (Hopkinton, MA) TurboVap-LV under nitrogen gas (7 lb/in2) and reconstituted in 50 μl of HPLC solvent A (described in detail in “HPLC analysis of DB75 and DB820” below). Concentrations were determined as described below.
In vivo antitrypanosomal activity.
Dose escalation studies in infected mice were used to determine in vivo antitrypanosomal activity. All animal experiments adhered to guidelines outlined by the University of North Carolina—Chapel Hill Institutional Animal Care and Use Committee. Each test group consisted of six mice obtained from Charles River (Wilmington, MA). Male Swiss Webster (20 to 30 g) mice were inoculated with 105 S427 trypanosomes intraperitoneally on day 0. On day 3, mice were treated with a single intravenous (IV) dose (ranging from 0.63 to 10 μmol/kg of body weight) of either compound. The compounds were administered in 0.9% (wt/vol) saline. The dose volume administered to each mouse was less than 0.2 ml. Untreated mice were administered the same saline vehicle. A drop of tail vein blood from each mouse was collected and examined for trypanosomes using a light microscope (×40, magnification) daily for 2 weeks posttreatment. After that, tail vein blood was examined twice a week for 60 days. Mice were considered cured when no parasites were found in blood after the 60-day period. Untreated mice typically died within 5 to 6 days of infection. The percentage of animals cured in each test group was used to determine the dose-response curve. Fifty-percent-effective-dose (ED50) values were calculated in Prism using the Hill equation.
Plasma concentrations of DB75 and DB820 in infected mice.
Plasma concentrations of DB75 and DB820 were determined in infected animals. In these experiments, mice were treated with intravenous 7.5 μmol/kg of DB75 or DB820 on the third day after infection. Parasitemia was typically about 107 to 108 trypanosomes per milliliter. Animals with lower levels of parasitemia were excluded from the experiments before treatment.
Plasma samples were prepared as described previously (24), with the following modifications. Mice were euthanized by CO2 asphyxiation, blood was collected by cardiac puncture, and the blood was separated into two lithium heparin-coated BD Microtainer tubes (Fisher Scientific), each with about 400 to 500 μl of blood. One Microtainer tube of the blood was used for determining DB75 and DB820 plasma concentrations after an IV dose of 7.5 μmol/kg through the tail vein, and the second was used to determine concentrations of DB75 and DB820 in trypanosomes isolated from the infected mice in certain experiments.
Blood was centrifuged at 3,000 rpm (1,500 × g) in a Marathon 8K centrifuge (Fisher Scientific) for 5 min to separate plasma from red blood cells. Plasma was removed, ensuring that the buffy coat was undisturbed. The buffy coat contains white blood cells and trypanosomes in infected animals. Approximately 5 to 10 μl of plasma was examined under a light microscope (×40 to ×100 magnification) to ensure that it was not contaminated with trypanosomes. DB75 and DB820 were extracted from plasma in 4 volumes of methanol-0.1 N HCl (8:1 vol/vol), vortexed briefly, and centrifuged at 3,000 rpm (1,500 × g) for 5 min. A total of 100 μl of supernatant was removed and evaporated as described previously (24). The residue was reconstituted in 50 μl of HPLC solvent A (for each respective compound) and transferred to 200 μl polypropylene inserts for use in 2.0-ml glass HPLC vials (Agilent Technologies, Palo Alto, CA). Samples were analyzed by HPLC as described below.
Area under the concentration-time curve (AUC) and half life (t1/2) values were determined from plasma concentrations using noncompartmental analysis in WinNonlin 4.1 (Pharsight, Mountain View, CA). The t1/2 value was calculated from the terminal elimination rate constant calculated between 4 and 24 h. The AUC was not extrapolated beyond 24 h as concentrations in plasma were often below the limit of quantification after this time (21).
In vivo accumulation of DB75 and DB820 in trypanosomes.
Male Swiss Webster mice were infected with 105 trypanosomes per ml intraperitoneally. On the third day postinfection, mice were treated with DB75 or DB820 by a single tail vein injection at a dose level of 7.5 μmol/kg. This dose level is curative for both compounds in this model of infection. At various time points after treatment, mice were euthanized by CO2 inhalation, and blood was collected by cardiac puncture. The trypanosomes were collected by centrifuging the blood on a Percoll (Sigma) gradient as described previously by Grab and Bwayo (12), with minor modifications to the centrifugation conditions. Briefly, blood was mixed with an equal volume of 100% Percoll supplemented with 0.25 M sucrose and 0.11 M glucose, in 2.0-ml centrifuge tubes. The blood and Percoll mixture was centrifuged for 16,100 × g for 35 min in an Eppendorf 5415R microcentrifuge (Fisher Scientific). Trypanosomes were removed from the supernatant, washed twice, and counted. Compounds were extracted from the trypanosomes using 4 volumes of methanol-0.1 N HCl (8:1 vol/vol). Samples were evaporated under nitrogen gas (7 lb/in2) in a Zymark TurboVap and resuspended in 100 μl of HPLC solvent A for each respective compound. The average concentration of DB75 and DB820 in trypanosomes was determined using an estimated cell volume of 58 μm3 (or 58 fl) for the S427 trypanosome (17). The AUC from 0 to 24 h (AUC0—24) and t1/2 values were calculated from trypanosome concentrations using noncompartmental analysis in WinNonlin 4.1. As with plasma, the t1/2 value was calculated from the terminal elimination rate constant between 4 and 24 h.
Intracellular localization of DB75 and DB820 in trypanosomes.
To determine the localization of DB75 and DB820 in trypanosomes, blood smears were made using a drop of tail blood at each time point after treatment with DB75 or DB820. Blood smears prepared from untreated mice were also examined. Blood smears were examined using a Nikon (Garden City, NJ) Microphot FXA with an objective lens (×60 dark medium, 1.4 numerical aperture), a mercury lamp, and an Optronics (Goleta, CA) DEI 750 charge-coupled device camera. The microscope was equipped with a Nikon UV2A cube that limits excitation wavelengths to 330 to 380 nm and emission wavelengths to ≥420 nm.
HPLC analysis of DB75 and DB820.
Analytical methods for DB75 were conducted as described previously (24). Briefly, the solvent system for DB75 analysis consisted of solvent A (15 mM ammonium formate-30 mM formic acid in 100% HPLC-grade water) and solvent B had the same components in 4:1 acetonitrile-HPLC-grade water. A gradient elution was used to resolve DB75, with a starting concentration of 88% solvent A-12% solvent B. This gradient increased linearly to 80% solvent B over 40 min, followed by an 8-min reequilibration time to initial solvent conditions.
Analytical methods for DB820 were slightly different. For DB820, solvent A contained 30 mM ammonium formate-60 mM formic acid in 100% HPLC-grade water, and solvent B was the same buffer in 4:1 acetonitrile-HPLC-grade water. A gradient elution was used to resolve the compound, with a starting concentration of 94% solvent A-6% solvent B. This gradient increased linearly to 80% solvent B over 40 min, followed by reequilibration back to starting concentrations by 48 min.
An Agilent 1100 series HPLC system equipped with a fluorescence detector (Agilent Technologies) was used for analytical procedures. DB75 and DB820 were eluted on a 5.0-μm Bonus-RP (Agilent Technologies) 2.1-by-150-mm column with a flow rate of 0.35 ml/min. The column was maintained at 40°C throughout the analytical method. The injection volume for each sample was 25 μl. The excitation and emission wavelengths used for fluorescence detection were 365 and 462 nm, respectively. Compounds were quantified by comparing to standards injected during each analytical run. This was used to determine the concentration of DB75 and DB820 in plasma and in trypanosomes. Concentrations for both compounds were expressed as μM and mM for plasma and trypanosomes, respectively. Concentrations are reported as plus or minus the standard errors (SEs).
RESULTS
In vitro activity of DB75 and DB820.
Both DB75 and DB820 had potent activity against the T. b. brucei S427 strain. The IC50 values were less than 10 nM (Table 1). The related compound pentamidine was slightly more potent than DB75 and DB820 in vitro with an IC50 value of 1.1 nM.
TABLE 1.
In vitro and in vivo efficacies of compounds against S427 trypanosomes
| Compound | In vitro IC50 (nM) | Dosea (μmol/kg) | Cure rate (%) | Mean survival time (days) |
|---|---|---|---|---|
| Pentamidine | 1.1 | 10 | 67 | 51 |
| 5 | 67 | 50 | ||
| 1.25 | 0 | 10 | ||
| 0.63 | 0 | 7 | ||
| DB75 | 3.4 | 10 | 100 | 60 |
| 5 | 67 | 47 | ||
| 1.25 | 36 | 37 | ||
| 0.63 | 17 | 24 | ||
| DB820 | 7.3 | 10 | 100b | 60 |
| 7.5 | 83 | 58 | ||
| 5 | 75 | 49 | ||
| 1.25 | 25 | 35 | ||
| 0.63 | 0 | 9 |
All single doses.
One animal was excluded from test group due to death on the day following treatment. The death may have been related to toxicity.
In vitro accumulation of DB75 and DB820.
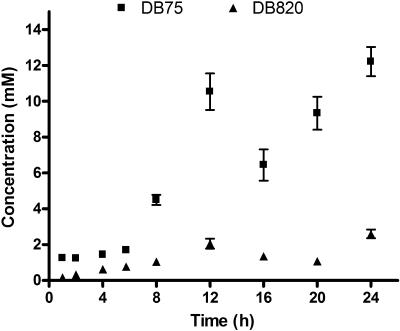
Trypanosomes growing in vitro were exposed to DB75 or DB820 at concentrations of 7.5 μM, approximately 1,000 times the IC50 values. This concentration is similar to that used in previous studies with pentamidine (5). Uptake of both DB75 and DB820 increased over time, with millimolar concentrations in trypanosomes at 24 h (Fig. 2). There appear to be two rises in accumulation for both compounds; after an initial rise in concentrations, there is a slight plateau before concentrations rise again. Although DB75 and its aza analog had similar in vitro activities, DB75 accumulated to a greater extent in trypanosomes than DB820 did over the 24 h period. At 24 h, trypanosomes accumulated DB75 to a concentration of 12.2 ± 0.8 mM (± SE), or 70.8 ± 4.7 nmol/108 trypanosomes, almost five times the concentration of DB820 in trypanosomes at that time (2.6 ± 0.2 mM or 15.0 ± 1.4 nmol/108 trypanosomes). Thus, DB820 and DB75 were concentrated inside trypanosomes approximately 400- to 2,000-fold the extracellular incubation concentration at 24 h.
FIG. 2.
In vitro accumulation of DB75 (▪) and DB820 (▴) over 24 h (n = 3). Trypanosomes were incubated with 7.5 μM of either compound. Concentrations are presented ± SE.
In vivo activity of DB75 and DB820.
In vivo efficacy experiments showed that DB75 and DB820 were both very potent in vivo, with comparable dose-response effects in terms of cure rates and survival times (Table 1). A single IV dose of either DB75 or DB820 at 10 μmol/kg cured all mice infected with T. b. brucei S427. Cure was defined as survival with no parasitemia for 60 days after treatment. ED50 values calculated from the cure rates were comparable (1.3 μmol/kg for DB75 and 1.6 μmol/kg for DB820). Pentamidine was less active in vivo, failing to completely cure all animals at 10 μmol/kg IV. The extrapolated ED50 value for pentamidine was 3.2 μmol/kg. This ED50 value is higher than that previously reported for pentamidine against the S427 strain, which was between 0.9 and 1.0 μmol/kg (or 0.5 to 0.6 mg/kg) (3). However, in this experiment, mice were infected with trypanosomes and treatment was begun 24 h later. In the experiments reported here, treatment was begun 3 days postinfection, which is a more stringent efficacy model. The values reported here are still very similar to those determined previously.
In vivo accumulation of DB75 and DB820 in trypanosomes and plasma.
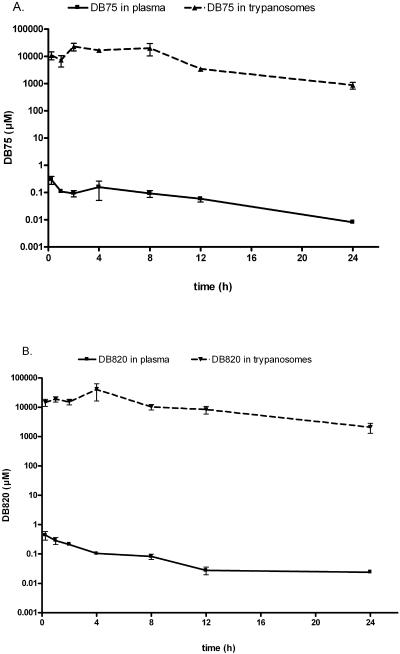
Mice infected with T. b. brucei S427 were dosed with DB75 or DB820 at 7.5 μmol/kg IV. Mouse plasma concentrations and trypanosome intracellular concentrations for both compounds were measured over a 24-h period (Fig. 3).
FIG. 3.
Concentration of DB75 (A) and DB820 (B) in S427 trypanosomes and plasma after intravenous administration of 7.5 μmol/kg of DB75 or DB820. Experiments were performed with 3 to 6 mice per time point. Concentrations in trypanosomes were determined based on a volume determination of 58 μm3, as determined by Opperdoes et al. (17). Concentrations are presented ± SE.
Plasma concentrations of DB75 and DB820 in infected animals.
The plasma time concentration profiles for DB75 and DB820 in infected mice were similar (Fig. 3). The peak plasma concentration of DB75 in infected mice after IV administration was 295 ± 93 nM at 15 min posttreatment (Fig. 3A), the earliest time point measured. The AUC0—24 of DB75 in the plasma-infected mice was 1.8 μM · h. The plasma t1/2 of DB75 was 4.5 h. The peak concentration of DB820 in plasma was 442 ± 145 nM at 15 min posttreatment (Fig. 3B). The AUC for DB820 in infected plasma was 1.9 μM · h, with a t1/2 of 12.4 h. The half lives for DB75 and DB820 in plasma were calculated between 4 and 24 h.
In vivo accumulation of DB75 and DB820 in trypanosomes.
To determine concentrations of DB75 and DB820 in trypanosomes, trypanosomes were isolated from blood at multiple time points after the dosing. Concentrations of DB75 and DB820 that accumulated in trypanosomes after a single 7.5 μmol/kg dose are plotted in Fig. 3. Both compounds were rapidly taken up and concentrated within trypanosomes, with 0.5 mM intracellular concentrations at 15 min posttreatment, the earliest time point measured. DB75 peaked in trypanosomes at a concentration of 0.9 ± 0.2 mM (5.4 ± 1 nmol/108 trypanosomes) at 4 h after administration, approximately 6,000 times the corresponding plasma concentration. The AUC0—24 for DB75 in trypanosomes was approximately 9,400 μM · h. The concentration of DB820 peaked in trypanosomes at 4 h, with a maximum concentration of 1.8 ± 0.7 mM (9.6 ± 4.3 nmol/108 trypanosomes), which is about 17,000 times the concentration in plasma at 4 h. The AUC0—24 for DB820 in trypanosomes was approximately 17,000 μM · h. Both DB75 and DB820 concentrations in trypanosomes declined over time with half-lives of 4.9 and 6.4 h, respectively.
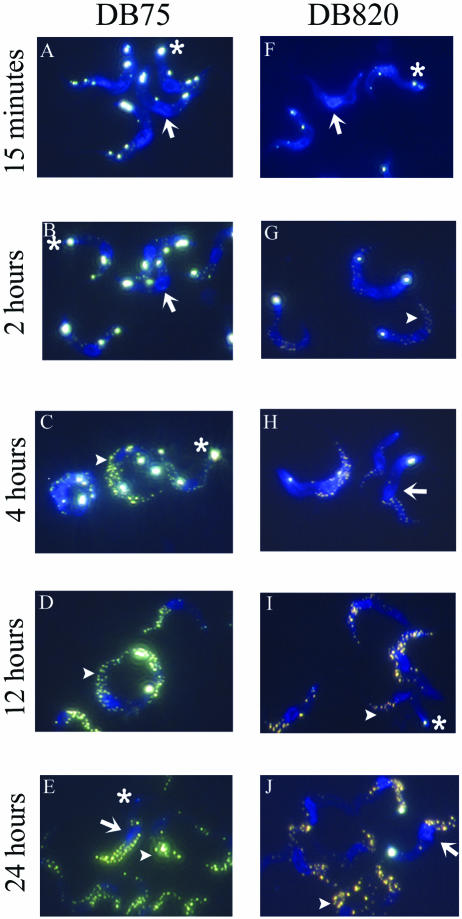
Intracellular distribution of DB75 and DB820 in trypanosomes.
Fluorescence microscopy was used to determine the intracellular distributions of DB75 and DB820 within organelles of trypanosomes. The fluorescence micrographs (Fig. 4) show that DB75 and DB820 rapidly accumulated in trypanosomes, concentrating initially in the DNA-containing nucleus and kinetoplast. At 15 min, when intracellular concentrations of DB75 and DB820 were approximately 0.5 mM, cell nuclei showed a bright blue fluorescence. The kinetoplast was also clearly visible at this early time point, with very intense white-yellow fluorescence observed (Fig. 4A and F). Nuclear and kinetoplast fluorescence is apparent at all time points examined to some degree, but the kinetoplast is not always yellow, as seen in Fig. 4E. Occasionally, some of the trypanosomes depicted at 24 h in the micrographs do not have fluorescent kinetoplasts (Fig. 4J). This appears to be a true phenomenon, with approximately one-fourth of trypanosomes in multiple fields showing no kinetoplast fluorescence at 24 h.
FIG. 4.
Fluorescence micrographs of DB75 and DB820 in trypanosomes after 7.5 μmol/kg intravenous dose of either compound. Panels A to E show selected time points from DB75-treated mice, while panels F to J are from DB820-treated mice. In select micrographs, the nucleus is represented by an arrow, the kinetoplast is depicted by an asterisk, and the acidocalcisomes are indicated by an arrowhead.
Additionally, DB75 appears in unidentified punctate organelles in the perinuclear region and at the tip of the trypanosome where the flagellum emerges as a yellow fluorescence (Fig. 4A). This also occurs with DB820 (Fig. 4F), but not as much as with DB75. As early as 2 h posttreatment, both compounds are found in organelles believed to be the acidocalcisomes based on a fluorescence pattern similar to acridine orange in trypanosomes (10). DB75 appears as a yellowish color in these organelles, while DB820 is a yellowish-orange color. The DB75- and DB820-accumulating organelles are usually concentrated at the posterior end of the trypanosome. By 4 h, the number of yellow fluorescent organelles increases (Fig. 4E and J) and the number remains that way through 24 h. It is interesting to note that after 4 h, concentrations of both compounds in trypanosomes begin declining.
DISCUSSION
DB75 and DB820 are potent analogs of pentamidine. Although all three have very similar in vitro activities, in vivo, it is clear that both DB75 and DB820 have better antitrypanosomal activity than pentamidine. Even at the highest dose tested, 20 μmol/kg IV of pentamidine, we were unable to cure the infection in all animals (unpublished results). Additionally, in the more stringent model of first-stage trypanosomiasis, the STIB 900 T. b. rhodesiense model, DB820 is the most potent of these three compounds (13). Both DB75 and DB820 can also be administered as lipophilic prodrugs, which enables an oral formulation for the treatment of trypanosomiasis (24). This is useful, especially for early-stage disease, because it eliminates the need for parenteral administration. DB289, the prodrug of DB75, is currently in phase III clinical trials for sleeping sickness. DB844, the prodrug of DB820, is an excellent drug candidate for the treatment of CNS trypanosomiasis. DB844 is able to cure mouse models infected with the chronic GVR35 strain of T. b. brucei after an oral dose of 5 × 100 mg/kg and is being investigated in further animal models.
In these studies, T. b. brucei accumulates millimolar concentrations of both DB75 and DB820 after both in vitro incubation and in vivo administration to infected mice (Fig. 2 and 3). The in vitro concentrations are very similar to concentrations previously reported by Carter et al. for pentamidine accumulation (6). They found that pentamidine was accumulated in 108 cells to a concentration of 1 mM, or 6.05 nmol/108 cells, after a 3-h in vitro incubation with 1 μM pentamidine. This concentration of pentamidine is approximately 1,000-fold the IC50 value. In the experiments presented here, trypanosomes were incubated with 7.5 μM of DB75 or DB820, which was approximately 1,000 times the IC50 value for both compounds, and both accumulated at millimolar levels in vitro over a 24-h period (Fig. 2). After 4 h, DB75 and DB820 are concentrated in trypanosomes at almost 200 times the initial concentration, whereas in Carter's experiments, pentamidine reaches levels inside trypanosomes that are approximately 1,000 times the incubation concentration.
The in vitro accumulation differences of pentamidine and DB75 and DB820 could be explained by the differing mechanisms for transport into trypanosomes. Pentamidine is transported into trypanosomes by three transporters, the high- and low-affinity pentamidine transporters (HAPT1 and LAPT1) and the P2 transporter (8). The P2 transporter has also been shown to accumulate DB75 and related diamidines (23), but to our knowledge, there is no evidence that HAPT1 or LAPT1 can transport DB75 and DB820 into trypanosomes. Therefore, pentamidine could have accumulated to a greater extent in trypanosomes due to the multiple routes of drug entry, which may not be available to DB75 and DB820. A more accurate comparison for DB75 and DB820 and related diamidines may be diminazene, which is taken up into trypanosomes almost exclusively through the P2 transporter (9).
DB75 and DB820 were shown to accumulate in trypanosomes to concentrations of 1-to-2-mM levels after in vivo treatment (Fig. 3). DB820 has a higher peak concentration and higher AUC0—24 in trypanosomes than does DB75. The same holds in plasma; DB820 had a higher peak concentration and slightly higher AUC0—24 in infected plasma than did DB75. For most time points, DB820 concentrations in infected plasma were higher than DB75 plasma concentrations. Plasma concentrations were also variable, which could be a result of the infection itself. At the peak concentration in trypanosomes, the organisms were able to accumulate between 6,000 and 17,000 times the plasma concentrations, which is a very large concentration factor.
Previously published data (6) suggests that the concentration of pentamidine in vivo in trypanosomes after a 4 mg/kg dose administered intraperitoneally to rats is approximately 0.84 mM, which was lower than what was shown here for DB75 and DB820. A 4-mg/kg dose of pentamidine is equivalent to approximately 6.75 μmol/kg of the isethionate salt, and therefore the dose of pentamidine administered by Carter et al. was slightly lower than the doses of DB75 and DB820 administered in our studies, which was 7.5 μmol/kg. Also, the route of administration used for pentamidine in those experiments was intraperitoneal injection. Thus, pentamidine required additional time to enter circulation and distribute into trypanosomes, and the peak concentration may not have occurred at their tested time point of 4 h. DB75 and DB820 were administered by intravenous injection, and do not require extra time to distribute into circulation. Both the lower dosage and the different route of administration could explain the lower concentration of pentamidine in trypanosomes at 4 h. It is also our experience that diamidines need to be administered at a much higher dose intraperitoneally to achieve comparable cure rates to a dose after intravenous administration. Although the routes of administration are different in the two studies and there are other confounding factors, it is clear that trypanosomes are able to accumulate high concentrations of diamidines, which lead to the killing of the organisms.
Diamidines, such as DB75, DB820, and pentamidine, are able to bind DNA in AT-rich regions of the minor groove (5, 28). In fact, one of the long-hypothesized mechanisms of action has been binding to DNA and/or interference with DNA-associated enzymes such as topoisomerase II (21, 22). We expected to, and did, see accumulation of DB75 and DB820 in the nucleus and the kinetoplast of trypanosomes, the two DNA-containing organelles. Although DB75 and DB820 bind to DNA in both the nucleus and the kinetoplast, it is not known for certain what effects this may have, or whether accumulation in one organelle has a greater impact on the death of the trypanosome. Future studies are planned to investigate whether different amounts of compound bind to the DNA in these organelles and how this affects the mechanism of action of the compounds.
Accumulation in other organelles, such as the organelles believed to be acidocalcisomes and several unknown perinuclear organelles, was unexpected. Accumulation in these organelles begins about 1 h after treatment is administered. This organelle accumulation could represent some unknown mechanism of action for diamidines, or it could be a source of loss for the compounds. If these organelles are acidocalcisomes, it is possible that the diamidines may have some kind of interaction with polyphosphates that are sequestered there. Acidocalcisomes have been postulated to be involved in many processes, including pH regulation and osmoregulation within trypanosomes. Disruption of these processes could potentially be a mechanism of action. Additionally, these diamidines could interfere in polyphosphate accumulation and hydrolysis in acidocalcisomes, a mechanism that has previously been shown to interfere with trypanosome virulence in animals (16). Supporting the idea of accumulation in the acidocalcisomes as a mechanism of action of these compounds is the investigation by Ormerod and Shaw (19). In this study, it was found that stilbamidine and hydroxystilbamidine accumulate in “volutin granules” in trypanosomes. One of these authors also noted a shift in the fluorescence of hydroxystilbamidine in these granules, much as is seen here with DB75 and DB820 (18). It is important to consider that there may be more than one mechanism of action for diamidine compounds as a class. Additionally, individual diamidines may display one or more of these mechanisms of action. In future studies, we hope to examine the mechanism of action for these and other diamidine compounds.
Another question that arises from the distribution micrographs shown in Fig. 3 is what could explain the shift in fluorescence seen with DB75 and DB820 in the kinetoplast, acidocalcisomes, and other organelles. DAPI (4′,6′-diamidino-2-phenylindole) has been postulated to undergo a red shift in its fluorescence emission when accumulated in volutin granules of yeast (1). Volutin granules or the acidocalcisomes in trypanosomes concentrate polyphosphates and pyrophosphates at high concentrations, and it has been hypothesized that diamidines such as DAPI interact with phosphates, leading to a shift in the fluorescence emission (25). It appears that the same phenomenon occurs with DB75 and DB820 in the posterior organelles, but this does not explain why there is a shift in the fluorescence emission in the kinetoplast. Potentially, two different mechanisms could cause the shifts in fluorescence in the organelles. Fluorescence scans of DB75 solutions prepared at low pH indicated no shift in the fluorescence spectrum to longer wavelengths; therefore the shift that occurs in these organelles probably does not occur due to low pH found inside any of the organelles (J. E. Hall, unpublished data).
The studies described here have opened many opportunities for the further study of diamidine compounds. The use of fluorescence to track the distribution of compounds in trypanosomes will enable us to examine uptake and intracellular accumulation as well as distribution and mechanism of action. When combined with HPLC analysis of concentrations of drugs in trypanosomes, this is an exciting technique to evaluate drugs and their antitrypanosomal activity. The studies described here will be extended to other compounds in a library of almost 2,000 structurally diverse diamidines in order to assess distribution and accumulation over time and how they relate to activity. Furthermore, using techniques to isolate trypanosome organelles, it will be possible to evaluate the accumulation of the compounds in each organelle in order to determine how it may relate to mechanism of action.
Acknowledgments
This work was supported by a grant from the Bill and Melinda Gates Foundation. A. M. Mathis was supported by a predoctoral fellowship from Amgen.
We are also grateful to Sam Black at the University of Massachusetts for the donation of the S427 T. b. brucei strain.
REFERENCES
- 1.Allan, R. A., and J. J. Miller. 1980. Influence of S-adenosylmethionine on DAPI-induced fluorescence of polyphosphate in the yeast vacuole. Can. J. Microbiol. 26:912-920. [DOI] [PubMed] [Google Scholar]
- 2.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, B. J., N. S. Carter, and A. H. Fairlamb. 1995. Characterisation of pentamidine-resistant Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 69:289-298. [DOI] [PubMed] [Google Scholar]
- 4.Bouteille, B., O. Oukem, S. Bisser, and M. Dumas. 2003. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 17:171-181. [DOI] [PubMed] [Google Scholar]
- 5.Boykin, D. 2002. Antimicrobial activity of the DNA minor groove binders furamidine and analogs. J. Braz. Chem. Soc. 13:763-771. [Google Scholar]
- 6.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153-28157. [DOI] [PubMed] [Google Scholar]
- 7.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 8.de Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512-522. [DOI] [PubMed] [Google Scholar]
- 9.de Koning, H. P., L. F. Anderson, M. Stewart, R. J. Burchmore, L. J. Wallace, and M. P. Barrett. 2004. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 48:1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Docampo, R., and S. N. Moreno. 1999. Acidocalcisome: A novel Ca2+ storage compartment in trypanosomatids and apicomplexan parasites. Parasitol. Today 15:443-448. [DOI] [PubMed] [Google Scholar]
- 11.Fairlamb, A. H. 2003. Chemotherapy of human African trypanosomiasis: current and future prospects. Trends Parasitol. 19:488-494. [DOI] [PubMed] [Google Scholar]
- 12.Grab, D. J., and J. J. Bwayo. 1982. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 39:363-366. [PubMed] [Google Scholar]
- 13.Ismail, M. A., R. Brun, J. D. Easterbrook, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2003. Synthesis and antiprotozoal activity of aza-analogues of furamidine. J. Med. Chem. 46:4761-4769. [DOI] [PubMed] [Google Scholar]
- 14.Jannin, J., and P. Cattand. 2004. Treatment and control of human African trypanosomiasis. Curr. Opin. Infect. Dis. 17:565-571. [DOI] [PubMed] [Google Scholar]
- 15.Legros, D., G. Ollivier, M. Gastellu-Etchegorry, C. Paquet, C. Burri, J. Jannin, and P. Buscher. 2002. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2:437-440. [DOI] [PubMed] [Google Scholar]
- 16.Lemercier, G., B. Espiau, F. A. Ruiz, M. Vieira, S. Luo, T. Baltz, R. Docampo, and N. Bakalara. 2004. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J. Biol. Chem. 279:3420-3425. [DOI] [PubMed] [Google Scholar]
- 17.Opperdoes, F. R., P. Baudhuin, I. Coppens, C. De Roe, S. W. Edwards, P. J. Weijers, and O. Misset. 1984. Purification, morphometric analysis, and characterization of the glycosomes (microbodies) of the protozoan hemoflagellate Trypanosoma brucei. J. Cell Biol. 98:1178-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ormerod, W. 1961. The study of volutin granules in trypanosomes. Trans. R. Soc. Trop. Med. Hyg. 55:313-332. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod, W., and J. Shaw. 1963. A study of granules and other changes in phase-contrast appearance produced by chemotherapeutic agents in trypanosomes. Br. J. Pharmacol. Chemother. 21:259-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro, T. A., and P. T. Englund. 1990. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. USA 87:950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro, T. A., V. A. Klein, and P. T. Englund. 1989. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J. Biol. Chem. 264:4173-4178. [PubMed] [Google Scholar]
- 23.Stewart, M. L., S. Krishna, R. J. Burchmore, R. Brun, H. P. de Koning, D. W. Boykin, R. R. Tidwell, J. E. Hall, and M. P. Barrett. 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366:486-487. [DOI] [PubMed] [Google Scholar]
- 24.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91:131-143. [DOI] [PubMed] [Google Scholar]
- 25.Tijssen, J. P., H. W. Beekes, and J. Van Steveninck. 1982. Localization of polyphosphates in Saccharomyces fragilis, as revealed by 4′,6-diamidino-2-phenylindole fluorescence. Biochim. Biophys. Acta 721:394-398. [DOI] [PubMed] [Google Scholar]
- 26.Welburn, S. C., and M. Odiit. 2002. Recent developments in human African trypanosomiasis. Curr. Opin. Infect. Dis. 15:477-484. [DOI] [PubMed] [Google Scholar]
- 27.WHO. March. 2001, posting date. African trypanosomiasis or sleeping sickness. WHO. [Online.] http://www.who.int/mediacentre/factsheets/fs259/en/.
- 28.Wilson W. D., B. Nguyen, F. Tanious, A. Mathis, J. E. Hall, C. Stephens, D. W. Boykin. 2005. Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution, and biological activity. Cur. Med. Chem. Anticancer Agents 5:389-408. [DOI] [PubMed] [Google Scholar]