Abstract
Brecanavir (BCV, 640385) is a novel, potent protease inhibitor (PI) with low nanomolar 50% inhibitory concentrations against PI-resistant human immunodeficiency virus (HIV) in vitro. This phase I, double-blind, randomized, placebo-controlled, two-part single-dose study (first time with humans) was conducted to determine the safety, tolerability, and pharmacokinetics of BCV administered at 10 mg/ml in a tocopherol-polyethylene glycol succinate-polyethylene glycol 400-ethanol 50:40:10 solution. In part 1 of the study, single oral doses of BCV ranged from 25 mg to 800 mg. In part 2, single oral doses of BCV ranged from 10 mg to 300 mg and were coadministered with 100-mg oral ritonavir (RTV) soft gel capsules. Single doses of BCV and BCV/RTV were generally well tolerated. There were no severe adverse events (SAEs), and no subject was withdrawn due to BCV. The most commonly reported drug-related AEs during both parts of the study combined were gastrointestinal disturbances (similar to placebo) and headache. BCV was readily absorbed following oral administration with mean times to maximum concentration from >1 h to 2.5 h in part 1 and from 1.5 h to 3 h in part 2. Administration of BCV without RTV resulted in BCV exposures predicted to be insufficient to inhibit PI-resistant virus based on in vitro data. Coadministration of 300 mg BCV with 100 mg RTV, however, significantly increased the plasma BCV area under the concentration-time curve and maximum concentration 26-fold and 11-fold, respectively, achieving BCV concentrations predicted to inhibit PI-resistant HIV.
Protease inhibitors (PIs) are administered in combination with other antiviral agents as part of highly active antiretroviral therapy for the treatment of human immunodeficiency virus (HIV) infection. PI resistance, however, remains a significant obstacle to achieving and maintaining viral suppression of HIV (5).
Brecanavir (BCV) (USAN approved, 640385; GlaxoSmithKline, Research Triangle Park, NC) is a novel, potent PI with in vitro activity against both wild-type and PI-resistant strains of HIV in the low nanomolar range. With an MT-4 assay, BCV demonstrated 20 to 100 times higher potency against both wild-type and PI-resistant HIV than other currently marketed protease inhibitors including lopinavir (LPV), saquinavir (SQV), indinavir (IDV), nelfinavir (NFV), and amprenavir (R. Hazen, M. St. Clair, M. Hanlon, S. Danehower, I. Kaldor, V. Samano, J. Miller, J. Ray, A. Spaltenstein, D. Todd, and M. Hale, Abstr. 2nd IAS Conf. HIV Pathog. Treat., abstr. 541, 2003). In addition, BCV exhibited greater potency in vitro than the same PIs against a panel of 55 clinical isolates with an average of 2.6 primary PI mutations and 5.4 secondary PI mutations per virus. BCV maintained low nanomolar 50% inhibitory concentrations (IC50s) for all 55 PI-resistant isolates, 80% of which had an IC50 at or below 0.8 nM. The resistance profile of BCV supports development of this compound for patients who experienced PI treatment.
The pharmacokinetic (PK) properties of HIV PIs, including high protein binding, low oral bioavailability, and short half-life, present challenges to their development as a pharmaceutical agents. Human plasma protein binding for BCV is estimated be 97 to 98%, similar to those for SQV, which is 98.8% bound to plasma proteins (3); NFV, which is >98% bound (2); and LPV, which is 98 to 99% bound (1). Protein binding is lower for IDV, which was 64% bound (3), and for amprenavir, which is 90% bound (2, 8). By an adjustment for a free fraction of 2%, 80% of the 55 clinical PI-resistant isolates would have predicted in vivo BCV IC50s of ≤40 nM or 28 ng/ml, a 50-fold shift from an in vitro IC50 of 0.8 nM. Achievement of plasma PI trough concentrations above the protein binding-corrected IC50 (i.e., trough concentration/IC50 ratio > 1) has been correlated with achievement of a >1-log drop in HIV RNA for NFV, IDV, and SQV (4). Therefore, an a priori PI resistance clinical target trough of 28 ng/ml was chosen to determine the viability of BCV as an antiretroviral agent in the target population. The validity of this target will be assessed for HIV-infected patients in ongoing phase II studies.
Coadministration with ritonavir (RTV), a potent CYP3A inhibitor (11), has been shown to improve oral bioavailability of many CYP3A substrates, including HIV PIs. BCV, a CYP3A4 substrate, demonstrated low oral bioavailability in animals (0 to 30%), which increased to 60 to 100% following coadministration with oral RTV (data not published). Interspecies scaling and simulations suggested that coadministration of RTV with BCV would achieve BCV trough concentrations above the estimated target for resistant virus.
This study (GlaxoSmithKline protocol number HPR10001), involving the first administration of BCV in humans, was undertaken to determine the safety, tolerability, and pharmacokinetic profiles of BCV following single-dose administration in healthy subjects. This study was conducted in two parts. In part 1, ascending single doses of BCV were administered until plasma exposures sufficient to characterize the single-dose PK and safety of BCV as a single agent were achieved. In part 2, ascending single doses of BCV were coadministered with 100 mg RTV capsules to achieve plasma BCV concentrations above the 28 ng/ml estimated target trough concentration.
MATERIALS AND METHODS
Study design and population.
This was a double-blind, randomized, placebo-controlled, single-dose, first-time-in-human study in two parts. Throughout the study, BCV was administered as a 10 mg/ml viscous oral solution in tocopherol-polyethylene glycol succinate-polyethylene glycol 400-ethanol (TPGS-PEG-EtOH) 50:40:10 vehicle. Vehicle alone was administered as a placebo for BCV, and placebo vehicle volumes were adjusted to match the volume of active BCV administered in a given period to maintain the blind. In part 1, single ascending doses of BCV were administered to an alternating panel of two cohorts of healthy male subjects. Subjects were randomized to receive one placebo treatment and a maximum of three doses of BCV, which included BCV at 25 mg, 50 mg, 150 mg, 450 mg, and/or 800 mg. Preliminary PK and safety data from the preceding cohort were reviewed prior to escalation to each subsequent dose.
In part 2, single ascending doses of BCV solution were coadministered with a single 100 mg RTV soft gel capsule in a nonoverlapping sequential panel design with two cohorts of healthy male subjects. The 10 mg BCV/100 mg RTV starting dose in part 2 was chosen based on the PK and safety data of BCV alone in part 1, assuming an impact of RTV on BCV similar to that observed in animal studies, and was predicted not to exceed the highest plasma BCV exposures achieved in part 1. As in part 1, preliminary PK and safety data from the preceding cohort were reviewed prior to escalation to each subsequent dose. Subjects in cohort 1 of part 2 were randomized to receive one placebo dose, one placebo/100 mg RTV dose, and three doses of BCV/RTV, which included 10 mg BCV/100 mg RTV, 30 mg BCV/100 mg RTV, and 100 mg BCV/100 mg RTV. Subjects in cohort 2 of part 2 were randomized to receive placebo, placebo/100 mg RTV, 150 mg BCV/100 mg RTV, 300 mg BCV/100 mg RTV, and 300 mg BCV without RTV. BCV at 300 mg without RTV was included to determine the intrasubject relative bioavailability of a given dose of BCV with and without RTV. Subjects were withdrawn prior to escalation if they were predicted to exceed BCV PK stopping criteria based on exposures (BCV area under the plasma concentration-time curve from time zero to 24 h [AUC0-24] of 11,474 ng · h/ml and maximum concentration [Cmax] of 1,442 ng/ml) observed in preclinical toxicology studies.
Safety assessments.
Clinical laboratory tests including hematology, chemistries (electrolytes, liver function tests, amylase, and lipase), urinalysis, and urine drug and alcohol screens were conducted and reviewed prior to each dosing period. In addition, thyroid function tests and cholesterol panel were obtained prior to dosing for review postdosing. Clinical laboratory tests were repeated prior to discharge from each dosing period. Supine blood pressure and heart rate were measured prior to dosing and at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 18, 24, and 48 h postdosing. In addition, a standing blood pressure was obtained at 4 h postdosing. Full 12-lead electrocardiograms were measured prior to dosing and at 1, 2, 4, 6, 8, 10, 12, 24, and 48 h postdosing. Subjects also underwent continuous telemetry from 1 h prior to dosing until 48 h postdosing. Physical examinations were performed predose and prior to discharge for each treatment period.
Blood collection.
Subjects observed an overnight fast (>10 h) prior to administration of study medications until 4 h postdose. Water was permitted ad libitum. In part 1 of the study, 19 blood samples (3 ml each) were collected predose and 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 18, 24, 36, and 48 h postdose for the determination of plasma BCV concentrations. In part 2 of the study, 17 blood samples (3 ml each) were collected at predose and 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 18, 24, 36, and 48 h postdose for the determination of BCV and RTV concentrations. Plasma samples were stored at −70°C prior to analysis.
Bioanalysis.
Following extraction from plasma by protein precipitation, BCV and RTV concentrations were determined by high-performance liquid chromatography-tandem mass spectrometry (PE Sciex Analyst, version 1.1, and SMS2000, version 1.4) using TurboIonspray and multiple-reaction monitoring. [2H9]BCV and paclitaxel (Taxol) were used as internal standards. The validated linear concentration range was 1 to 1,000 ng/ml for BCV and 10 to 10,000 ng/ml for RTV. Bias and precision were ≤6.0% and >90%, respectively.
Pharmacokinetic evaluation.
Plasma BCV and RTV pharmacokinetic parameters following single-dose administration were determined by noncompartmental analysis of individual concentration-actual time data using WinNonLin Professional, version 3.2 (Pharsight Corporation, Mountain View, California), for extravascular administration (model 200) with linear-up/log-down implementation of the trapezoidal rule. Single-dose PK parameters included Cmax, time of Cmax (Tmax), time to first quantifiable concentration, apparent terminal-phase elimination rate constant (λz), estimated terminal-phase half-life (t1/2), area under the plasma concentration-time curve from time zero to the time of last quantifiable concentration (AUC0-t), area under the plasma concentration-time curve from time zero extrapolated to infinity (AUC0-∞), and the percentage of AUC0-∞ extrapolated to infinity (%AUCex). Individual λz values were estimated by log-linear regression of the terminal phases of plasma concentration-time curves using a minimum of three data points; t1/2 was calculated as (ln 2)/λz. AUC0-t was determined using the linear-up/log-down application of the trapezoidal rule from time zero to the last quantifiable concentration (Clast). AUC0-∞ was calculated by adding Clast/λz to AUC0-t. The %AUCex was calculated as 100 × (AUC0-∞ − AUC0-t/AUC0-∞).
Statistical analysis.
Plasma BCV and RTV PK parameters were summarized for each dose level using descriptive statistics. In addition, plasma RTV PK parameters following administration of BCV/100 mg RTV and placebo/100 mg RTV were summarized by coadministered vehicle volume. Dose proportionality of plasma BCV PK parameters was evaluated separately for BCV and BCV/RTV by using the power model described by the equation log Y = a + b × log (BCV dose), where Y is the parameter of interest and a and b are the estimated coefficients. The slope and corresponding 90% confidence interval (CI) were calculated from the power model which was fitted by restricted maximum likelihood using SAS Proc Mixed. Analysis of variance was performed using the SAS Mixed Linear Models procedure to assess the BCV intrasubject relative bioavailability following 300 mg BCV/100 mg RTV and 300 mg BCV without RTV. The geometric least-squares mean ratios and associated 90% CIs for this comparison were estimated for BCV AUC0-t, AUC0-∞, and Cmax.
RESULTS
Demographics.
A total of 53 male subjects enrolled in the study and were included in the safety population. Fifty-two subjects received at least one dose containing BCV or RTV and were included in the PK summary population (n = 52). One subject in part 1 withdrew following period 1 (placebo treatment) and was not included in the PK summary. The 23 subjects in part 1 had a median (range) age of 31 (20 to 52) years, mean (standard deviation [SD]) weight of 79 (10) kg, and mean (SD) height of 177.8 (7.9) cm; 13 (57%) were white, 8 (35%) black, 1 (4%) Hispanic, and 1 (4%) other. The 30 subjects in part 2 had a median (range) age of 30 (22 to 52) years, mean (SD) weight of 79 (11) kg, and mean (SD) height of 177.1 (5.8) cm; 17 (57%) were white, 10 (33%) black, 1 (3%) Hispanic, and 2 (7%) other races.
Safety.
Single doses of BCV and BCV/RTV were generally well tolerated throughout the study. No severe adverse events (SAEs) were observed. The most commonly reported AEs and drug-related AEs are summarized by treatment type (BCV, BCV/RTV, placebo/RTV, and placebo) in Table 1. Three subjects were withdrawn from the study due to AEs—one for an eye irritation (placebo/RTV; considered drug related), another for accelerated idioventricular rhythm (150 mg BCV/100 mg RTV; not drug related), and the third for elevated predose amylase (4 weeks after receiving 150 mg BCV alone in period 2 and prior to period 3). No dose-related changes or trends in clinical laboratory, vital signs, or electrocardiogram parameters were observed in either part of the study.
TABLE 1.
Summary of commonly reported adverse events
| Adverse event | No. (%) of subjects with AE by treatment
|
|||
|---|---|---|---|---|
| BCV (n = 35) | BCV/RTV (n = 30) | Placebo/RTV (n = 26) | Placebo (n = 45) | |
| Overall AEs | ||||
| Any AE | 17 (49) | 18 (60) | 13 (50) | 18 (40) |
| Headache | 5 (14) | 4 (13) | 4 (15) | 9 (20) |
| Dizziness | 1 (3) | 4 (13) | 0 | 1 (2) |
| Diarrhea | 2 (6) | 3 (10) | 0 | 2 (4) |
| Nausea | 3 (9) | 2 (7) | 0 | 0 |
| Flatulence | 3 (9) | 0 | 1 (4) | 0 |
| Constipation | 0 | 2 (7) | 0 | 0 |
| Application site irritation | 1 (3) | 0 | 2 (8) | 0 |
| Back pain | 2 (6) | 1 (3) | 0 | 0 |
| Myalgia | 0 | 0 | 2 (8) | 0 |
| Pharyngolaryngeal pain | 2 (6) | 0 | 0 | 1 (2) |
| Throat irritation | 0 | 0 | 2 (8) | 0 |
| Supraventricular tachycardia | 2 (6) | 0 | 1 (4) | 1 (2) |
| Acne | 1 (3) | 0 | 0 | 2 (4) |
| Drug-related AEs | ||||
| Any drug-related AE | 4 (11) | 4 (13) | 2 (8) | 6 (13) |
| Diarrhea | 2 (6) | 3 (10) | 0 | 2 (4) |
| Flatulence | 3 (9) | 0 | 1 (4) | 0 |
Pharmacokinetics.
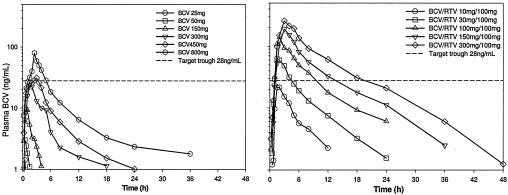
Plasma BCV parameters following administration of single doses of BCV alone are summarized in Table 2. Median plasma BCV concentration-time profiles following administration of single doses of BCV without RTV are shown in Fig. 1 (left panel). BCV was readily absorbed with median Tmax observed from <1 to 2.5 h postdose. BCV concentrations were measurable to <6 h following 25 to 150 mg BCV and to 24 h following 450 mg and 800 mg doses of BCV, resulting in an apparent increase in the estimated terminal-phase half-life. For part 1, the BCV AUC and Cmax increased greater than proportional to dose.
TABLE 2.
BCV pharmacokinetic parameters following single-dose administration of BCV alone
| BCV dose (mg) | No. of subjects receiving a dose | Median Tmax (range) (h) | Geometric mean (95% CI)
|
||
|---|---|---|---|---|---|
| AUC0-∞ (ng · h/ml) | Cmax (ng/ml) | t1/2 (h) | |||
| 25 | 6 | 0.50 (0.28-1.00) | 2.51a | 1.71 (1.18, 2.49) | 0.300a |
| 50 | 6 | 0.50 (0.47-0.73) | 4.90b (2.40, 10.0) | 3.32 (2.03, 5.42) | 0.677b (0.416, 1.10) |
| 150 | 9 | 0.77 (0.48-2.00) | 21.5 (11.2, 41.1) | 12.7 (7.65, 21.0) | 0.993 (0.566, 1.74) |
| 300 | 13 | 1.53 (0.98-4.02) | 105 (52.7, 210) | 29.9 (16.3, 54.8) | 2.63 (1.85, 3.74) |
| 450 | 10 | 2.48 (0.73-3.97) | 203 (116, 354) | 41.3 (20.8, 82.2) | 6.41 (3.38, 12.2) |
| 800 | 11 | 1.48 (0.58-3.97) | 396 (242, 647) | 73.7 (47.2, 115) | 12.3 (7.72, 19.4) |
One subject.
Five subjects.
FIG. 1.
Median plasma BCV concentration-time profiles following single-dose administration of BCV (left panel) and BCV/100 mg RTV (right panel).
Plasma BCV parameters following administration of single doses of BCV/100 mg RTV (part 2) are summarized in Table 3. Median plasma BCV concentration-time profiles following administration of single doses of BCV/RTV are shown in Fig. 1 (right panel). BCV was readily absorbed with median Tmax observed from 2 to 3 h postdose. Median BCV terminal-phase half-life was consistent across doses of BCV/RTV (range, 4.9 to 6.6 h). BCV AUC increased in a dose-proportional manner following administration of single doses of BCV/RTV; Cmax increased less than proportional to dose. Median plasma BCV concentrations were sustained above the 28 ng/ml estimated target for 12 h postdose following 150 mg BCV/100 mg RTV and for 18 h postdose following 300 mg BCV/100 mg RTV. All subjects had concentrations at 12 h postdose at or above the estimated target following 300 mg BCV/100 mg RTV.
TABLE 3.
BCV pharmacokinetic parameters following single-dose administration of BCV/100 mg RTV (part 2)
| BCV/RTV dose (mg/mg) | No. of subjects receiving a dose | Median Tmax (range) (h) | Geometric mean (95% CI)
|
||
|---|---|---|---|---|---|
| AUC0-∞ (ng · h/ml) | Cmax (ng/ml) | t1/2 (h) | |||
| 10/100 | 15 | 1.95 (0.98-3.00) | 101 (70.8, 144) | 20.2 (14.0, 29.2) | 4.87 (4.30, 5.51) |
| 30/100 | 15 | 1.98 (1.02-4.03) | 295 (176, 493) | 57.6 (34.5, 95.9) | 5.20 (4.36, 6.19) |
| 100/100 | 12 | 2.00 (1.48-4.02) | 810 (530, 1,238) | 137 (92.4, 203) | 6.04 (5.25, 6.95) |
| 150/100 | 15 | 2.98 (1.98-4.07) | 1,158 (690, 1,945) | 175 (109, 281) | 6.49 (5.43, 7.77) |
| 300/100 | 11 | 3.02 (2.00-5.02) | 2,010 (1,375, 2,939) | 258 (182, 366) | 6.61 (5.23, 8.35) |
BCV geometric least-squares mean ratios (90% CI) comparing 300 mg BCV/100 mg RTV to 300 mg BCV were determined to evaluate the boost effect of 100 mg RTV on a given single dose of BCV. RTV increased BCV AUC0-∞ 26-fold (range, 19- to 35-fold) and increased BCV Cmax 11-fold (range, 7- to 16-fold).
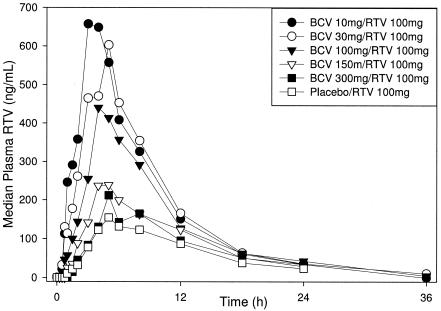
Plasma RTV parameters following the administration of BCV/RTV and placebo/RTV are summarized in Table 4. Median plasma RTV concentration-time profiles following the administration of BCV/RTV and placebo/RTV are shown in Fig. 2. Following the administration of BCV/RTV, RTV Cmax and AUC0-∞ decreased as BCV dose and corresponding TPGS-PEG-EtOH vehicle volume increased. The RTV Tmax increased with increasing vehicle volume. RTV t1/2 values were similar across doses and vehicle volumes.
TABLE 4.
Geometric mean (95% CI) RTV pharmacokinetic parameters by volume of vehicle (TPGS-PEG-EtOH) following administration of 10 mg/ml BCV/100 mg RTV and placebo/100 mg RTV (part 2)
| Vol (ml) | Value for RTV following administration with BCV in vehicle
|
Value for RTV following administration with vehicle alone
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects receiving a dose | Geometric mean (95% CI)
|
Median Tmax (range) (h) | No. of subjects receiving a dose | Geometric mean (95% CI)
|
Median Tmax (range) (h) | |||||
| AUC0-∞ (ng · h/ml) | Cmax (ng/ml) | t1/2 (h) | AUC0-∞ (ng · h/ml) | Cmax (ng/ml) | t1/2 (h) | |||||
| 1 | 15 | 5,108 (3,827, 6,818) | 610 (466, 798) | 5.81 (4.62, 7.29) | 2.97 (0.98-5.02) | 2 | 4,746a (2,943, 7,653) | 537a (353, 816) | 5.76a (5.07, 6.54) | 2.98 (2.95-3.00) |
| 3 | 15 | 4,818 (3,654, 6,355) | 520 (385, 702) | 5.77 (5.06, 6.57) | 4.02 (1.50-5.02) | 3 | 4,284a (1,584, 7,881) | 569a (196, 1,092) | 5.58a (5.24, 5.91) | 4.95 (3.98-5.03) |
| 10 | 12 | 3,760 (2,702, 5,233) | 414 (293, 584) | 5.81 (5.11, 6.62) | 4.52 (2.97-5.03) | 8 | 3,294 (1,841, 5,889) | 336 (184, 615) | 6.45 (4.36, 9.54) | 4.99 (4.02-5.02) |
| 15 | 15 | 2,995 (2,242, 4,001) | 295 (231, 376) | 6.52 (5.36, 7.93) | 4.97 (1.98-5.97) | 2 | 1,312a (890, 1,934) | 106a (55, 204) | 5.93a (4.68, 7.51) | 6.45 (4.97-7.92) |
| 30 | 11 | 2,455 (1,724, 3,496) | 217 (167, 281) | 6.15 (5.12, 7.38) | 4.98 (3.95-6.07) | 11 | 1,256 (769, 2,052) | 93 (61, 142) | 7.64 (6.21, 9.39) | 5.00 (1.00-8.07) |
Presented as geometric mean (minimum, maximum).
FIG. 2.
Median plasma RTV concentration-time profiles following single-dose administration of BCV/100 mg RTV and placebo/100 mg RTV (part 2).
DISCUSSION
Single doses of BCV, ranging from 25 mg to 800 mg alone and from 10 mg to 300 mg in combination with 100 mg RTV, were generally well tolerated by healthy male subjects. No SAEs or deaths occurred during the study. The most commonly reported drug-related AEs experienced throughout the study were gastrointestinal in nature and were similar in BCV, BCV/RTV, and BCV/placebo, but not RTV/placebo, treatment groups, suggesting that they may be attributable, in part, to the TPGS-PEG-EtOH vehicle used to formulate BCV and placebo in this study.
Following the dose escalation (single-dose) of BCV alone in part 1, BCV was readily absorbed, reaching Tmax within 2.5 h of dosing. The apparent increase in terminal-phase half-life with increasing BCV doses likely represents an artifact of the detection limits of the assay rather than nonlinear PK across doses. The BCV concentration-time profile was defined better following BCV at 450 mg and 800 mg, with concentrations measurable to >24 h postdose, than following 25 to 150 mg of BCV with concentrations measurable to <6 h postdose. For the lower doses, the half-life likely represents a distributional half-life rather than true terminal-phase half-life. No dose of BCV alone achieved sustained plasma BCV concentrations above the 28 ng/ml estimated target for potential treatment of PI-resistant virus. Based on modeling and simulation, BCV doses of >1 g twice daily would be required to achieve sustained plasma BCV levels above the 28 ng/ml target for resistant virus.
Following the single-dose escalation of BCV/100 mg RTV in part 2 of this study, BCV was readily absorbed, reaching Tmax at 2 to 3 h postdose. Plasma BCV AUC0-∞ increased proportional to dose; plasma BCV Cmax increased less than proportional to dose. Median plasma BCV concentrations exceeded the 28 ng/ml target to 12 h postdose following 150 mg BCV/100 mg RTV and to 18 h postdose following 300 mg BCV/100 mg RTV. All subjects achieved concentrations at 12 h postdose at or above 28 ng/ml following 300 mg BCV/100 mg RTV. These results suggest that 300 mg BCV/100 mg RTV twice daily may be a viable regimen in the target population.
Coadministration of 100 mg RTV with 300 mg BCV increased plasma BCV AUC0-∞ 26-fold and plasma BCV Cmax 11-fold relative to BCV at 300 mg alone. This is consistent with prior studies indicating RTV exerts a greater impact on PI exposure for PIs with low oral bioavailability. SQV, which has an oral bioavailability in humans of 4 to 12% depending on the formulation (7), exhibited a 63- to 83-fold increase in AUC0-40 following single-dose administration of 200 mg RTV with 600 mg SQV in healthy subjects (10). LPV, shown to have low oral bioavailability in animals, exhibited a 77-fold increase in AUC0-24 following coadministration of 50 mg RTV with 400 mg LPV in healthy human subjects (13). IDV has an absolute oral bioavailability in humans of ∼60% (14), and oral clearance of single-dose 800-mg IDV was decreased ∼60% (i.e., bioavailability increased 2.4-fold) when administered following repeat administration of 200 mg RTV daily (9). The NFV AUC increased slightly (20 to 39%) following coadministration with RTV to healthy subjects given that its primary route of metabolism is CYP2C19 rather than CYP3A; the M8 metabolite of NFV, a substrate for CYP3A, exhibited a 74 to 86% increase in AUC (12). The significant increase in BCV following coadministration with RTV is consistent with the part 1 data of this study and preclinical data that suggest that BCV has low oral bioavailability (<10%) in humans secondary to high first-pass intestinal and hepatic metabolic clearance, similar to other RTV-boosted PIs, including LPV and SQV.
Plasma RTV AUC0-∞ and Cmax following coadministration of BCV/100 mg RTV decreased with increasing BCV dose and corresponding vehicle volume. The same trend was observed following coadministration of placebo TPGS-PEG-EtOH/100 mg RTV. At a given vehicle volume, RTV exposures appeared higher following coadministration with BCV than with placebo. These observations suggest that the negative effect on RTV soft gel capsules was precipitated by the vehicle rather than BCV and that BCV mitigates the effect of vehicle on RTV. TPGS, a P-glycoprotein (P-gp) inhibitor (6), would be expected to increase the oral bioavailability of P-gp substrates such as RTV. Given the observed decrease in RTV exposure, the interaction between RTV and TPGS-PEG-EtOH observed in this study was not mediated by TPGS effects on P-gp. TPGS enhances solubility of compounds by micelle formation and optimizes uptake in Caco-2 cells at a compound-specific critical micelle concentration, above which permeability may be reduced (15). At the higher volumes of TPGS-containing vehicle, RTV may have become sequestered in the gut lumen by micelle entrapment resulting in reduced plasma RTV exposure. Alternatively, the trend of increasing Tmax values may suggest that the decreased plasma RTV exposure resulted from delayed gastric emptying, although the volumes of vehicle administered in this study are lower than those thought to impact gastric emptying. Despite the incremental decrease in RTV exposure with increasing vehicle volume, BCV AUC0-∞ increased proportionally to the dose. TPGS-PEG-EtOH is not a component of the BCV tablet formulation used in clinical studies.
Conclusions.
Single doses of BCV and BCV/100 mg RTV were safe and well tolerated. The results of this study support further development of BCV coadministered with RTV for the treatment of HIV infection in the population of patients who experienced PI treatment.
REFERENCES
- 1.Abbott Laboratories. 2003. Kaletra product information. Abbott Laboratories, Chicago, Ill.
- 2.Agouron Pharmaceuticals. 2004. Viracept product information. Agouron Pharmaceuticals, San Diego, Calif.
- 3.Boffito, M., P. G. Hoggard, H. E. Reynolds, S. Bonora, E. R. Meaden, A. Sinicco, G. Di Perri, and D. J. Back. 2002. The unbound percentage of saquinavir and indinavir remains constant throughout the dosing interval in HIV positive subjects. Br. J. Clin. Pharmacol. 54:262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casado, J. L., A. Moreno, R. Sabido, P. Marti-Belda, A. Antela, F. Dronda, M. J. Perez-Elias, and S. Moreno. 2003. Individualizing salvage regimens: the inhibitory quotient (Ctrough/IC50) as predictor of virological response. AIDS 17:262-264. [DOI] [PubMed] [Google Scholar]
- 5.Clotet, B., F. Raffi, D. Cooper, J. F. Delfraissy, A. Lazzarin, G. Moyle, J. Rockstroh, V. Soriano, and J. Schapiro. 2004. Clinical management of treatment-experienced, HIV-infected patients with the fusion inhibitor enfuvirtide: consensus recommendations. AIDS 18:1137-1146. [DOI] [PubMed] [Google Scholar]
- 6.Dintaman, J. M., and J. A. Silverman. 1999. Inhibition of P-glycoprotein by d-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 16:1550-1556. [DOI] [PubMed] [Google Scholar]
- 7.Flexner, C. 1998. HIV-protease inhibitors. N. Engl. J. Med. 338:1281-1292. [DOI] [PubMed] [Google Scholar]
- 8.GlaxoSmithKline. 2004. Lexiva product information. GlaxoSmithKline, Research Triangle Park, N.C.
- 9.Hugen, P. W., D. M. Burger, H. J. ter Hofstede, P. P. Koopmans, M. Stek, Y. A. Hekster, P. Reiss, and J. M. Lange. 2000. Dose-finding study of a once-daily indinavir/ritonavir regimen. J. Acquir. Immune Defic. Syndr. 25:236-245. [DOI] [PubMed] [Google Scholar]
- 10.Kempf, D. J., K. C. Marsh, G. Kumar, A. D. Rodrigues, J. F. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. 1997. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar, G. N., A. D. Rodrigues, A. M. Buko, and J. F. Denissen. 1996. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J. Pharmacol. Exp. Ther. 277:423-431. [PubMed] [Google Scholar]
- 12.Kurowski, M., B. Kaeser, A. Sawyer, M. Popescu, and A. Mrozikiewicz. 2002. Low-dose ritonavir moderately enhances nelfinavir exposure. Clin. Pharmacol. Ther. 72:123-132. [DOI] [PubMed] [Google Scholar]
- 13.Sham, H. L., D. J. Kempf, A. Molla, K. C. Marsh, G. N. Kumar, C. M. Chen, W. Kati, K. Stewart, R. Lal, A. Hsu, D. Betebenner, M. Korneyeva, S. Vasavanonda, E. McDonald, A. Saldivar, N. Wideburg, X. Chen, P. Niu, C. Park, V. Jayanti, B. Grabowski, G. R. Granneman, E. Sun, A. J. Japour, J. M. Leonard, J. J. Plattner, and D. W. Norbeck. 1998. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 42:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh, K. C., J. A. Stone, A. D. Carides, P. Rolan, E. Woolf, and W. D. Ju. 1999. Simultaneous investigation of indinavir nonlinear pharmacokinetics and bioavailability in healthy volunteers using stable isotope labeling technique: study design and model-independent data analysis. J. Pharm. Sci. 88:568-573. [DOI] [PubMed] [Google Scholar]
- 15.Yu, L., A. Bridgers, J. Polli, A. Vickers, S. Long, A. Roy, R. Winnike, and M. Coffin. 1999. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm. Res. 16:1812-1817. [DOI] [PubMed] [Google Scholar]