Abstract
Invasive aspergillosis, an important cause of morbidity and mortality in immunosuppressed (IS) patients, is often treated with amphotericin B lipid formulations. In the present study, liposomal amphotericin B (L-AMB) and amphotericin B lipid complex (ABLC) were compared in treatment of murine pulmonary aspergillosis. Uninfected, IS mice were treated for 4 days with 1, 4, 8, or 12 mg L-AMB or ABLC/kg of body weight, and their lungs were analyzed by high-performance liquid chromatography for drug concentrations. IS mice intranasally challenged with Aspergillus fumigatus were treated with 12, 15, or 20 mg/kg L-AMB or ABLC and monitored for survival, fungal burden (CFU), and tissue drug concentration. Blood urea nitrogen (BUN) levels and kidney histopathology were determined for uninfected and infected mice given 15 or 20 mg/kg L-AMB or ABLC. The results showed that both drugs had therapeutic levels of drug (>3.0 μg/g) in the lungs of uninfected or infected mice, and 24 h after the last dose, ABLC levels were significantly higher than L-AMB levels (P < 0.02). L-AMB and ABLC at 12 mg/kg both produced 57% survival, but only L-AMB at 15 or 20 mg/kg further increased survival to 80 to 90%, with BUN levels and kidney morphology similar to those of controls. Survival at 15 or 20 mg/kg ABLC was not significantly different than that of controls, and BUN levels were significantly elevated, with tubular alterations in uninfected animals and acute necrosis in kidney tubules of infected animals. In conclusion, although both drugs were effective in prolonging survival at 12 mg/kg, the reduced nephrotoxicity of L-AMB increased its therapeutic index, allowing for its safe and effective use at 15 or 20 mg/kg.
Invasive aspergillosis (IA) has become an increasingly important cause of morbidity and mortality in patients who have been immunocompromised due to allogeneic bone marrow transplantation or intensive chemotherapy (4, 36, 42, 45). Although newer triazoles with activity against IA have reached the commercial market (11, 27, 30), amphotericin B deoxycholate (D-AMB) remains as one of the drugs used against severe cases of IA (40). Even so, infusion-related toxicities and nephrotoxicity limit the optimal use of D-AMB for many patients (10, 47). To reduce these toxicities, the lipid formulations of amphotericin B, i.e., liposomal amphotericin B (L-AMB) (AmBisome) and amphotericin B lipid complex (ABLC) (Abelcet), have been used to treat IA patients (9, 19, 34, 44, 46).
With the reduced toxicity of the lipid formulations, a number of preclinical studies have been done which show that doses of L-AMB and ABLC ranging from 5 to 15 mg/kg of body weight have improved therapeutic efficacy in different animal models of invasive fungal infection (3, 13-18, 21, 28). These preclinical studies would suggest that improved efficacy might be correlated with increased levels of drug in tissues. Several investigations have, in fact, shown that increased tissue concentrations of L-AMB do correlate with increased antifungal activity. Garcia and coworkers (23) found that mice given a single prophylactic dose of L-AMB (1, 5, 10, or 20 mg/kg) 7 or 9 days prior to fungal challenge with Candida albicans or Histoplasma capsulatum had dose-dependent increases in efficacy and amphotericin B concentrations in both kidneys and spleen. By using a murine model of visceral leishmaniasis, investigators compared the levels of amphotericin B in the livers, spleens, and kidneys of mice treated with L-AMB at 0.8, 5, and 50 mg/kg and reported that L-AMB efficacy could be correlated with increasing drug levels in the tissues, but not plasma (22).
The studies cited above indicate that there may be a correlation between improved efficacy and tissue concentration of amphotericin B in some animal models, although increased tissue levels of amphotericin B could also have detrimental effects on these tissues. Various degrees of ABLC and L-AMB nephrotoxicity have been reported for test animals, such as the dog (5), mouse (12, 38), and rat (7, 31), when lipid formulations of amphotericin B have been administered at doses generally ranging from 4 to 50 mg/kg. However, both animal and human data have shown that L-AMB is significantly less nephrotoxic than ABLC (8, 48) and D-AMB (43). We selected an immunosuppressed murine model of pulmonary aspergillosis to further investigate the effects of drug tissue concentrations on therapeutic efficacy and the nephrotoxicity that might follow use of high-dose L-AMB or ABLC to achieve elevated tissue levels.
MATERIALS AND METHODS
Animals.
Four-week-old, female DBA/2 mice were obtained from Taconic Farms (Germantown, NY) and maintained in microisolator boxes with standard rodent diet and water ad libitum. All animal research procedures were approved by the Institutional Animal Care and Use Committee of California State Polytechnic University, Pomona.
Fungal culture.
Aspergillus fumigatus ATCC 13073 was cultured on Sabouraud dextrose agar slants at 30°C for 9 to 10 days. Conidia were dislodged from the slants by dispersal in 0.9% saline with 0.05% Tween 80 (Sigma, St. Louis, MO) and stored at 4°C. The conidial count was determined with a hemacytometer and the conidial suspension adjusted to the desired concentration by dilution in phosphate-buffered saline (PBS). The viability of the conidial stock was assessed by plating 200 μl of the suspension on inhibitory mold agar plates (Hardy Diagnostics, Santa Maria, CA), followed by incubation at 30°C for 48 h.
Test substances.
L-AMB (AmBisome; Gilead Sciences, Inc., San Dimas, CA) was reconstituted according to the manufacturer's instructions to give 4 mg/ml in 12 ml sterile water for injection (WFI) (AgriLabs, St. Louis, MO). ABLC (Abelcet; Enzon Pharmaceuticals, Piscataway, NJ) at a concentration of 5 mg/ml was shaken gently to resuspend any sediment and then filtered through a 5-μm filter according to the manufacturer's instructions. D-AMB (Fungizone; Apothecon, Princeton, NJ) was reconstituted with 10 ml WFI to give a 5-mg/ml solution. Further dilutions of each drug were prepared in 5% dextrose in water (D5W) (Baxter Healthcare Corp., Deerfield, IL).
Pulmonary challenge.
Three days prior to fungal challenge, animals (12 to 15 g) were immunosuppressed with a single intraperitoneal dose of 75 mg/kg cyclophosphamide (Sigma, St. Louis, MO) in WFI. On the day of challenge (day 0), mice were sedated with an intraperitoneal injection of 80 mg/kg ketamine (Ketaset; Fort Dodge Laboratories, Inc., Fort Dodge, IA) and 16 mg/kg xylazine (Anased; Lloyd Laboratories, Shenandoah, IA) in PBS. Using a micropipettor, 6 × 104 to 1.7 × 105 viable conidia in 20 μl PBS were applied to the nares of sedated mice. The mice were then placed on a warm surface in a supine position for 5 min with their heads slightly elevated to promote the deposition of conidia in their lungs.
Drug biodistribution.
To determine drug concentrations in the tissues of uninfected animals, mice were treated intravenously with 1, 4, 8, or 12 mg/kg L-AMB or ABLC or 1 mg/kg D-AMB on day 0 and then again every 24 h for three more days. Drug concentrations in the lungs of the immunosuppressed, uninfected animals (n = 5 mice/time point) were measured 24 h after the first and fourth drug treatments. Tissue concentrations in infected animals were determined by challenging cyclophosphamide-immunosuppressed mice with 8.6 × 104 A. fumigatus conidia and treating them intravenously with 15 mg/kg L-AMB or ABLC beginning 2 h postchallenge and then again every 24 h for two more days. The lungs, liver, spleen, kidneys, and blood were collected from five infected mice/group 24 h after the third drug treatment. At the time of tissue collection, mice were exposed to halothane inhalation (Halocarbon Laboratories, River Edge, NJ), blood was obtained via cardiac puncture, and the serum was stored at −20°C. The other tissues were removed, weighed, transferred into 0.5-ml ring seal screw-cap tubes (United Scientific Products, San Leandro, CA), and stored at −20°C.
Tissue analysis.
Tissues were thawed and homogenized by addition of six 3.2-mm chrome steel beads, 450 μl methanol, and 40 μl of the internal standard (0.1 mg/ml N-acetyl amphotericin B; Xechem, Inc., New Jersey) to each sample. The tubes with the samples were placed in a locked position on a MiniBeadbeater 8 homogenizer (BioSpec International, Bartlesville, OK), homogenized for 60 s, and placed in an ice bath for approximately 1 min. The homogenization process was repeated three more times, the homogenized tissues were pelleted, and the supernatants were passed through a 0.45-μm GHP Acrodisc syringe filter (Gelman Sciences, Ann Arbor, MI) into siliconized microfuge tubes. Amphotericin B concentrations were determined by high-performance liquid chromatography (Waters photodiode array 996; Waters Corp., Milford, MA), with data acquisition, integration, and processing done by using Waters Millennium 2020 software. The separation was achieved with a Luna C18 (2) column (Phenomenex, Torrance, CA) and a mobile phase mixture of 25:35:40 (vol/vol/vol) acetonitrile:methanol:2.5 mM EDTA. A portion (30 μl) of each sample was injected for analysis and the column kept at 30°C throughout the assay (24). The inter- and intra-assay precision and accuracy of this method were less than 10%, and the limit of quantitation was 0.5 μg/ml (24).
Efficacy experiments.
In each of three experiments, drug treatment of the cyclophosphamide-immunosuppressed, infected mice was administered via the lateral tail vein 2 h post-intranasal challenge and repeated every 24 h for an additional three days. In the first experiment, mice were infected with 6 × 104 A. fumigatus conidia, treated with 12 mg/kg L-AMB, ABLC, or D5W (n = 10 mice/group), and followed for morbidity for 9 days postchallenge. In the second experiment, mice were challenged with 8 × 104 A. fumigatus conidia, given 15 mg/kg L-AMB or ABLC or 1 mg/kg D-AMB or D5W (n = 7 mice/group), and followed for morbidity for 9 days postchallenge. In the third experiment, mice were challenged with 1.6 × 105 A. fumigatus conidia and given 15 or 20 mg/kg L-AMB or ABLC or 1 mg/kg D-AMB or D5W (n = 20 mice/group). To assess the fungal burden in the lungs in the third experiment, 10 mice in each treatment group were sacrificed 24 h after the third drug treatment. The lungs were aseptically removed, homogenized in 1 ml PBS, diluted in PBS, plated (200 μl/dilution) in duplicate on Sabouraud dextrose agar with 0.05% chloramphenicol, and incubated at 30°C for 48 h to determine CFU/g lung tissue. The other 10 mice in each treatment group were followed for morbidity for 9 days postchallenge.
Evaluation of toxicity.
Uninfected, immunosuppressed mice (n = 5/group) were intravenously treated with 15 or 20 mg/kg L-AMB or ABLC on day 0 and then again every 24 h for two more days. Blood was collected by cardiac puncture from these uninfected animals 24 h after the third treatment and the sera analyzed for blood urea nitrogen (BUN) levels. The BUN levels in the sera of immunosuppressed mice intranasally challenged with 1.6 × 105 A. fumigatus conidia (n = 5/group) were also determined 24 h after the third treatment with 15 or 20 mg/kg L-AMB or ABLC.
Histopathological evaluation was also done on the kidneys and lungs of cyclophosphamide-immunosuppressed, uninfected mice as well as intranasally infected (1.7 × 105 A. fumigatus conidia) mice following intravenous treatment with 15 or 20 mg/kg L-AMB, ABLC, or D5W at 2 h and then again every 24 h for three more days (n = 10 to 17/group). The tissues were collected 24 h after the last drug treatment. At the time of necropsy, kidneys and lungs were collected and fixed in 10% neutral buffered formalin. Fixed tissues were processed routinely. They were dehydrated in alcohol, cleared in xylene, and infiltrated and embedded in paraffin. Sections (5 μm thick) were cut and mounted on glass slides. The slides were then rehydrated in water, stained with hematoxylin and eosin (H&E), dehydrated, cleared in xylene, mounted with a synthetic resin, and covered with a coverslip. Additional tissue sections were cut and stained with Gridley's fungal stain to demonstrate fungal hyphae and conidia in the tissue sections (26). Briefly, deparaffinized slides were washed in distilled water, stained with Coleman's preparation of Feulgen reagent, rinsed in sulfurous acid rinse, washed in water, stained with aldehyde-fuchsin solution, rinsed in alcohol, washed in water, and counterstained with Metanil yellow. All tissue sections were examined and photographed with a light microscope (Olympus BX45 and BH-2) by a board-certified veterinary pathologist.
Statistical analysis.
Kaplan-Meier analysis was used to compare the survival rates of mice in all of the treatment groups. Tissue drug concentrations (μg/g) and tissue fungal burdens (CFU/g) were analyzed by using GraphPad Prism, version 4.0 (GraphPad Software, Inc., San Diego, CA). A one-way analysis of variance was applied to compare the control to all groups in each experiment, and where differences occurred, a two-tailed Mann-Whitney test was done between paired groups. A P value of <0.05 was considered significant. Incidences of histopathological findings were compared among groups using the chi-square test. If a significant effect occurred (P < 0.05), Fisher's exact test was used for pairwise comparisons to the control group and other treatment groups.
RESULTS
Drug concentrations in lungs of uninfected mice.
To better understand the relationship of dose to drug level in the lungs, the target tissue for pulmonary aspergillosis, we initially examined drug concentrations in the lungs of uninfected mice treated with increasing doses of amphotericin B formulations, i.e., D-AMB, L-AMB, and ABLC. As shown in Table 1, lung amphotericin B concentrations 24 h after one treatment with 1, 4, 8, or 12 mg/kg showed dose-dependent increases, and after the fourth treatment, the levels in the lungs of ABLC-treated animals were significantly higher than the levels in mice given L-AMB.
TABLE 1.
Concentrations of amphotericin B in lungs of uninfected mice 24 h after one dose or four doses of the indicated amphotericin B formulation
| Treatment dosage (mg/kg) | Concn (μg/g [mean ± SD]) of amphotericin B in lungs after treatment witha:
|
|||||
|---|---|---|---|---|---|---|
| D-AMB
|
L-AMB
|
ABLC
|
||||
| One dose | Four doses | One dose | Four doses | One dose | Four doses | |
| 1 | 0.44 ± 0.12 | 0.59 ± 0.22 | ND | 0.24 ± 0.07 | ND | ND |
| 4 | — | — | 0.41 ± 0.10 | 1.30 ± 0.51b | 1.91 ± 0.71 | 3.02 ± 0.67b |
| 8 | — | — | 0.92 ± 0.18 | 2.12 ± 0.70c | 4.23 ± 1.35 | 5.65 ± 1.70c |
| 12 | — | — | 1.93 ± 0.37 | 3.60 ± 1.23d | 5.26 ± 0.98 | 10.07 ± 2.20d |
—, not tested; ND, not detected.
P = 0.016 for four doses of 4 mg/kg L-AMB versus four doses of 4 mg/kg ABLC.
P = 0.008 for four doses of 8 mg/kg L-AMB versus four doses of 8 mg/kg ABLC.
P = 0.016 for four doses of 12 mg/kg L-AMB versus four doses of 12 mg/kg ABLC.
Drug efficacy.
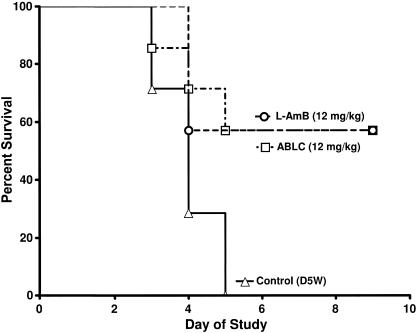
Having confirmed that, for the doses examined, a dose of 12 mg/kg delivered the most drug to the lungs, we tested the efficacy of the lipid amphotericin B formulations at 12 mg/kg in a murine model of pulmonary aspergillosis. In this experiment, mice (seven mice/group) were challenged intranasally with A. fumigatus conidia. Beginning 2 h postchallenge, the mice were treated daily for four consecutive days with D5W (control) or 12 mg/kg L-AMB or ABLC. Both groups treated with the lipid amphotericin B formulations had survival rates of 57%, and no control mice survived (Fig. 1). Thus, with this challenge dose, comparable efficacies of the lipid amphotericin B formulations were demonstrated despite the accumulation of higher drug concentrations in the lungs of ABLC-treated animals.
FIG. 1.
Survival of mice (n = 7 mice/group) challenged with 6 × 104 A. fumigatus conidia and then intravenously treated daily for four days with D5W (control), 12 mg/kg L-AMB, or 12 mg/kg ABLC.
Drug nephrotoxicity in uninfected mice.
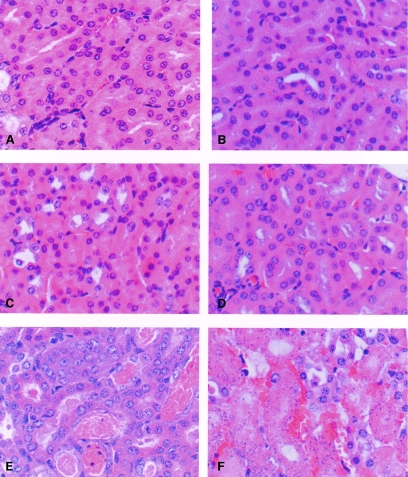
To try to improve survival, higher doses of L-AMB and ABLC were tested. To evaluate if the higher doses would produce nephrotoxicity in the mice, uninfected, immunosuppressed animals were given 15 or 20 mg/kg of L-AMB or ABLC daily for three days. Comparator groups were given 1 mg/kg of either D-AMB or D5W. Only the ABLC groups showed elevated BUN values (Table 2). Toxicity in the kidneys was also evaluated histologically for uninfected, immunosuppressed mice 24 h after 4 days of dosing with 15 or 20 mg/kg L-AMB, ABLC, or D5W (Fig. 2). The renal tubule morphology was normal for D5W and L-AMB treatment groups (Fig. 2A and C, respectively), but nephrosis characterized by regeneration with minimal ongoing tubular damage was observed with the 20-mg/kg ABLC treatment group (Fig. 2E).
TABLE 2.
BUN concentrations in uninfected and infected mice 24 h after the third daily dose of the indicated amphotericin B formulation
| Groupa | BUN concn (mg/dl [mean ± SD])
|
|||||
|---|---|---|---|---|---|---|
| D5W (control) | D-AMB (1 mg/kg) | L-AMB (15 mg/kg) | L-AMB (20 mg/kg) | ABLC (15 mg/kg) | ABLC (20 mg/kg) | |
| Uninfected mice | 26.4 ± 5.7 | 17.8 ± 3.0 | 19.4 ± 2.4 | 23.0 ± 1.8b | 59 ± 17.5c | 59.3 ± 8.1c |
| Infected mice | 22.3 ± 2.5d | 14.0 ± 1.8e | 18.5 ± 4.5b | 14.8 ± 3.1 | 40.8 ± 15.0f | 71.0 ± 19.4g |
Except where noted, n = 5/group.
n = 4.
P = 0.036 for 15 and 20 mg/kg ABLC in uninfected mice (n = 3/group) versus the control (n = 5/group) (same P value for both comparisons).
n = 3.
n = 6.
P = 0.036 for 15 mg/kg ABLC in infected mice (n = 5/group) versus the control (n = 3/group).
P = 0.057 for 20 mg/kg ABLC in infected mice (n = 4/group) versus the control (n = 3/group).
FIG. 2.
Histopathology of kidneys from cyclophosphamide-immunosuppressed, uninfected (A, C, and E) and intranasally infected (1.7 × 105 A. fumigatus conidia) (B, D, and F) mice following intravenous treatment with D5W or 20 mg/kg L-AMB or ABLC at 2 h and then again every 24 h for three more days. Kidneys were collected 24 h after the last drug treatment and processed for microscopic evaluation. (A and B) D5W (control); (C and D) 20 mg/kg L-AMB; (E and F) 20 mg/kg ABLC. The renal tubule morphology was normal in uninfected and infected D5W and L-AMB treatment groups (A, B, C, and D). In uninfected, ABLC-treated animals, the primary finding was regeneration with minimal ongoing tubular damage (E). In infected, ABLC-treated animals, tubular damage was more severe, characterized by acute tubular necrosis and accompanied by surrounding interstitial hemorrhage (F). The term nephrosis was used to describe extensive tubular damage in which the normal tubules were replaced by dilated, regenerative, and/or necrotic tubules containing intraluminal granular casts. The tubular changes were considered to be consistent with amphotericin B nephrotoxicity. H&E staining is shown. Magnification, ×400.
Drug efficacy at elevated doses.
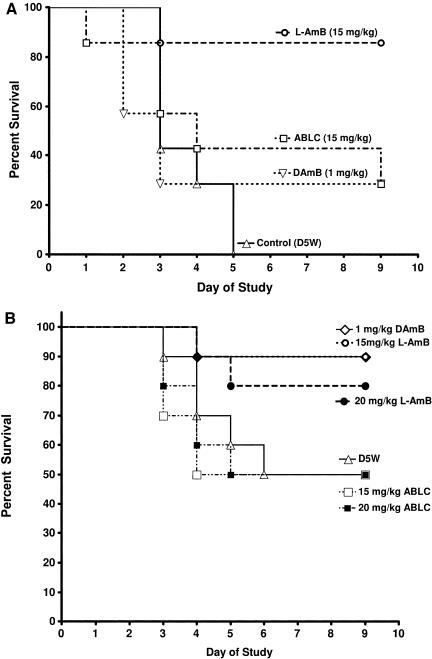
Doses above 12 mg/kg were tested to determine if they would provide more protection against acute pulmonary infection. In one experiment (Fig. 3A), 15 mg/kg L-AMB was very effective, with 86% of the animals surviving to at least day 9. ABLC at 15 mg/kg and D-AMB at 1 mg/kg produced only 29% survival, which was significantly less efficacious than 15 mg/kg L-AMB (P = 0.039 and P = 0.025, respectively). In another experiment (Fig. 3B), 1 mg/kg D-AMB and 15 or 20 mg/kg L-AMB showed a trend toward increased survival (80 to 90% survival), although results did not reach statistical significance due to the relatively high survival rate (50%) in the control group. In comparison, 15 or 20 mg/kg of ABLC was not protective.
FIG. 3.
(A) Survival of mice (n = 7 mice/group) challenged with 8 × 104 conidia of A. fumigatus and treated intravenously daily for four days with D5W (control), 1 mg/kg D-AMB, 15 mg/kg L-AMB, or 15 mg/kg ABLC. (B) Survival of mice (n = 10 mice/group) challenged with 1.6 × 105 conidia of A. fumigatus and treated intravenously daily for four days with D5W (control), 1 mg/kg D-AMB, 15 mg/kg L-AMB, 20 mg/kg L-AMB, 15 mg/kg ABLC, or 20 mg/kg ABLC.
Drug nephrotoxicity in infected mice.
To evaluate the nephrotoxicities of the untreated and drug-treated infected mice, BUN levels in the sera of immunosuppressed, A. fumigatus-infected mice were assessed (Table 2). As was seen with the uninfected mice, the BUN levels of the ABLC-treated, infected mice were significantly elevated compared to those of the D5W- and L-AMB-treated, infected mice. Toxicity was also evaluated by histological examination of kidneys from immunosuppressed, A. fumigatus-infected mice treated for 4 days with 15 or 20 mg/kg L-AMB, ABLC, or D5W. As shown in Fig. 2, there were no changes in the normal morphology of the kidneys of mice given D5W or L-AMB. In comparison, nephrosis was observed in the kidneys of ABLC-treated, infected mice, with tubular damage being more severe than that seen in the uninfected kidneys of similarly treated mice.
Drug biodistribution in infected mice.
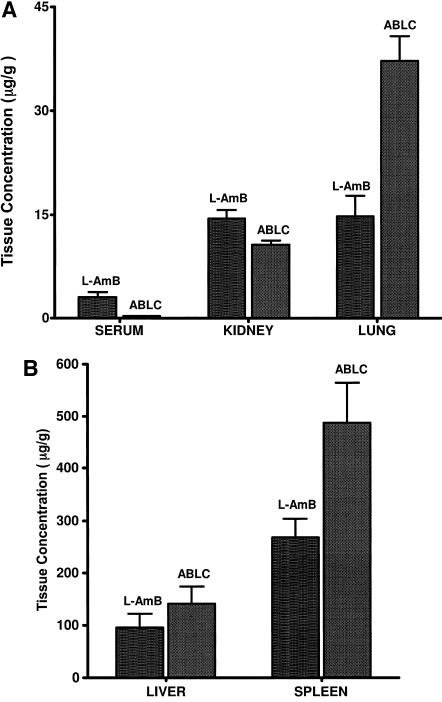
To determine if differences in toxicity were due to higher concentrations of ABLC in infected kidneys, we examined drug concentrations in the kidneys and in various other tissues. The tissue concentrations of amphotericin B in infected mice were determined 24 h after the third dose of either 15 mg/kg L-AMB or 15 mg/kg ABLC (Fig. 4A and B). ABLC treatment resulted in significantly more drug in the lungs (P = 0.002) and the spleen (P = 0.03) than treatment with L-AMB, while L-AMB treatment produced significantly more drug in the kidneys (P = 0.03) and the serum (P = 0.004). The drug levels in the liver were comparable for both treatments (P = 0.33). Although ABLC delivered about 2.5-fold-higher drug levels to the infected lungs than L-AMB, ABLC was significantly less efficacious as measured by survival (Fig. 3). Notably, the ABLC concentrations in the kidneys were significantly lower than those of L-AMB, indicating that the nephrotoxicity observed with the ABLC-treated mice was not due to higher kidney drug levels.
FIG. 4.
(A) Mean (±standard deviation) amphotericin B concentrations (μg/g) in (A) sera, kidneys, and lungs and (B) livers and spleens of mice challenged with 8 × 104 conidia of A. fumigatus and intravenously treated daily for three days with L-AMB (15 mg/kg) or ABLC (15 mg/kg). Tissue samples were collected from five mice/group 24 h after the third daily treatment with L-AMB (15 mg/kg) or ABLC (15 mg/kg).
Fungal burden in infected mice.
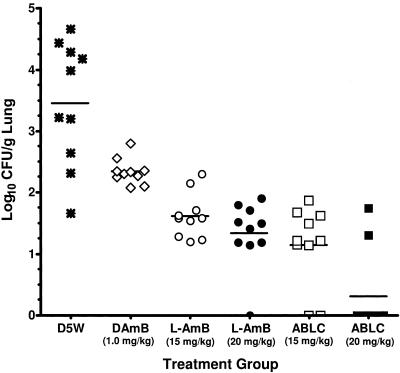
To evaluate the antifungal activities of ABLC and L-AMB in A. fumigatus-infected mice, the lungs of mice treated with D5W, 1 mg/kg D-AMB, or 15 or 20 mg/kg ABLC or L-AMB were collected 24 h after the third drug treatment and evaluated for CFU/g. The results demonstrated that both ABLC and L-AMB (P < 0.0001 for each group), as well as D-AMB (P = 0.009), significantly reduced the fungal burden compared to D5W (Fig. 5). The 15- and 20-mg/kg ABLC and L-AMB treatments also significantly reduced the fungal burden compared to the 1-mg/kg D-AMB treatment (P ≤ 0.0003 for each group). In addition, CFU/g for the 20-mg/kg ABLC treatment group were significantly lower than those for the 15-mg/kg L-AMB group (P = 0.002) or the 20-mg/kg L-AMB group (P = 0.009).
FIG. 5.
Fungal burdens (log10 CFU/g lung tissue) in mice infected with A. fumigatus and treated intravenously daily for three days with D5W (control), 15 mg/kg L-AMB, 20 mg/kg L-AMB, 15 mg/kg ABLC, or 20 mg/kg ABLC. For each group, the solid horizontal bar represents the mean log10 CFU/g lung tissue (n = 7/group).
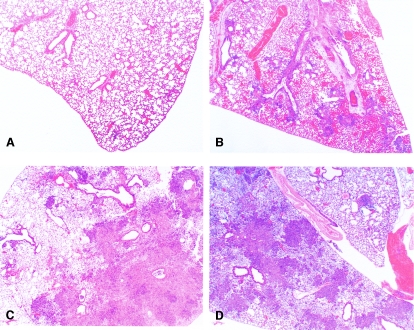
Additional observations of the degree of infection and fungal burden were obtained by comparing histological sections of drug-treated, uninfected mouse lungs with those of infected mouse lungs. The tissue was collected 24 h following 4 days of dosing with 15 or 20 mg/kg L-AMB or ABLC. Normal lung morphology was seen with the uninfected mouse lungs regardless of treatment (Fig. 6A), and pneumonia was identified in all infected animals. In the infected animals given D5W, there was marked vascular congestion, perivascular edema, and fibrin exudation, hemorrhage, and necrosis consistent with acute necrotizing pneumonia (NP) (Fig. 6B). All drug treatments significantly reduced NP compared to D5W (Table 3). The infected lungs of the L-AMB- and ABLC-treated mice had multifocal to coalescing pyogranulomatous inflammation consistent with pyogranulomatous bronchopneumonia (PGP) (Fig. 6C and D and Table 3). Elongated, invasive hyphae with minimal neutrophil response were visualized by H&E and Gridley staining in the lungs of mice given D5W (Fig. 7). In contrast, the lungs of mice treated with ABLC or L-AMB showed degenerative hyphae surrounded by large numbers of neutrophils. The histological observations supported the CFU results, demonstrating that ABLC and L-AMB were both effective in markedly reducing the fungal burden in the lungs.
FIG. 6.
Lung histopathology. Pneumonia was identified in all A. fumigatus-infected animals and separated into two distinct morphological patterns, acute NP with active fungal invasion and PGP with minimal necrosis and minimal fungal invasion. (A) Tissue from uninfected, D5W-treated mouse, showing normal lung morphology. (B) Tissue from A. fumigatus-infected, D5W-treated mouse. Marked vascular congestion, perivascular edema, and fibrin exudation, hemorrhage, and necrosis consistent with NP was observed. Tissue from (C) A. fumigatus-infected, L-AMB (20 mg/kg)-treated mouse and (D) A. fumigatus-infected, ABLC (15 mg/kg)-treated mouse. Multifocal to coalescing pyogranulomatous inflammation consistent with PGP was observed. Magnification, ×20.
TABLE 3.
Incidence of lung histopathological findings following infection of mice with 1.7 × 105 A. fumigatus conidia and treatment with the indicated amphotericin B formulation
| Lung histopathology | No. of observations/no. of mice in group
|
||||
|---|---|---|---|---|---|
| D5W (control) | L-AMB (15 mg/kg) | L-AMB (20 mg/kg) | ABLC (15 mg/kg) | ABLC (20 mg/kg) | |
| NP with active fungal invasion | 17/17 | 6/10a,b | 0/10a,b,c | 0/12a,b | 1/12a,b,c |
| PGP with minimal fungal invasion | 0/17 | 4/10a,b | 10/10a,b,c | 12/12a,b | 11/12a,b,c |
All treatments were significantly different from the controls (control versus 15 mg/kg L-AMB, P < 0.05; control versus 20 mg/kg L-AMB, P < 0.01; control versus 15 mg/kg ABLC, P < 0.01; and control versus 20 mg/kg ABLC, P < 0.01).
15 mg/kg L-AMB was significantly different from 15 mg/kg ABLC (P < 0.01) and from 20 mg/kg L-AMB and 20 mg/kg ABLC (P < 0.05).
20 mg/kg L-AMB was not significantly different from 20 mg/kg ABLC.
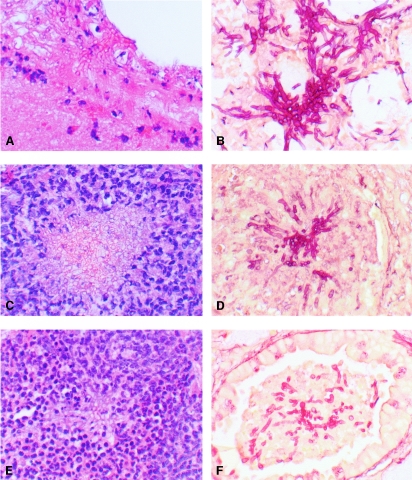
FIG. 7.
A. fumigatus-infected lungs. (A, C, and E) H&E-stained paraffin sections. (B, D, and F) Gridley-stained paraffin sections. (A and B) Tissue from D5W-treated controls, showing elongated invasive hyphae with minimal neutrophil response. Tissue from (C and D) ABLC (15 mg/kg)-treated mouse and (E and F) L-AMB (20 mg/kg)-treated mouse, showing degenerative hyphae surrounded by neutrophils. Magnification, ×400.
DISCUSSION
Several investigators reported that increasing the daily dosing of L-AMB or ABLC up to 13 mg/kg was needed to provide significant protection against severe pulmonary aspergillosis in animal models (12, 14, 21). In the present study, we found that a multidosing drug regimen of 12 mg/kg of either L-AMB or ABLC protected immunosuppressed 4-week-old mice against A. fumigatus intranasal challenge, with 57% survival. Although uninfected, ABLC-treated animals had about 2.5-fold-higher drug levels in their lungs than L-AMB-treated mice, the efficacies of both agents in the young infected mice were comparable, indicating that survival could be obtained with a range of drug levels in the lungs. The efficacy of the lower concentrations of L-AMB may be related to several factors, including the ability of L-AMB to localize at sites of infection, to bind to fungal cell walls, and to release the drug directly into the fungus, with subsequent fungal cell death (1).
When the drug dose was increased to 15 or 20 mg/kg, the ABLC-treated mice were no longer protected. In comparison, mice given L-AMB at 15 or 20 mg/kg showed increased protection, with 80 to 90% survival compared to 57% survival with the 12-mg/kg dosing regimen. The drug concentrations in the lungs of infected mice given repeated dosing with 15 mg/kg were well above the MIC for A. fumigatus (29, 37) for both drugs (for L-AMB, 14.7 μg/g; for ABLC, 36.6 μg/g), which produced significant reduction in the fungal burden in the infected lungs. Thus, the difference in survival between ABLC and L-AMB was probably not related to the amount of drug in the lungs since the reductions in CFU/g were comparable for both drugs. More likely, it was related to differences in nephrotoxicity at these higher doses. For uninfected and infected mice, the BUN levels of the ABLC-treated mice, unlike those of the L-AMB-treated mice, were significantly elevated. Histological observations of the kidneys of ABLC-treated mice showed regeneration with minimal ongoing tubular damage in the uninfected mice and acute, tubular necrosis in the kidneys of the infected mice. In comparison, there was no nephrosis in the kidneys of either uninfected or infected L-AMB-treated mice. These results are consistent with preclinical reports by other investigators that L-AMB is less toxic than D-AMB and ABLC at the cellular level (32, 39) and is less toxic than D-AMB and amphotericin B colloidal dispersion (Amphotec) in organs (8, 33, 41).
The enhanced nephrotoxicity of ABLC at elevated doses was not related to higher drug concentrations in the kidneys since L-AMB had higher kidney drug levels than ABLC. With a multidosing drug regimen, L-AMB levels were significantly higher in the serum and kidneys, whereas ABLC levels were significantly higher in the spleen and lungs. The concentrations of amphotericin B in the liver were comparably elevated for both L-AMB and ABLC treatments. These differences are probably the result of each drug's unique pharmacokinetics (8). With increasing doses, L-AMB demonstrates nonlinear pharmacokinetics, with redistribution into the kidneys and lungs (33). L-AMB circulates at high levels for much longer periods of time than ABLC (8), allowing greater penetration in these tissues. In contrast, ABLC is reported to be rapidly removed from the circulation and taken up primarily by the liver and spleen, with some localization in the lungs (12, 31). Other investigators have reported that dose-dependent increases in amphotericin B levels could be achieved in kidneys and lungs of both rats and dogs given multiple-dose L-AMB regimens (2, 5, 6, 7).
For both ABLC and L-AMB at 15 and 20 mg/kg, the fungal burden (assessed by CFU/g) in the lungs was significantly reduced compared to that with no drug treatment, with enhanced neutrophil responses as evidenced by multifocal pyogranulomatous sites of inflammation (PGP) surrounding degenerating hyphae. In comparison, branching, long hyphae were present in the hemorrhagic, edematous, necrotizing lung lesions (NP) of non-drug-treated mice. Thus, although both lipid formulations produced sufficiently high levels of drug in the lungs to decrease the fungal burden, the absence of nephrotoxicity with L-AMB and the severe kidney changes with ABLC probably contributed to the significantly better survival profile with L-AMB. Increased mortality in ABLC-treated animals, compared to L-AMB-treated animals, may have been due to a critical pH imbalance caused by the combined effect of respiratory acidosis (20) resulting from the severe pneumonia and renal tubular acidosis, a well-known side effect of amphotericin B nephrotoxicity (10, 25). As noted by others, normal kidneys are thought to play a key role in the amelioration of respiratory acid-base disorders (35).
In conclusion, the present investigation demonstrated that doses of 12 mg/kg of L-AMB or ABLC significantly prolonged survival of mice with acute, murine pulmonary aspergillosis. However, the reduced toxicity of L-AMB increased its therapeutic index compared to ABLC and allowed it to be safely used at doses of 15 or 20 mg/kg to further enhance its efficacy against this difficult-to-treat fungal infection. The results underscore the importance of understanding how dosing levels affect the efficacy and toxicity of a given drug.
Acknowledgments
Invaluable technical support for these studies was provided by Van T. Huynh, Tarquinus H. Bunch, Peter Smith, and David Constable.
The study was supported by research grants from Gilead Sciences, Inc., and Astellas, Inc.
REFERENCES
- 1.Adler-Moore, J. 1994. AmBisome targeting to fungal infections. Bone Marrow Transplant. 14(Suppl. 5):S3-S7. [PubMed] [Google Scholar]
- 2.Adler-Moore, J., and R. T. Proffitt. 2003. Effect of tissue penetration on AmBisome efficacy. Curr. Opin. Investig. Drugs 4:179-185. [PubMed] [Google Scholar]
- 3.Albert, M. M., L. Stahl-Carroll, M. F. Luther, and J. R. Graybill. 1995. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J. Mycol. Med. 5:1-6. [Google Scholar]
- 4.Baddley, J. W., T. P. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319-1324. [DOI] [PubMed] [Google Scholar]
- 5.Bekersky, I., G. W. Boswell, R. Hiles, R. M. Fielding, D. Buell, and T. J. Walsh. 1999. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm. Res. 16:1694-1701. [DOI] [PubMed] [Google Scholar]
- 6.Bekersky, I., G. W. Boswell, R. Hiles, R. M. Fielding, D. Buell, and T. J. Walsh. 2000. Safety, toxicokinetics and tissue distribution of long-term intravenous liposomal amphotericin B (AmBisome): a 91-day study in rats. Pharm. Res. 17:1494-1502. [DOI] [PubMed] [Google Scholar]
- 7.Boswell, G. W., I. Bekersky, D. Buell, R. Hiles, and T. J. Walsh. 1998. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob. Agents Chemother. 42:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boswell, G. W., D. Buell, and I. Bekersky. 1998. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 38:583-592. [DOI] [PubMed] [Google Scholar]
- 9.Bowden, R., P. Chandrasekar, M. H. White, X. Li, L. Pietrelli, M. Gurwith, J. A. van Burik, M. Laverdiere, S. Safrin, and J. R. Wingard. 2002. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35:359-366. [DOI] [PubMed] [Google Scholar]
- 10.Burges, J. L., and R. Birchall. 1972. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am. J. Med. 53:77-84. [PubMed] [Google Scholar]
- 11.Caillot, D., H. Bassaris, A. McGeer, C. Arthur, H. G. Prentice, W. Seifert, and K. De Beule. 2001. Intravenous itraconazole followed by oral itraconazole in the treatment of invasive pulmonary aspergillosis in patients with hematologic malignancies, chronic granulomatous disease, or AIDS. Clin. Infect. Dis. 33:e83-e90. [DOI] [PubMed] [Google Scholar]
- 12.Clark, J. M., R. R. Whitney, S. J. Olsen, R. J. George, M. R. Swerdel, L. Kunselman, and D. P. Bonner. 1991. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob. Agents Chemother. 35:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemons, K. V., R. A. Sobel, P. L. Williams, D. Pappagianis, and D. A. Stevens. 2002. Efficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbits. Antimicrob. Agents Chemother. 46:2420-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clemons, K. V., and D. A. Stevens. 2004. Comparative efficacies of four amphotericin B formulations—Fungizone, Amphotec (Amphocil), AmBisome, and Abelcet—against systemic murine aspergillosis. Antimicrob. Agents Chemother. 48:1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemons, K. V., and D. A. Stevens. 1993. Comparison of a liposomal amphotericin B formulation (AmBisome) and deoxycholate amphotericin B (Fungizone) for the treatment of murine paracoccidioidomycosis. J. Med. Vet. Mycol. 31:387-394. [Google Scholar]
- 16.Clemons, K. V., and D. A. Stevens. 1993. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J. Antimicrob. Chemother. 32:465-472. [DOI] [PubMed] [Google Scholar]
- 17.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacies of amphotericin B lipid complex and amphotericin B deoxycholate suspension against murine blastomycosis. Antimicrob. Agents Chemother. 35:2144-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft, S. L., R. N. Davidson, and E. A. Thornton. 1991. Liposomal amphotericin B in the treatment of visceral leishmaniasis. J. Antimicrob. Chemother. 28(Suppl. B):111-118. [DOI] [PubMed] [Google Scholar]
- 19.Ellis, M., D. Spence, B. de Pauw, F. Meunier, A. Marinus, L. Collette, R. Sylvester, J. Meis, M. Boogaerts, D. Selleslag, V. Krcmery, W. von Sinner, P. MacDonald, C. Doyen, and B. Vandercam. 1998. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin. Infect. Dis. 27:1406-1412. [DOI] [PubMed] [Google Scholar]
- 20.Epstein, S. K., and N. Singh. 2001. Respiratory acidosis. Respir. Care 46:366-383. [PubMed] [Google Scholar]
- 21.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 22.Gangneux, J. P., A. Sulahian, Y. J. Garin, R. Farinotti, and F. Derouin. 1996. Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisome. Antimicrob. Agents Chemother. 40:1214-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia, A., J. P. Adler-Moore, and R. T. Proffitt. 2000. Single-dose AmBisome (liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob. Agents Chemother. 44:2327-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garey, K. W., S. L. Pendland, V. T. Huynh, T. H. Bunch, G. M. Jensen, and K. J. Pursell. 2001. Cunninghamella bertholletiae infection in a bone marrow transplant patient: amphotericin lung penetration, MIC determinations, and review of the literature. Pharmacotherapy 21:855-860. [DOI] [PubMed] [Google Scholar]
- 25.Gouge, T. H., and V. T. Andriole. 1971. An experimental model of amphotericin B nephrotoxicity with renal tubular acidosis. J. Lab. Clin. Med. 78:713-724. [PubMed] [Google Scholar]
- 26.Gridley, M. F. 1953. A stain for fungi in tissue sections. Am. J. Clin. Pathol. 23:303-307. [DOI] [PubMed] [Google Scholar]
- 27.Groll, A. H. 2002. Itraconazole—perspectives for the management of invasive aspergillosis. Mycoses 45(Suppl. 3):48-55. [DOI] [PubMed] [Google Scholar]
- 28.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 29.Guinea, J., T. Peláez, L. Alcalá, M. J. Ruiz-Serrano, and E. Bouza. 2005. Antifungal susceptibility of 596 Aspergillus fumigatus strains isolated from outdoor air, hospital air, and clinical samples: analysis by site of isolation. Antimicrob. Agents Chemother. 49:3495-3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbrecht, R., D. W. Denning, T. F. Patterson, J. E. Bennett, R. E. Greene, J. W. Oestmann, W. V. Kern, K. A. Marr, P. Ribaud, O. Lortholary, R. Sylvester, R. H. Rubin, J. R. Wingard, P. Stark, C. Durand, D. Caillot, E. Thiel, P. H. Chandrasekar, M. R. Hodges, H. T. Schlamm, P. F. Troke, B. de Pauw, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408-415. [DOI] [PubMed] [Google Scholar]
- 31.Janoff, A. S., W. R. Perkins, S. L. Saletan, and C. E. Swenson. 1993. Amphotericin B lipid complex (ABLC): a molecular rationale for the attenuation of amphotericin B related toxicities. J. Liposome Res. 3:451-471. [Google Scholar]
- 32.Jensen, G. M., C. R. Skenes, T. H. Bunch, C. A. Weissman, N. Amirghahari, A. Satorius, K. Moynihan, and C. G. S. Eley. 1999. Determination of the relative toxicity of amphotericin B formulations: a red blood cell potassium release assay. Drug Deliv. 6:81-88. [Google Scholar]
- 33.Lee, J. W., M. A. Amantea, P. A. Francis, E. E. Navarro, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leenders, A. C., S. Daenen, R. L. Jansen, W. C. Hop, B. Lowenberg, P. W. Wijermans, J. Cornelissen, R. Herbrecht, H. van der Lelie, H. C. Hoogsteden, H. A. Verbrugh, and S. de Marie. 1998. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br. J. Haematol. 103:205-212. [DOI] [PubMed] [Google Scholar]
- 35.Madias, N. E., and H. J. Adrogue. 2003. Cross-talk between two organs: how the kidney responds to disruption of acid-base balance by the lung. Nephron Physiol. 93:61-66. [DOI] [PubMed] [Google Scholar]
- 36.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 37.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, and the Sentry Participants Group. 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proffitt, R. T., A. Satorius, S.-M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 39.Sperry, P. J., D. J. Cua, S. A. Wetzel, and J. P. Adler-Moore. 1998. Antimicrobial activity of AmBisome and non-liposomal amphotericin B following uptake of Candida glabrata by murine epidermal Langerhans cells. Med. Mycol. 36:135-141. [PubMed] [Google Scholar]
- 40.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, G. A. Pankey, et al. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 41.van Etten, E. W. M., C. van den Heuvel-de Groot, and I. A. Bakker-Woudenberg. 1993. Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic mice. J. Antimicrob. Chemother. 32:723-739. [DOI] [PubMed] [Google Scholar]
- 42.Wald, A., W. Leisenring, J. A. van Burik, and R. A. Bowden. 1997. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J. Infect. Dis. 175:1459-1466. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, J. S. Holcenberg, et al. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 44.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh, T. J., J. W. Hiemenz, and E. Anaissie. 1996. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect. Dis. Clin. N. Am. 10:365-400. [DOI] [PubMed] [Google Scholar]
- 46.Walsh, T. J., J. W. Hiemenz, N. L. Seibel, J. R. Perfect, G. Horwith, L. Lee, J. L. Silber, M. J. DiNubile, A. Reboli, E. Bow, J. Lister, and E. J. Anaissie. 1998. Amphotericin B lipid complex for invasive fungal infections: analysis of safety and efficacy in 556 cases. Clin. Infect. Dis. 26:1383-1396. [DOI] [PubMed] [Google Scholar]
- 47.Wingard, J. R., P. Kubilis, L. Lee, G. Yee, M. White, L. Walshe, R. Bowden, E. Anaissie, J. Hiemenz, and J. Lister. 1999. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29:1402-1407. [DOI] [PubMed] [Google Scholar]
- 48.Wingard, J. R., M. H. White, E. Anaissie, J. Raffalli, J. Goodman, A. Arrieta, et al. 2000. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. Clin. Infect. Dis. 31:1155-1163. [DOI] [PubMed] [Google Scholar]