Abstract
We compared the efficacy of human papillomavirus (HPV) DNA detection between a PCR-based genechip (Easychip HPV Blot [hereafter referred to as HPV Blot]; King Car, Taiwan) method and Hybrid Capture II (HCII; Digene, Gaithersburg, MD) in women with previous normal (n = 146) or abnormal (≥atypical squamous cells of undetermined significance [ASCUS] [n = 208]) cytology. A total of 354 cervical swab samples were collected for HPV DNA assay by both HCII and SPF1/GP6+ PCR followed by HPV Blot tests. Colposcopy-directed biopsy was performed if clinically indicated. Of the 354 samples, HPV-positive rates by these two methods (HCII and HPV Blot) were 12.6% and 18.2% in 143 normal samples, 36.2% and 45.7% in 105 ASCUS samples, 57.4% and 57.4% in 94 low-grade squamous intraepithelial lesion samples, and 83.3% and 75.0% in 12 high-grade squamous intraepithelial lesion samples, respectively. The concordance of HPV Blot and HCII was 80.8% (286/354), and the agreement between the methods (κ value, 0.68) was substantial. Discrepancies were further investigated by at least one of the following three methods: direct sequencing, type-specific PCR, and HPV Blot genotyping of cervical biopsy tissue. In the 15 HCII-positive samples, HPV Blot detected only non-HCII HPV genotypes; results of further verification methods were consistent with the latter test in the 15 samples. Of the 20 samples with HCII-negative and HPV Blot-positive results, 18 were found to contain the 13 HCII high-risk genotypes by verification methods. In only 16.7% (3/18) of the HCII-positive but HPV Blot-negative samples, further studies detected the 13 HCII genotypes. We conclude that HPV Blot seemed comparable to HCII for detection of HPV DNA in cervical swab samples.
Human papillomavirus (HPV), a small, nonenveloped, double-stranded DNA virus, is established as the key etiological factor in cervical neoplasms (24, 29, 30). More than 90% of cervical neoplasms are attributed to HPV infection. Persistence of high-risk HPV types is a major risk factor for the development of high-risk cervical intraepithelial neoplasia (CIN) (9). Although the regression of HPV infection commonly takes place within 3 years, compelling evidence indicated that a small but definite fraction of the infected population is at risk for developing invasive cervical cancer after many years or decades of a long latency period of primary infection (3, 10, 14).
Currently, HPV DNA testing has played a triage role for atypical squamous cells of undetermined significance (ASCUS), primary screening in conjunction with cytology for the detection of cervical cancer and CIN, and follow-up in a variety of clinical settings (4, 15, 16, 18, 25).
HPV DNA detection by the FDA-approved Hybrid Capture II HPV DNA test (HCII) (Digene Corporation, Gaithersburg, MD) is the most widely used method. The HCII system, a commercial liquid hybridization kit using RNA probes against HPV DNA genomic targets followed by signal amplification, has been validated for its reproducibility in HPV DNA detection (18, 26, 29). Thirteen carcinogenic types implicated in the pathogenesis of high-grade squamous intraepithelial lesions (HSILs) and invasive cancer, such as HPV type 16 (HPV-16), -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68, can be detected (18, 26). A positive cutoff value was set at 1 pg HPV DNA per milliliter in the specimen. However, information on HPV genotype is lacking in this cocktail detection method.
Other detection systems that determine genotyping include nonamplication Southern and dot blot hybridizations with type-specific probes (17), type-specific PCR (2), and general-primer PCR (6, 9). The disadvantage of type-specific PCR is the need for multiple hybridization reactions to achieve multiple HPV genotypes in a single sample, while general-primer PCR such as MY09/11 has the drawback of a large PCR fragment with less sensitivity. The development of PCR-reverse hybridization permits the use of amplimers generated by the MY09/11 as well as the SPF1/2 primers and allows the detection of 27 HPV genotypes (13).
In our group, a highly sensitive method using modified sets of primers (SPF1/GP6+), which synthesizes a PCR fragment of approximately 184 bp, followed by direct DNA sequencing as well as revert blotting with a commercialized macroarray genechip (Easychip HPV Blot [hereafter referred to as HPV Blot]; King Car, Taiwan), is used for identification of 39 HPV genotypes. In our previous report, HPV Blot was used to investigate HPV genotypes of cervical cancer (paraffin-embedded specimens) treated with neoadjuvant chemotherapy plus radical surgery and showed good sensitivity and reproducibility upon HPV genotyping (11). The association of HPV-18 or HPV-16 and -18 with poor outcome in cervical carcinoma treated with neoadjuvant chemotherapy plus radical surgery is confirmed. However, the utilization of HPV Blot in exfoliated cells collected from the lower genital tract has not been defined.
The purpose of this study is to compare the efficacy of HPV Blot with that of the HCII system for detection of HPV DNA in cervical swab samples.
MATERIALS AND METHODS
Patient recruitment.
Cervical swab samples (n = 354) were collected from women with previous normal (n = 146) or abnormal (≥ASCUS [n = 208]) cytology between January 2001 and September 2004 at the Department of Gynecology and Obstetrics, Chang Gung Memorial Hospital. Two HPV tests were performed and compared using simultaneously obtained cervical swab samples or samples obtained within 3 months for untreated patients with a recent abnormal Pap smear.
HCII system.
Procedures for collecting and transporting specimens for HPV DNA testing were performed as described previously (4, 14, 15). Briefly, specimens were denatured at 65°C for 45 min and hybridized under high-stringency conditions with a mixture of RNA probes that detects 13 different carcinogenic HPV types. The resultant DNA-RNA hybrids were captured on the surface of the microtiter plate wells coated with an anti-DNA-RNA hybrid antibody. The immobilized hybrids then reacted with an alkaline phosphatase-conjugated antihybrid monoclonal antibody. Light intensity was measured with a luminometer. A positive cutoff value was set at 1 pg HPV DNA per milliliter in the specimen.
DNA extraction.
A total of 0.2 ml of sample was obtained from Digene specimen transport medium. DNA was extracted according to the protocol for the isolation of total DNA from cultured animal cells (QIAGEN Inc., Valencia, CA). Briefly, the cell pellet was obtained by centrifugation for 5 min at 300 × g. The sample was lysed by adding 20 μl proteinase K and 0.2 ml AL buffer and incubated at 70°C for 10 min. A total of 0.2 ml of 96 to 100% ethanol was added for precipitation. The sample mixture was loaded onto a DNeasy Mini Spin column. DNA was selectively bound to the membrane. Two efficient wash steps removed the remaining contaminants and enzyme inhibitors. Finally, 100 μl of DNA solution was eluted, and 1 μl of the aliquot was used for PCR amplification.
SPF1/GP6+ PCR.
The SPF1/GP6+ (6) consensus primers (Table 1) were used to amplify a fragment of approximately 184 bp in the L1 open reading frame. Primer GP6+ was biotinylated at the 5′ end. SPF1/GP6+ PCR was performed in a final reaction mixture volume of 50 μl containing 1 μl of the isolated DNA, 20 mmol/liter Tris-HCl (pH 8.4), 3 mmol/liter MgCl2, 0.1% Triton X-100, 0.01% gelatin, 200 μmol/liter each deoxynucleoside triphosphate, 20 pmol of forward and reverse primers, and 0.25 U of AmpliTaq Gold polymerase (Applied Biosystems CA). PCR conditions were as follows: a preheating step for 10 min at 94°C followed by 40 cycles of 1 min at 94°C, 1 min at 45°C, and 1 min at 72°C and a final extension step of 5 min at 72°C. Each PCR experiment was performed with several positive and negative controls. Routine precautionary procedures were applied to avoid carryover or contamination (20). Controls lacking template DNA were also used throughout the entire procedure to monitor for sample contamination, and HeLa cell DNA was included in each batch of PCR to ensure the absence cross-hybridization with types other than HPV-18.
TABLE 1.
Primer sequences
| Primer | Amplimer size (bp) | Tm (°C)a | Sequence |
|---|---|---|---|
| SPF1A | 5′-GCICAGGGICACAATAATGG-3′ | ||
| SPF1B | 5′-GCICAGGGICATAACAATGG-3′ | ||
| SPF1C | 5′-GCICAGGGICATAATAATGG-3′ | ||
| SPF1D | 5′-GCICAAGGICATAATAATGG-3′ | ||
| GP6+ | 5′-GAAAAATAAACTGTAAATCATATTC-3′ | ||
| 16F | 5′-TGTGCTGCCATATCTACTTCAGAAACTAC-3′ | ||
| 16R | 186 | 59 | 5′-TAGACCAAAATTCCAGTCCTCCAAA-3′ |
| 18F | 5′-AGTCTCCTGTACCTGGGCAATATGAT-3′ | ||
| 18R | 177 | 59 | 5′-GAACACCAAAGTTCCAATCCTCTAAAATAC-3′ |
| 31F | 5′-CAATTGCAAACAGTGATACTACAT-3′ | ||
| 31R | 242 | 59 | 5′-TGTAATGGCCTGTGAGGTGAC-3′ |
| 33F | 5′-ACAAGTAACTAGTGACAGTACATA-3′ | ||
| 33R | 243 | 56 | 5′-CACGTAATAGCCTGAGAGGTAACA-3′ |
| 35F | 5′-GTGTCTTCTAGTGACAGTACATA-3′ | ||
| 35R | 233 | 59 | 5′-GCCTGTGATGTTACATAGCGAT-3′ |
| 39F | 5′-TAGAGTCTTCCATACCTTCTACA-3′ | ||
| 39R | 238 | 56 | 5′-ATGGCTGCAGACTGTAGGTATC-3′ |
| 45F | 5′-ACAAAATCCTGTGCCAAGTACATA-3′ | ||
| 45R | 251 | 59 | 5′-CCTTTTGACAGGTAACAGCAACT-3′ |
| 51F | 5′-CACTGCCACTGCTGCGGTTTCC-3′ | ||
| 51R | 239 | 59 | 5′-AGTAGCTGCATTTCTAACAAACCT-3′ |
| 52F | 5′-AGGTTAAAAAGGAAAGCACATAT-3′ | ||
| 52R | 256 | 56 | 5′-GGTGGTGTGTTTTTTTGACAAGT-3′ |
| 56F | 5′-GCTACAGAACAGTTAAGTAAATA-3′ | ||
| 56R | 241 | 56 | 5′-TCCCGTTGACATGTTATAGCTG-3′ |
| 58F | 5′-ATGCACTGAAGTAACTAAGGAAG-3′ | ||
| 58R | 240 | 59 | 5′-GCCTGGGAGGTAACAAATCTAT-3′ |
| 59F | 5′-ACTACTTCTTCTATTCCTAATGT-3′ | ||
| 59R | 233 | 56 | 5′-GCAGATTGAACAAAACGGTATG-3′ |
| 68F | 5′-TGAATCAGCTGTACCAAATA-3′ | ||
| 68R | 229 | 59 | 5′-TGATTGCAGATAGCGGTATGTA-3′ |
| GAPDHF | 136 | 60 | 5′-GGCAGCAGCAAGCATTCCT-3′ |
| GAPDHR | 5′-GCCCAACACCCCCAGTCA-3′ |
Tm, melting point temperature.
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) primers were included in each amplification as a control for the adequacy of the tissue DNA preparation in the pilot study phase. Because coamplification of housekeeping gene primers could reduce the efficiency of target HPV DNA amplification (5), GAPDH PCR was performed if negative results were obtained in this study. The internal control GAPDH PCR mixture contained 0.2 μM primers, 0.24 mM deoxynucleoside triphosphates, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 0.1 mg/ml bovine serum albumin, 1% glycerol, and 0.002 U/μl AmpliTaq Gold polymerase. Forty cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s were performed, and a final extension step was performed at 72°C for 5 min.
Type-specific PCR.
Type-specific primer sequences and annealing temperatures of the 13 HCII HPV genotypes are listed in Table 1. The annealing conditions were different for each set of primers. Type-specific PCR was performed for 50 cycles of 1 min at 94°C, 1 min at the specific annealing temperature, and 1 min at 72°C. A final extension step of 5 min at 72°C was added after 50 cycles. The total yield of each PCR product was analyzed by electrophoresis on 2% agarose gels stained with ethidium bromide (Fig. 1). The quality of isolated DNA was checked with GAPDH PCR, by which a 136 bp product was amplified.
FIG. 1.
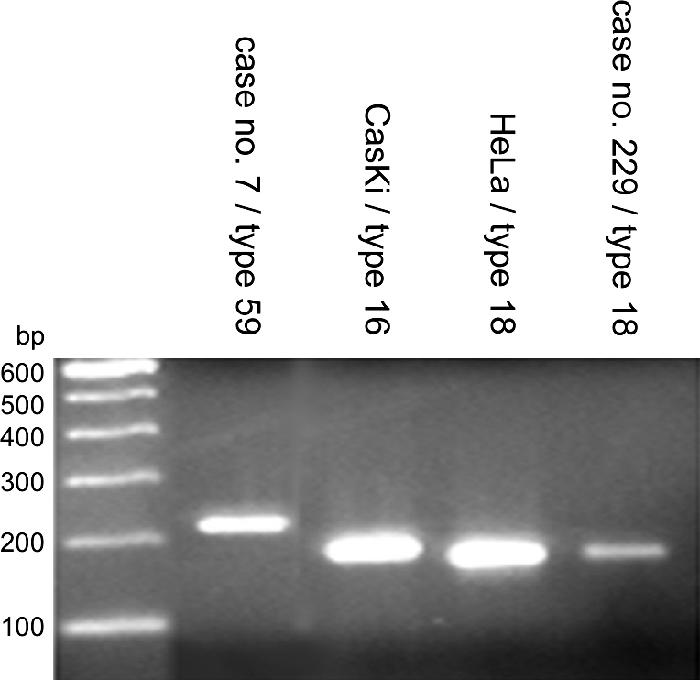
Examples of type-specific PCR. HPV-59 amplimers (233 bp) and HPV-18 amplimers (177 bp) were demonstrated in samples 7 and 229.
HPV genotyping by direct sequencing analyses.
PCR products amplified by SPF1/GP6+ were isolated from agarose gels using a QIAGEN gel extraction kit (QIAGEN Inc., Venlo, The Netherlands). Twenty microliters of amplimer solution was eluted, and 3 μl of eluent was used for direct sequencing with the GP6+ primer. Sequencing reaction of double-stranded PCR products was performed with a dye terminator cycle sequencing kit (ABI Prism dRhodamine Terminator cycle sequencing ready reaction kit; PE Applied Biosystems, Foster City, CA). The DNA sequences obtained from the patient samples were compared to the GenBank sequences by using the BLAST program at the National Center for Biotechnology Information website.
HPV genotyping by HPV Blot.
Fifteen microliters of the resultant amplimers was then hybridized with an Easychip HPV Blot (manufactured by King Car, I-Lan, Taiwan) membrane. Thirty-nine types of HPV (types 6, 11, 16, 18, 26, 31, 32, 33, 35, 37, 39, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 72, 74, 82, CP8061, CP8304, L1AE5, MM4, MM7, and MM8) oligonucleotide probes of 20 to 30 mers with an approximately 100- to 200-mer poly(T) tail were immobilized on a nylon membrane in a single reaction as previously described (11). Two independent PCR products of each sample were hybridized with an HPV Blot, and the two HPV Blot results were compared with each other and also cross-checked with the direct sequencing results. Type-specific PCRs were performed to resolve the discrepant results, if necessary.
HPV Blot membranes were equilibrated with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature for 10 min and prehybridized with prehybridization buffer (2× SSC, 0.5% blocking reagent, 5% dextran sulfate, 0.1% sodium dodecyl sulfate [SDS]) containing denatured salmon sperm DNA (50 μg/ml) by shaking at 35°C for 30 min. Membranes were hybridized with 500 μl of hybridization buffer (2× SSC, 0.5% blocking reagent, 5% dextran sulfate, 0.1% SDS, 50 μg/ml denatured salmon sperm DNA) containing denatured amplimers (15 μl) by shaking at 35°C for at least 3 h. The HPV Blot membranes were washed once in washing buffer 1 (2× SSC and 0.1% SDS) for 5 min at room temperature and then washed twice in washing buffer 2 (0.2× SSC, 0.1% SDS) for 5 min at 35°C. Following this stringent washing, the membranes were equilibrated with buffer 1 (1× phosphate-buffered saline [PBS], pH 7.4, 0.05% Tween-20, 0.1% SDS) by shaking at room temperature for 5 min twice. HPV Blot membranes were incubated in 500 μl of buffer 2 (1× PBS, pH 7.4, 0.05% Tween-20, 0.1% SDS, and 0.5% blocking reagent) containing Avidex-AP (alkaline phosphatase conjugates and biotinylated antibodies; 1:1,000 dilution) for 40 min. After alkaline phosphatase conjugation, HPV Blot membranes were washed in buffer 1 and rinsed with buffer 3 (0.1 M Tris-HCl, pH 9.5, 0.1 M NaCl) for 5 min. Seventy microliters of the substrate 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium was then added and incubated for 30 min at room temperature. The reaction was stopped by the aspiration of the substrate solution and the addition of distilled water. After drying, the HPV Blot results were determined visually according to the HPV type format of the membranes.
Serial dilutions of plasmid DNA containing the specific segment of the L1 open reading frame of the 39 HPV types and cell lines containing known HPV genotypes, such as HeLa and CaSki cells (American Type Culture Collection, Manassas, VA), were analyzed in parallel on every batch of primers to monitor the detection limit of the assay. The detection limit of this assay determined by serial dilution of plasmid DNA containing the specific segment of the L1 open reading frame of the 39 HPV types and cell lines containing known HPV genotypes, such as HeLa and CaSki cells, is about 10 to 100 copies per sample (11).
Comparison between HCII and HPV Blot.
Concordance was defined as detection of the 13 high-risk HPV genotypes contained in HCII by HPV Blot in those patients who were positive by HCII or if the HPV Blot was negative in those patients who had negative HCII results. All other discordant results were further investigated using one of the following three methods: direct sequencing analyses or type-specific PCR of the same specimen or HPV genotyping by HPV Blot on paraffin-embedded cervical biopsy tissue.
Statistical analysis.
The agreement between HCII and HPV Blot was measured by Cohen's κ statistic (poor if κ ≤ 0.20; fair if 0.21 ≤ κ ≤ 0.40; moderate if 0.41 ≤ κ ≤ 0.60; substantial if 0.61 ≤ κ ≤ 0.80; good if κ > 0.80). The McNemar test was used to compare paired HCII and HPV Blot positivities. The calculations were performed by using SPSS (Chicago, IL) version 10.0 statistical software.
RESULTS
HPV testing according to cytology.
Of the 146 patients with a previous normal Pap smear, 3 had abnormal cytology of this incidence. Therefore, of the 354 Pap smears, 143 were normal, 105 were ASCUS, 94 were low-grade squamous intraepithelial lesions (LSIL), and 12 were HSILs (Table 2). The overall HPV detection rates were 33.9% (120/354) by HCII and 38.7% (137/354) by HPV Blot. The HPV-positive rates by HPV Blot and HCII were 18.2% versus 12.6% for normal Pap smears, 45.7% versus 36.2% for ASCUS Pap smears, 57.4% versus 57.4% for LSIL Pap smears, and 75.0% versus 83.3% for HSIL Pap samples. Paired HPV Blot and HCII comparisons of HPV positivity by cytological grade using the McNemar test showed a difference in ASCUS (P = 0.021). When the 13 paired HCII high-risk HPV types were compared, the detection was not different in all cytological grades between HPV Blot and HCII (P > 0.05) (Table 2).
TABLE 2.
HPV test results according to Pap smears from 354 samples
| Cytological grade | No. (%) positive
|
Total no. | |||
|---|---|---|---|---|---|
| HCII | HPV Blot
|
||||
| 39 typesa | 13 typesb | Non-13 types | |||
| Normal | 18 (12.6) | 26 (18.2) | 16 (11.2) | 10 (7.0) | 143 |
| ASCUS | 38 (36.2) | 48 (45.7)d | 36 (34.3)e | 12 (11.4) | 105 |
| LSIL | 54 (57.4) | 54 (57.4) | 47 (50.0) | 7 (7.4) | 94 |
| HSIL | 10 (83.3) | 9 (75.0) | 8 (66.7) | 1 (8.3) | 12 |
HPV-6, -11, -16, -18, -26, -31, -32, -33, -35, -37, -39, -42, -43, -44, -45, -51, -52, -53, -54, -55, -56, -58, -59, -61, -62, -66, -67, -68, -69, -70, -72, -74, -82, -CP8061, -CP8304, -L1AE5, -MM4, -MM7, and -MM8. New carcinogenic and probably carcinogenic types are shown in boldface type.
HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68.
Non-13 types, HPV types not part of the HCII panel.
P = 0.021.
P = 0.791.
Among the 137 (38.7%) samples showing HPV DNA sequences by HPV Blot, 68.6% (n = 94) contained a single type and 31.4% (n = 43) had multiple types. The distribution of HPV genotypes among HPV Blot-positive samples in the order of highest to lowest was as follows: HPV-52, 15.1%; HPV-58, 9.5%; HPV-16, 8.5%; HPV-18 and -33, 5.5% each; HPV-70, 5.0%; HPV-51, -53, -56, and -MM8, 4.5% each; HPV-31 and -39, 4.0% each; HPV-CP8304, 3.5%; HPV-68, 2.0%; HPV-45, -59, -61, and -66, 1.5% each; HPV-11, -42, -43, -62, -69, -72, -82, and -CP8061, 1.0% each; and HPV-6, -26, -32, -35, -44, -55, -67, -74, and -L1AE5, 0.5% each.
Comparisons between Easychip HPV Blot and HCII.
The concordance of HPV detection between the HPV Blot and HCII was 80.8% (286/354), with a substantial agreement between the methods (κ, 0.68; P < 0.001), as demonstrated in Table 3. In analyses of discordant results, HPV Blot detected types other than the 13 HCII high-risk HPV types in 15 cases positive by HCII (Table 4). Verification methods using one of the three methods, direct sequencing, type-specific PCR, or HPV Blot genotyping of paraffin-embedded cervical biopsies, showed that 100% of the results (15 out of 15) were consistent with the cervical swab HPV Blot results. The probable cross-hybridization rate was 12.5% (15/120) in those samples that were positive by the HCII assay. The most common subtypes for cross-hybridization were types 70, CP8304, MM8, and 53. The five abnormal smears in these 15 samples contained HPV-74, -70, -53, and -66. In the 20 samples that were negative by HCII but for which HPV Blot detected the 13 HCII high-risk HPV types, 18 were verified as consistent with the latter (Table 5). All the five samples with ASCUS/LSIL harbored the oncogenic HPV types 16, 18, and 52. The other 15 samples were HCII negative, but HPV Blot detected non-HCII HPV genotypes, further verifying that the methods were consistent with HPV Blot results (Table 6). In only 16.7% (3/18) of the HCII-positive but HPV Blot-negative samples, further studies detected the 13 HCII high-risk genotypes. Nevertheless, HPV Blot missed three cases with high-risk HPV (types 16 and 52), one of which came from a patient with CIN grade 3 (CIN3). These cases were picked up by HCII, albeit at the price of a substantial false-positive rate (Table 7).
TABLE 3.
Analysis of concordance between HCII and HPV Blot results according to HPV positivitya
| HCII result | No. of samples tested by HPV Blot
|
||
|---|---|---|---|
| Positive
|
Negative | ||
| 13 HCII high-risk typesb | 26 non-HCII typesc | ||
| Positive | 87 | 15 | 18 |
| Negative | 20 | 15 | 199 |
n = 354.
HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68.
HPV-6, -11, -26, -32, -37, -42, -43, -44, -53, -54, -55, -61, -62, -66, -67, -69, -70, -72, -74, -82, -CP8061, -CP8304, -L1AE5, -MM4, -MM7, and -MM8. New carcinogenic and probably carcinogenic types are shown in boldface type.
TABLE 4.
| Case | Cytological finding | HPV type(s) | HPV type(s) determined by:
|
Pathology | |
|---|---|---|---|---|---|
| Direct sequencing | Other verification method | ||||
| 38 | Normal | 62 | 62 | NA | |
| 49 | Normal | CP8304 | CP8304 | NA | |
| 59 | Normal | L1AE5 | L1AE5 | NA | |
| 106 | Normal | CP8304 | CP8304 | NA | |
| 157 | LSIL | 74 | NAe | 74c | Negative |
| 182 | Normal | 53 | NA | 53c | NA |
| 193 | ASCUS | 70 | 70 | NA | |
| 197 | Normal | 82 | 82 | NA | |
| 214 | HSIL | 70 | 70 | 18, 70d | Negative |
| 293 | Normal | MM8 | MM8 | Negative | |
| 294 | Normal | MM8 | MM8 | NA | |
| 303 | Normal | 53, MM8 | NA | 53d | NA |
| 308 | LSIL | 53 | 53 | NA | |
| 309 | LSIL | 66 | 66 | CIN1 | |
| 312 | Normal | 11 | 11 | NA | |
HPV-6, -11, -26, -32, -37, -42, -43, -44, -53, -54, -55, -61, -62, -66, -67, -69, -70, -72, -74, -82, -CP8061, -CP8304, -L1AE5, -MM4, -MM7, and -MM8. New carcinogenic and probably carcinogenic types are shown in boldface type.
n = 15.
Type-specific PCR.
Paraffin-embedded cervical biopsy tissue for HPV genotyping using HPV Blot.
NA, not applicable. The amount of PCR product was insufficient for direct sequencing.
TABLE 5.
Analysis of cases for which HCII was negative but HPV Blot detected the 13 HCII high-risk typesa
| Case | Cytological finding | HPV type(s) | HPV type(s) determined by:
|
Pathology | |
|---|---|---|---|---|---|
| Direct sequencing | Other verification method | ||||
| 5 | Normal | 51 | 51 | NA | |
| 7 | Normal | 59 | NAd | 59b | NA |
| 24 | Normal | 16 | 16 | NA | |
| 52 | Normal | 52 | 33 | NA | |
| 89 | Normal | 39 | 39 | NA | |
| 92 | Normal | 56 | NA | NA | |
| 107 | Normal | 18, 52 | 52 | NA | |
| 110 | Normal | 52 | 52 | NA | |
| 114 | Normal | 18 | 18 | NA | |
| 186 | ASCUS | 52 | 52 | Negative | |
| 187 | Normal | 52 | 52 | NA | |
| 217 | Normal | 56, 62 | 56 | NA | |
| 229 | ASCUS | 18 | NA | 18b | NA |
| 295 | ASCUS | 52 | 52 | Negative | |
| 299 | LSIL | 16, 52 | 52 | CIN1 | |
| 300 | Normal | 52 | 52 | CIN1 | |
| 317 | LSIL | 16 | 16 | 16, 18c | NA |
| 363 | Normal | 45, 58, CP8304 | 58 | NA | |
| 364 | Normal | 16, 66 | 16 | NA | |
| 383 | Normal | 16, 31 | NA | 16, 31c | NA |
n = 20. The 13 high-risk types were HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, and -68.
Type-specific PCR.
Paraffin-embedded cervical biopsy tissue for HPV genotyping using HPV Blot.
NA, not applicable. The amount of PCR product was insufficient for direct sequencing.
TABLE 6.
Analysis of cases with negative HCII but positive HPV Blot results for the 26 non-HCII HPV typesa
| Case | Cytological finding | HPV type(s) | HPV type determined by:
|
Pathology | |
|---|---|---|---|---|---|
| Direct sequencing | Other verification method | ||||
| 13 | Negative | CP8304 | CP8304 | NA | |
| 36 | Negative | CP8304, 37, 53 | CP8304 | NA | |
| 57 | Negative | MM8 | MM8 | NA | |
| 77 | Negative | 70 | 70 | NA | |
| 84 | Normal | 33, 61, 70 | 61 | 61b | NA |
| 134 | Negative | MM8 | MM8 | NA | |
| 164 | ASCUS | CP8061 | CP8061 | CP8061b | Negative |
| 173 | Negative | 26 | 26 | NA | |
| 179 | ASCUS | 77 | NAc | 77b | NA |
| 191 | ASCUS | 53 | 53 | NA | |
| 301d | LSIL | MM8, 53 | NA | 53b | NA |
| 302 | Negative | 53 | NA | 53b | NA |
| 327 | ASCUS | 42 | 42 | NA | |
| 329 | Negative | 69 | NA | 69b | NA |
| 335 | ASCUS | 53 | 53 | NA | |
n = 15. The 26 non-HCII HPV types were HPV-6, -11, -26, -32, -37, -42, -43, -44, -53, -54, -55, -61, -62, -66, -67, -69, -70, -72, -74, -82, -CP8061, -CP8304, -L1AE5, -MM4, -MM7, -MM8. New carcinogenic and probably carcinogenic types are shown in boldface type.
Paraffin-embedded cervical biopsy tissue for HPV genotyping using HPV Blot.
NA, not applicable. The amount of PCR product was insufficient for direct sequencing.
Follow-up 6 months later showed that the sample was HCII positive and HPV Blot MM8 and 53 positive.
TABLE 7.
Cases with samples positive by HCII but negative by HPV Blota
| Case | Cytological finding | HPV type determined by other verification method | Pathologyd |
|---|---|---|---|
| 19 | Negative | Negative | NA |
| 22 | Negative | 52b | NA |
| 28 | Negative | Negative | NA |
| 35 | Negative | Negative | NA |
| 121 | Negative | Negative | NA |
| 128 | Negative | Negative | NA |
| 133 | Negative | Negative | NA |
| 196 | LSIL | Negative | CIN1 |
| 204 | Negative | Negative | NA |
| 215 | ASCUS | Negative | Negative |
| 224 | Negative | Negative | NA |
| 260 | Negative | Negative | NA |
| 261 | Negative | Negative | NA |
| 282c | Negative | 16b | NA |
| 313e | LSIL | Negative | NA |
| 314 | HSIL | 52b | CIN3 |
| 328 | Negative | Negative | NA |
| 384 | ASCUS | Negative | NA |
n = 18.
Type-specific PCR.
Follow-up 3 months later showed that the sample was both HPV Blot and HCII negative, and follow-up 27 months later showed that the sample was HCII positive and HPV-39 positive by HPV Blot, while colposcopy-directed cervical biopsy showed immature squamous metaplasia.
NA, not applicable.
Follow-up 8 months later showed that the sample was both HPV Blot and HCII negative and had normal cytology.
DISCUSSION
Evidence has shown that HPV is the main cause of most CIN and invasive cervical cancers (24, 29, 30). The determination of HPV status in initial Pap specimens of ASCUS has been proposed to play a pivotal role in detecting cervical lesions as well as in assisted decision-making regarding treatment (18, 26). In a large cohort study conducted in a population of 46,009 patients undergoing primary screening, HCII was demonstrated to increase the detection of high-grade CIN in 995 women with ASCUS Pap tests (18). Sixty-five women (6.7%) had histologically proven CIN2 or CIN3 or invasive cancer. The HPV test was positive in 89.2% of women with histologic CIN2 or CIN3 or invasive cancer, compared with 76.2% detection by cytologic testing (18). Another study that included 4,075 women for primary screening revealed a prevalence of 3.2% with CIN3 or higher and a sensitivity of 88.2% detection for HPV testing by PCR (15).
In this study, we compared the PCR-based HPV Blot to the well-established, FDA-approved HCII method. Data obtained from cervical swab samples analyzed using the two methods of HPV DNA detection were compared in women with previous normal Pap smears or ASCUS. The PCR-based method is based on using modified sets of primers (SPF1/GP6+) that synthesize a PCR fragment of approximately 184 bp followed by revert blotting in a macroarray genechip, and direct DNA sequencing was performed to cross-check results (11). It is a highly sensitive method that discriminates 39 types simultaneously in a single reaction, without performing 39 separate hybridizations. Other reports of similar strategies identified 25 genotypes using the SPF1/2 primer set amplifying a 65-bp fragment within the L1 region, designated the line blot assay (13), and 27 subtypes by a reverse line blot detection method employing the MY09/11 primer set, which amplifies a 450-bp fragment within the L1 region (7).
The HPV-positive rates by HPV Blot and HCII were 18.2% versus 12.6% in normal, 45.7% versus 36.2% in ASCUS, 57.4% versus 57.4% in LSIL, and 75.0% versus 83.3% in HSIL Pap samples, respectively. The HPV detection rate increases as the severity of cervical abnormalities increases. The performance of HPV detection (positive or negative) between the HPV Blot method (39 types) and HCII (13 types) was similar, except for ASCUS. Results from 13 paired HCII oncogenic-type comparisons showed no difference in all cytological grades. Additionally, 26 HPV types detected by HPV Blot significantly affected the matched specimens in the ASCUS group by increasing HPV positivity. If these 26 non-HCII genotypes are unrelated to HSIL or invasive cancer, the HPV positivity would be of no clinical significance. However, a previous study from our group showed that HPV types other than the 13 HCII genotypes were present in invasive cervical carcinoma tissues from Taiwanese women (n > 2,000) (our unpublished data). HPV genotypes other than the 13 types represented by HCII (such as HPV-26, -53, -66, and -82) are necessary for a thorough assessment of the risk of cervical neoplasms (19).
Although HPV-16 has been the most prevalent genotype in many studies worldwide (29, 30), the detailed HPV genotype distribution is varied in different areas (12, 28). Huang et al. previously investigated 40 cervical carcinoma biopsy specimens from China, 87.5% of which contained HPV DNA, and HPV-52 and -58 were equally represented, with 42.5% being HPV-52 and/or HPV-58 positive, while only 37.5% were HPV-16 and/or HPV-18 positive (12). In 159 cervical carcinomas from Russia, 100% were positive for HPV by SPF1/2 PCR. The three most prevalent types were HPV-16 (64.8%) followed by HPV-18 (10.7%) and HPV-45 (8.2%) (12, 28). The knowledge of HPV genotyping of cervical cancer within a country or region is important for both primary screening and vaccination policy.
In this study, multiple infections were detected in 31.4% of samples. In our previous study of 149 cervical cancer specimens from patients undergoing neoadjuvant chemotherapy plus radical surgery using SPF1/GP6+ PCR and HPV Blot, HPV DNA sequences were detected in 100% of the samples, and multiple infections (32.2%) were found (11). van Doorn et al. also addressed the issue of multiple infections in a sample by comparing the PGMY line blot assay and the SPF10 line probe assay of 400 cervical scrape specimens (27). They found that different concentrations of two HPV genotypes with greater molar amounts were preferentially amplified.
The “gold standard” for disease status is difficult define in this study, as most of the specimens were cytology samples (23). In an attempt to reduce the limitations of comparisons between the two methods, a third detection method, such as direct sequencing, type-specific PCR, or HPV Blot genotyping of paraffin-embedded cervical biopsy, was employed to elucidate discrepant results. Data from multiple follow-up studies could facilitate comparisons of HPV DNA assays. A good concordance rate (80.8%) and substantial agreement (κ = 0.68) between the two methods were observed. Proficiencies of various HPV detection assays vary for different types of HPV, which was demonstrated in a recently published international collaborative study using a recombinant HPV DNA standard reagent panel in 29 laboratories from 12 countries (23).
In the discordant samples (n = 68), further studies seemed to be more consistent with HPV Blot in 92.6% of the samples (n = 63). Further investigation of the 15 HCII-positive samples but HPV Blot detected HPV types other than the 13 HCII high-risk types indicated cross-reactivity in all of them (Table 4). The most common subtypes for cross-reaction were types 70, CP8304, MM8, and 53. The dilemma of cross-reactivity entrapped in the cocktail was previously reported for comparisons with other PCR-based detection methods (1, 21, 22). In a subset of 210 paired specimens tested by HCII and the MY09/MY11/HMB01 PCR-based assay, The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study Group showed that 11.7% of LSIL specimens that were positive by the HCII high-risk cocktail were positive only by the PCR method for low-risk HPV types (HPV-6, -40, -42, -53, or -66), which are not included in the 13 HCII high-risk HPV types (1). Using restriction fragment analysis of PGMY09/PGMY11 PCR products, Poljak et al. successfully amplified a 450-bp fragment in 312 out of 325 HCII-positive specimens. They suspected that at least 15 HPV types cross-hybridized with the current HCII high-risk probe cocktail (22). The most frequent cross-hybridization types were HPV-53, -66, -54, -6, -40, and -42 in the 30 samples (9.6%; 30/312) (22). In our series, the probable cross-hybridization rate was 12.5% (15/120) in those samples that were positive by HCII. On the other hand, some reasons for possible false negatives by HPV Blot include suboptimal primer/probe design, low viral load, and false positivity on the part of the HCII assay.
The clinical significance of latent infection was still unsettled. In this series, 12.6% and 11.2% of samples with normal cytology contained 13 carcinogenic types by HCII and HPV Blot, respectively. In our previous study, the positive predictive value (recurrent high-grade CIN) of an abnormal follow-up cytology after conization for CIN3 was only 27%, while the positive predictive value of persistent HPV status (by HCII) was 19.8% with one test and 36% with at least three consecutive positive HPV tests (4). Follow-up of these women with latent infection is ongoing in another study from our group. Herrington et al. previously reported a follow-up study of 167 women with persistent borderline, wart virus, or mildly dyskaryotic changes in cervical cytology. CIN grade 2 or 3 was identified in 46 (31.3%) patients after a median follow-up period of 27 months. HPV positivity (types 6/11, 16, 18, 31, and 33) by both nonisotopic in situ hybridization and PCR-based methods was associated with high-grade CIN (8).
In conclusion, HPV Blot tends to detect more samples with HPV infection than HCII in those patients with normal and ASCUS cytology for a broader HPV type range. Besides, it is comparable to HCII with regard to the identification of oncogenic HPV genotypes.
Acknowledgments
This study was supported by grants from the Chang Gung Memorial Hospital (CMPRG32016-II) and National Science Council-Taiwan (NSC93-2314-B-182-036).
We thank Chee-Jen Chang from the Graduate Institute of Basic Medical Sciences, Chang Gung University, and Chun-Houh Chen from the Institute of Statistical Science, Academia Sinica, for assistance in statistical analysis.
REFERENCES
- 1.Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. 2000. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J. Natl. Cancer Inst. 92:397-402. [DOI] [PubMed] [Google Scholar]
- 2.Baay, M. F., W. G. Quint, J. Koudstaal, H. Hollema, J. M. Duk, M. P. Burger, E. Stolz, and P. Herbrink. 1996. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J. Clin. Microbiol. 34:745-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campion, M. J., D. J. McCance, J. Cuzick, and A. Singer. 1986. Progressive potential of mild cervical atypia: prospective cytological, colposcopic, and virological study. Lancet ii:237-240. [DOI] [PubMed] [Google Scholar]
- 4.Chao, A., C. T. Lin, S. Hsueh, H. H. Chou, T. C. Chang, M. Y. Chen, and C. H. Lai. 2004. Usefulness of human papillomavirus testing in the follow-up of patients with high-grade cervical intraepithelial neoplasia after conization. Am. J. Obstet. Gynecol. 190:1046-1051. [DOI] [PubMed] [Google Scholar]
- 5.Coutlee, F., P. Gravitt, H. Richardson, C. Hankins, E. Franco, N. Lapointe, H. Voyer, and the Canadian Women's HIV Study Group. 1999. Nonisotopic detection and typing of human papillomavirus DNA in genital samples by the line blot assay. J. Clin. Microbiol. 37:1852-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Roda Husman, A. M., J. M. Walboomers, A. J. van den Brule, C. J. Meijer, and P. J. Snijders. 1995. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J. Gen. Virol. 76:1057-1062. [DOI] [PubMed] [Google Scholar]
- 7.Gravitt, P. E., C. L. Peyton, R. J. Apple, and C. M. Wheeler. 1998. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J. Clin. Microbiol. 36:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrington, C. S., M. F. Evans, F. M. Charnock, W. Gray, and J. O'D McGee. 1996. HPV testing in patients with low grade cervical cytological abnormalities: a follow up study. J. Clin. Pathol. 49:493-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildesheim, A., M. H. Schiffman, P. E. Gravitt, A. G. Glass, C. E. Greer, T. Zhang, D. R. Scott, B. B. Rush, P. Lawler, M. E. Sherman, R. J. Kurman, and M. M. Manos. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. J. Infect. Dis. 169:235-240. [DOI] [PubMed] [Google Scholar]
- 10.Ho, G. Y., R. D. Burk, S. Klein, A. S. Kadish, C. J. Chang, P. Palan, J. Basu, R. Tachezy, R. Lewis, and S. Romney. 1995. Persistent genital human papillomavirus infection as a risk factor for persistent cervical dysplasia. J. Natl. Cancer Inst. 87:1365-1371. [DOI] [PubMed] [Google Scholar]
- 11.Huang, H. J., S. L. Huang, C. Y. Lin, R. W. Lin, F. Y. Chao, M. Y. Chen, T. C. Chang, S. Hsueh, K. H. Hsu, and C. H. Lai. 2004. Human papillomavirus genotyping by a polymerase chain reaction-based genechip method in cervical carcinoma treated with neoadjuvant chemotherapy plus radical surgery. Int. J. Gynecol. Cancer 14:639-649. [DOI] [PubMed] [Google Scholar]
- 12.Huang, S., I. Afonina, B. A. Miller, and A. M. Beckmann. 1997. Human papillomavirus types 52 and 58 are prevalent in cervical cancers from Chinese women. Int. J. Cancer 70:408-411. [DOI] [PubMed] [Google Scholar]
- 13.Kleter, B., L. J. van Doorn, L. Schrauwen, A. Molijn, S. Sastrowijoto, J. ter Schegget, J. Lindeman, B. ter Harmsel, M. Burger, and W. Quint. 1999. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 37:2508-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutsky, L. A., K. K. Holmes, C. W. Critchlow, C. E. Stevens, J. Paavonen, A. M. Beckmann, T. A. DeRouen, D. A. Galloway, D. Vernon, and N. B. Kiviat. 1992. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N. Engl. J. Med. 327:1272-1278. [DOI] [PubMed] [Google Scholar]
- 15.Kulasingam, S. L., J. P. Hughes, N. B. Kiviat, C. Mao, N. S. Weiss, J. M. Kuypers, and L. A. Koutsky. 2002. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 288:1749-1757. [DOI] [PubMed] [Google Scholar]
- 16.Lin, C. T., C. J. Tseng, C. H. Lai, S. Hsueh, K. G. Huang, H. J. Huang, and A. Chao. 2001. Value of human papillomavirus deoxyribonucleic acid testing after conization in the prediction of residual disease in the subsequent hysterectomy specimen. Am. J. Obstet. Gynecol. 184:940-945. [DOI] [PubMed] [Google Scholar]
- 17.Low, S. H., T. W. Thong, T. H. Ho, Y. S. Lee, T. Morita, M. Singh, E. H. Yap, and Y. C. Chan. 1990. Prevalence of human papillomavirus types 16 and 18 in cervical carcinomas: a study by dot and Southern blot hybridization and the polymerase chain reaction. Jpn. J. Cancer Res. 81:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manos, M. M., W. K. Kinney, L. B. Hurley, M. E. Sherman, J. Shieh-Ngai, R. J. Kurman, J. E. Ransley, B. J. Fetterman, J. S. Hartinger, K. M. McIntosh, G. F. Pawlick, and R. A. Hiatt. 1999. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 281:1605-1610. [DOI] [PubMed] [Google Scholar]
- 19.Munoz, N., F. X. Bosch, S. de Sanjose, R. Herrero, X. Castellsague, K. V. Shah, P. J. Snijders, and C. J. Meijer. 2003. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 348:518-527. [DOI] [PubMed] [Google Scholar]
- 20.Pao, C. C., S. S. Lin, C. Y. Lin, J. S. Maa, C. H. Lai, and T. T. Hsieh. 1991. Identification of human papillomavirus DNA sequences in peripheral blood mononuclear cells. Am. J. Clin. Pathol. 95:540-546. [DOI] [PubMed] [Google Scholar]
- 21.Peyton, C. L., M. Schiffman, A. T. Lorincz, W. C. Hunt, I. Mielzynska, C. Bratti, S. Eaton, A. Hildesheim, L. A. Morera, A. C. Rodriguez, R. Herrero, M. E. Sherman, and C. M. Wheeler. 1998. Comparison of PCR- and hybrid capture-based human papillomavirus detection systems using multiple cervical specimen collection strategies. J. Clin. Microbiol. 36:3248-3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poljak, M., I. J. Marin, K. Seme, and A. Vince. 2002. Hybrid Capture II HPV Test detects at least 15 human papillomavirus genotypes not included in its current high-risk probe cocktail. J. Clin. Virol. 25:S89-97. [DOI] [PubMed] [Google Scholar]
- 23.Quint, W. G., S. R. Pagliusi, N. Lelie, E. M. de Villiers, C. M. Wheeler, and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. 2006. Results of the first World Health Organization international collaborative study of detection of human papillomavirus DNA. J. Clin. Microbiol. 44:571-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffman, M. H., H. M. Bauer, R. N. Hoover, A. G. Glass, D. M. Cadell, B. B. Rush, D. R. Scott, M. E. Sherman, R. J. Kurman, S. Wacholder, C. K. Stanton, and M. M. Manos. 1993. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 85:958-964. [DOI] [PubMed] [Google Scholar]
- 25.Sherman, M. E., M. H. Schiffman, A. T. Lorincz, M. M. Manos, D. R. Scott, R. J. Kuman, N. B. Kiviat, M. Stoler, A. G. Glass, and B. B. Rush. 1994. Toward objective quality assurance in cervical cytopathology. Correlation of cytopathologic diagnoses with detection of high-risk human papillomavirus types. Am. J. Clin. Pathol. 102:182-187. [DOI] [PubMed] [Google Scholar]
- 26.Solomon, D., M. Schiffman, and R. Tarone. 2001. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J. Natl. Cancer Inst. 93:293-299. [DOI] [PubMed] [Google Scholar]
- 27.van Doorn, L.-J., W. Quint, B. Kleter, A. Molijn, B. Colau, M.-T. Martin, K.-In, N. Torrez-Martinez, C. L. Peyton, and C. M. Wheeler. 2002. Genotyping of human papillomavirus in liquid cytology cervical specimens by the PGMY line blot assay and the SPF10 line probe assay. J. Clin. Microbiol. 40:979-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Muyden, R. C., B. W. ter Harmsel, F. M. Smedts, J. Hermans, J. C. Kuijpers, N. T. Raikhlin, S. Petrov, A. Lebedev, F. C. Ramaekers, J. B. Trimbos, B. Kleter, and W. G. Quint. 1999. Detection and typing of human papillomavirus in cervical carcinomas in Russian women: a prognostic study. Cancer 85:2011-2016. [PubMed] [Google Scholar]
- 29.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 30.zur Hausen, H. 1996. Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta 1288:F55-F78. [DOI] [PubMed] [Google Scholar]


