Pseudomonas aeruginosa is an opportunistic human pathogen that causes nosocomial infections. This bacterium is characterized by inherent resistance to a wide variety of antimicrobials; the inherent resistance is always mediated by antibiotic resistance genes (3, 10). A genetic element, the integron, is potentially a major agent in the dissemination of multidrug resistance among gram-negative bacteria, especially enteric bacteria and Pseudomonas (2, 6). Class 1 integrons are predominant among integrons that carry resistance cassettes (11, 12).
The goal of our study was to analyze the gene cassettes and a noncoding cassette in a class-1-integron-positive clinical Pseudomonas aeruginosa strain isolated from a sputum specimen of a respiratory disease patient in Hebei Province, People's Republic of China.
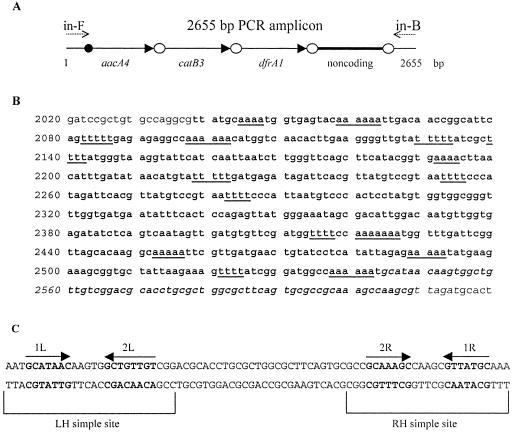
P. aeruginosa strain P11 was screened for antimicrobial susceptibility by using the disk agar dilution method (7). Results of antimicrobial susceptibility testing showed that strain P11 was resistant to several antibiotics, including cefaclor, cefazolin, cefuroxime, chloramphenicol, gentamicin, trimethoprim-sulfamethoxazole, tetracycline, and tobramycin. The intI1 gene, an integron-encoded integrase gene, was amplified by primers specific for intI1 (intM1-U [5′-ACGAGCGCAAGGTTTCGGT-3′] and intM1-D [5′-GAAAGGTCTGGTCATACATG-3′]). The variable region of the integron was determined to be 2,655 bp by PCR with primers specific for the variable region between the 5′ conserved sequence (5′-CS) (in-F [5′-GGCATCCAAGCAGCAAGC-3′]) and the 3′-CS (in-B [5′-AAGCAGACTTGACCTGAT-3′]) (8). The 3′-CS of the class 1 integron was also specifically amplified by primers (qacEΔ1-F [5′-ATCGCAATAGTTGGCGAAGT-3′] and sul1-B [5′-GCAAGGCGGAAACCCGCGCC-3′]) (5), indicating that the 3′-CS of the class 1 integron possessed the qacEΔ1 and sul1 genes. In order to identify the gene cassettes harbored in this integron, the 2,655-bp amplicon was purified by using the QIAquick PCR purification kit (QIAGEN, Hilden, Germany) and cloned into the pMD18-T vector (TaKaRa, Japan). The sequencing was done by an ABI PRISM 310 genetic analyzer. Analysis of the sequence by BLASTX and BLASTN revealed that the 2,655-bp amplicon (GenBank accession number AB195796) contained aacA4, catB3, and dfrA1 gene cassettes and the noncoding cassette, a cassette without any open reading frames (Fig. 1). It is noteworthy that aacA4-catB3-dfrA1 is a novel rearrangement in the class 1 integron. Also, this noncoding cassette had no significant BLASTN hits and had not been reported before. Noncoding cassettes were reported in vibrios only recently (1), but the details of their structures were not analyzed. Interestingly, this noncoding cassette has many adenines and thymines repeated in the sequence. In generic gene cassettes, 59-base elements (59-be) are typical and important components, because they can serve as integration sites for IntI1-catalyzed site-specific recombination (4, 9). Downstream, this noncoding cassette also contained a 59-be with an imperfectly inverted repeat sequence that was 71 bp long. Despite the fact that the 59-be had not been reported in other gene cassettes, it contained features common to 59-base elements, such as 1L, 2L, 2R, and 1R (13). As reported, a few scattered attC sites, apparently not associated with structural genes and not making up parts of integrons, were found in some partially sequenced genomes (3). Therefore, it is possible that the 59-be of this cassette could originally be derived from the scattered attC sites. However, the sequence of this noncoding cassette has not been reported before, which makes the mechanism of this cassette formation questionable.
FIG. 1.
(A) Schematic representation of the 2,655-bp fragment of class 1 integron in P. aeruginosa strain P11. Cassettes are represented by arrowheads, and their 59-base elements are represented by ellipses. (B) Sequence of the noncoding cassette (boldface type). Adenines and thymines of this cassette sequence repeated more than four times are underlined. The 59-be fragment in the linear form is shown in italic type. (C) 59-be structure of the noncoding cassette. The sequence shown is that present in circular form. Putative intI1 binding domains at the LH (1L and 2L) and RH (1R and 2R) sites described by Stokes et al. (13) are shown in boldface type, with their orientations indicated by the arrows above the sequences.
Our study provides a new array of aacA4-catB3-dfrA1 gene cassettes in a class 1 integron and analyzes the structure of a new noncoding cassette which offers information for the formation and evolution of cassettes harbored by integrons.
Acknowledgments
This work was supported by the National Natural Science Foundation of the People's Republic of China (20436020), Guangdong Prov-ince Science Foundation (04020050), and the Scientific and Technological Project of Guangzhou City (2004J1-C0161).
REFERENCES
- 1.Boucher, Y., C. L. Nesbo, M. J. Joss, A. Robinson, B. C. Mabbutt, M. R. Gillings, W. F. Doolittle, and H. W. Stokes. 2006. Recovery and evolutionary analysis of complete integron gene cassette arrays from Vibrio. BMC Evol. Biol. 6:3. (First published 18 January 2006; doi: 10.1186/1471-2148-6-3.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunny, K. L., R. M. Hall, and H. W. Stokes. 1995. New mobile gene cassettes containing an aminoglycoside resistance gene, aacA7, and a chloramphenicol resistance gene, catB3, in an integron in pBWH301. Antimicrob. Agents Chemother. 39:686-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centrón, D., and P. H. Roy. 2002. Presence of a group II intron in a multiresistant Serratia marcescens strain that harbors three integrons and a novel gene fusion. Antimicrob. Agents Chemother. 46:1402-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collis, C. M., G. Grammaticopoulos, J. Briton, H. W. Stokes, and R. M. Hall. 1993. Site-specific insertion of gene cassettes into integrons. Mol. Microbiol. 9:41-52. [DOI] [PubMed] [Google Scholar]
- 5.Fluit, A. C., and F. J. Schmitz. 2004. Resistance integrons and super-integrons. Clin. Microbiol. Infect. 10:272-288. [DOI] [PubMed] [Google Scholar]
- 6.Lee, M. D., S. Sanchez, M. Zimmer, U. Idris, M. E. Berrang, and P. F. McDermott. 2002. Class 1 integron-associated tobramycin-gentamicin resistance in Campylobacter jejuni isolated from the broiler chicken house environment. Antimicrob. Agents Chemother. 46:3660-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 1998. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 8.Navia, M. M., J. Ruiz, and J. Vila. 2004. Molecular characterization of the integrons in Shigella strains isolated from patients with traveler's diarrhea. Diagn. Microbiol. Infect. Dis. 48:175-179. [DOI] [PubMed] [Google Scholar]
- 9.Partridge, S. R., C. M. Collis, and R. M. Hall. 2002. Class 1 integron containing a new gene cassette, aadA10, associated with Tn1404 from R151. Antimicrob. Agents Chemother. 46:2400-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer, J., M. A. Toleman, L. M. Deshpande, W. Kaminska, D. Dzierzanowska, P. M. Bennett, R. N. Jones, and T. R. Walsh. 2004. Pseudomonas aeruginosa strains harbouring an unusual blaVIM-4 gene cassette isolated from hospitalized children in Poland (1998-2001). J. Antimicrob. Chemother. 53:451-456. [DOI] [PubMed] [Google Scholar]
- 11.Plante, I., D. Centron, and P. H. Roy. 2003. An integron cassette encoding erythromycin esterase, ere(A), from Providencia stuartii. J. Antimicrob. Chemother. 51:787-790. [DOI] [PubMed] [Google Scholar]
- 12.Recchia, G. D., and R. M. Hall. 1997. Origins of the mobile gene cassettes found in integron. Trends Microbiol. 5:389-394. [DOI] [PubMed] [Google Scholar]
- 13.Stokes, H. W., D. B. O'Gorman, G. D. Recchia, M. Parsekhian, and R. M. Hall. 1997. Structure and function of 59-base element recombination sites associated with mobile genetic elements. Mol. Microbiol. 26:731-745. [DOI] [PubMed] [Google Scholar]