Abstract
Invasive disease due to Corynebacterium diphtheriae is rare in North America. Here we describe the emergence of a predominant clone of a nontoxigenic strain of C. diphtheriae in the impoverished population of Vancouver's downtown core. This clone has caused significant morbidity and contributed to at least two deaths. Over a 5-year period, seven cases of bacteremia due to C. diphtheriae were detected in patients admitted to Vancouver hospitals. Injection drug use, diabetes mellitus, skin colonization/infection with C. diphtheriae, and homelessness all appeared to be related to the development of bacteremia with the organism. Ribotyping of isolates recovered from blood culture revealed a predominant ribotype pattern that has not previously been reported in North America.
Over the past 15 years, there has been an increase in the incidence of infections caused by Corynebacterium diphtheriae in many parts of the world. The recent epidemic of respiratory diphtheria caused by toxigenic strains of C. diphtheriae in the Newly Independent States of the former Soviet Union is a prime example of this epidemiological shift (12, 24).
Systemic infections due to C. diphtheriae are also being increasingly reported. Cases of invasive disease with nontoxigenic strains have been described for injection drug users in Switzerland (15), Aborigines in Australia (17, 18), homeless alcoholics in France (22), and patients with bone or joint infections in Germany (11). In addition, an increasing proportion of strains isolated in the United Kingdom are nontoxigenic (29, 30). In Canada, diphtheria has been limited to mainly small outbreaks in First Nations (i.e., indigenous North American Indian) populations, with the most recent outbreaks occurring over 30 years ago (3).
More recently, seven patients with bacteremia due to C. diphtheriae were admitted to three hospitals in greater Vancouver over a 5-year period. Six of seven isolates from those patients were found to be nontoxigenic C. diphtheriae subsp. mitis. To the best of our knowledge, we are the first to report the emergence of invasive bloodstream infections caused by nontoxigenic C. diphtheriae in North America.
Clinical and laboratory findings for seven patients with C. diphtheriae bacteremia are summarized in Table 1.
TABLE 1.
Clinical and laboratory data for seven patients with C. diphtheriae bacteremia
| Patient number | Age/sex | Related factorsa | Organisms from wounds | Evidence of endocarditis | Toxin status | Outcome | Isolate number |
|---|---|---|---|---|---|---|---|
| 1 | 45/M | DM, EtOH | C. diphtheriae, A. haemolyticum, S. agalactiae | No | Nontoxigenic | Survived | 00/515/CD |
| 2 | 51/M | DM, EtOH | C. diphtheriae, S. aureus, E. avium | No | Nontoxigenic | Survived | 00/516/CD |
| 3 | 67/M | EtOH | S. aureus, group G streptococci, P. mirabilis, Alcaligenes sp., A. haemolyticum | No | Nontoxigenic | Survived | 00/517/CD |
| 4 | 58/M | IDU, EtOH | No wounds | No | Nontoxigenic | Died | 00/518/CD |
| 5 | 49/F | EtOH, PulmCa | No wounds | No | Nontoxigenic | Died | 00/519/CD |
| 6 | 29/F | IDU | C. diphtheriae, S. aureus, A. haemolyticum (on subsequent admission) | Yes, TVb | Nontoxigenic | Survived | 00/520/CD |
| 7 | 33/M | ALL, IDU | S. aureus, coagulase-negative staphylococci, “diphtheroids” | No | Toxigenic | Survived | 00/514/CD |
DM, diabetes mellitus; EtOH, alcohol abuse; IDU, injection drug use; Pulm Ca, pulmonary cancer; ALL, acute lymphoblastic leukemia.
TV, tricuspid valve.
MATERIALS AND METHODS
Microbiological investigation.
Blood cultures were collected from hospitalized patients and incubated in either a BacT/Alert analyzer (bioMérieux, Durham, North Carolina) or a Bactec 9420 analyzer (Becton Dickinson, Sparks, Maryland). Specimens from skin and miscellaneous wounds were inoculated onto standard microbiological culture media, including 5% sheep's blood agar, chocolate agar, and MacConkey agar. Positive cultures revealing gram-positive bacilli in palisade formation on Gram stain and exhibiting typical V and L forms were tested for catalase and oxidase and subcultured to Tinsdale medium. Coryneform bacteria with black colonies and brown halos on Tinsdale medium were further identified by using the API Coryne reaction strip (bioMérieux, Durham, NC). All organisms identified as C. diphtheriae were sent to the National Microbiology Laboratory of the Public Health Agency of Canada for confirmatory testing by conventional methods, including starch fermentation/utilization tests.
Toxin studies were carried out using the modified Elek test (10) and PCR for the diphtheria toxin gene (6, 21). Isolates from patients 1 to 4 were also tested for toxin production by the in vivo guinea pig inoculation test.
Molecular investigation.
Ribotyping is currently considered the preferred molecular method for subtyping C. diphtheriae (4, 5, 13). Within the European Laboratory Working Group on Diphtheria (9), standardization of the protocol has been established and the ribotype patterns so obtained have been used to construct a database of C. diphtheriae ribotype patterns generated by BstEII (14). In this study, ribotyping was employed to determine the genetic diversity of strains that were isolated from blood cultures. Automatic identification of a ribotype pattern was achieved by interrogating the profiles against the reference patterns held in the database of the C. diphtheriae ribotype patterns generated by BstEII.
A total of seven C. diphtheriae isolates were sent as freeze-dried preparations to the WHO Collaborating Centre for Diphtheria and Streptococcal Infections, Diphtheria Reference Unit, Health Protection Agency Centre for Infections (HPA CfI), London, United Kingdom, for ribotyping (4, 5, 9). The specimens were reconstituted in 1 ml of nutrient broth, left for 1 min before streaking onto Columbia blood agar plates, and incubated aerobically for 24 h at 37°C. All cultures were biotyped at the HPA CfI to validate the strain identity by using conventional biochemical methods, and toxigenicity testing was performed by using the Elek test as described previously (7, 8, 10).
Chromosomal DNA was prepared by a modified version of the method described by Pitcher et al. (23). Purified DNA (3 to 5 μg) from the corynebacteria was cleaved with BstEII (Sigma, St. Louis, MO), and the marker strain, Citrobacter koseri CIP 105177, was cleaved with MluI according to the manufacturer's instructions. Restriction fragments were separated by agarose (0.7%) (Seakem ME; FMC Bioproducts, Rockland, ME) gel electrophoresis in 0.5× Tris-borate-EDTA buffer for 16 h at 1.5 V/cm to separate fragments of 1 to 20 kb. The DNA fragments were transferred onto a nylon membrane (HybondN+; Amersham International, Buckinghamshire, United Kingdom) and hybridized with a digoxigenin (DIG)-labeled OligoMix5 probe (2 pmol/ml) in DIG Easy Hyb (Boehringer, Milan, Italy). The procedures of hybridization with the DIG oligonucleotide probe and detection of bound DIG were performed according to modified versions described by Regnault et al. (25). Hybridization was performed at 37°C for 4 h, and posthybridization washes were performed at 41°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate, (two 5-min washes) and then in 0.1× SSC, 0.1% sodium dodecyl sulfate (two 5-min washes). Ribotype patterns were interpreted using the Taxotron software package (Taxolab; Institut Pasteur, Paris, France) according to Regnault et al. (25).
A tolerance function (percent tolerated error) was set as a fixed value of 5%, indicating that two fragments were considered identical if their sizes did not differ by more than 5%. Clustering was achieved by the Adanson program following the single-linkage algorithm and the unweighted-pair group method using average linkages (27). Finally, a dendrogram was generated by the Dendrograf program of the Taxotron package. A ribotype was defined as an isolated pattern or as a group of patterns clustered at distance zero. Ribotypes were unaffected by the error setting (within the limits of 4 to 5%).
RESULTS
Seven patients had blood cultures that were positive for bacteria that were subsequently identified as C. diphtheriae. Time to positivity for blood cultures ranged from 17 to 48 h. All of the C. diphtheriae isolates recovered from blood were catalase positive and oxidase negative and exhibited typical stacking formations on Gram stain. All of the isolates grew on Tinsdale medium as black colonies surrounded by brown halos. Using the API Coryne reaction strip, six of the seven isolates were identified as C. diphtheriae subsp. mitis, while a single isolate (from patient 7) was identified as C. diphtheriae subsp. gravis. The identification of all of the isolates was confirmed at the National Microbiology Laboratory of the Public Health Agency of Canada by conventional methods (Table 2). Results from starch utilization/fermentation and glycogen fermentation tests were negative for the C. diphtheriae subsp. mitis isolates and positive for the C. diphtheriae subsp. gravis isolate. Seven out of seven isolates were reported as susceptible to penicillin G and erythromycin.
TABLE 2.
Summary of subtype, toxigenicity, and ribotype of C. diphtheriae isolates from blood cultures
| Patient no. | HPAa reference | Subtype | Toxigenicity | Ribotypeb
|
||
|---|---|---|---|---|---|---|
| Cluster | Provisional name | Old name | ||||
| 1 | 00/515/CD | mitis | Nontoxigenic | SMC | Novel | NA |
| 2 | 00/516/CD | mitis | Nontoxigenic | 1 | Tunisia | NA |
| 3 | 00/517/CD | mitis | Nontoxigenic | 1 | Tunisia | NA |
| 4 | 00/518/CD | mitis | Nontoxigenic | 1 | Tunisia | NA |
| 5 | 00/519/CD | mitis | Nontoxigenic | 1 | Tunisia | NA |
| 6 | 00/520/CD | mitis | Nontoxigenic | 1 | Novel | NA |
| 7 | 00/514/CD | gravis | Toxigenic | SMC | Pamiers | D44 |
HPA, Health Protection Agency.
SMC, single-member cluster; NA, not applicable.
All of the isolates that had been identified as C. diphtheriae subsp. mitis were found to be nontoxigenic by both the modified Elek test and PCR (isolates from patients 1 to 4 were also found to be nontoxigenic by in vivo testing). The single isolate from patient 7 which had been identified as C. diphtheriae subsp. gravis was found to be toxigenic by both the modified Elek test and PCR.
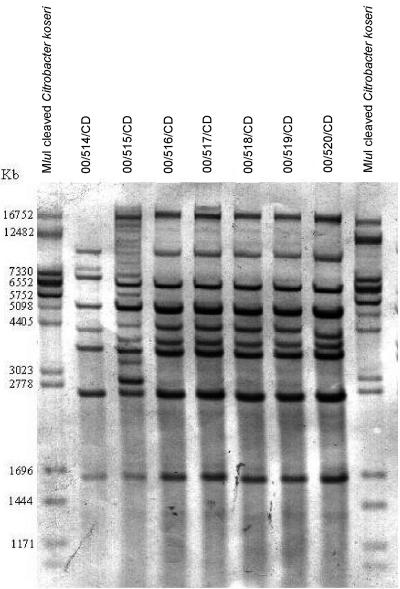
The hybridization of genomic DNA digested with BstEII revealed characteristic rRNA gene restriction patterns among the seven strains isolated from blood culture (Fig. 1). An examination of the restriction fragment length polymorphisms of ribosomal genes of the seven strains revealed three distinct ribotype clusters. The numbers of rRNA gene restriction fragments ranged from seven to nine for the BstEII digests.
FIG. 1.
BstEII ribotype profiles of C. diphtheriae isolated from blood cultures. Lanes 1 and 9, MluI-cleaved Citrobacter koseri as a size standard (sizes are indicated on the left); lanes 2 to 8, ribotypes of 00/514/CD, 00/515/CD, 00/516/CD, 00/517/CD, 00/518/CD, 00/519/CD, and 00/520/CD.
The nomenclature for C. diphtheriae ribotypes generated by BstEII is based on geographic location (14). Five of the six nontoxigenic C. diphtheriae subsp. mitis strains (isolates 00/516/CD, 00/517/CD, 00/518/CD, 00/519/CD, and 00/520/CD) were grouped together in cluster 1. Of these, four strains shared the reference ribotype pattern “Tunisia” held in the ribotype database. To date, the ribotype pattern Tunisia is represented by only one strain in the database and therefore the ribotype pattern of strain 00/520/CD (showing very slight fragment size variation) could not be confirmed as Tunisia.
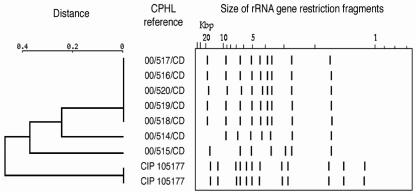
Cluster analysis did not provide information about the phylogenetic relationship between strains (2); however, the dendrogram did reveal a single, predominant ribotype pattern (Fig. 2).
FIG. 2.
Dendrogram and schematic representation of the ribotype profiles obtained by comparison of BstEII ribotype patterns from seven strains of C. diphtheriae. Citrobacter koseri (CIP 105177) served as the molecular weight marker on the agarose gel.
DISCUSSION
We report a cluster of seven cases of nontoxigenic C. diphtheriae bacteremia that occurred in Vancouver over a period of 5 years. Invasive infections with this organism are rare: during the entire period from 1893 to 1985, only 13 cases of bacteremia with toxigenic and nontoxigenic C. diphtheriae infections were reported in the medical literature (26). In Vancouver, the last and perhaps only previous case of invasive infection with C. diphtheriae was over 20 years ago when a man with leukemia developed sepsis due to a nontoxigenic strain of C. diphtheriae subsp. gravis (19).
All but one of the isolates were found to be nontoxigenic C. diphtheriae subsp. mitis. A single, predominant ribotype, which has not previously been reported in North America, was recovered from five of the seven patients. The lone toxigenic C. diphtheriae subsp. gravis isolate was identified as Pamiers, an outbreak strain isolated in 1989 from four different members of a family in France.
Most patients in this series presented concurrently with skin lesions (e.g., cutaneous ulcers, pustules, and diabetic foot ulcers), three of which grew nontoxigenic C. diphtheriae. Although the portal of entry for invasive cases of nontoxigenic C. diphtheriae has yet to be fully elucidated, skin and breaks therein appear to be the most likely sources (1). Person-to-person transmission of the organism seems to be more efficient via the cutaneous route than the respiratory route (20); moreover, skin colonized with C. diphtheriae may act as a reservoir for the organism between epidemics. Cutaneous diphtheria is a persistent problem in Vancouver's skid row area, and outbreaks of cutaneous disease in this population have previously been described (3). It is not unusual for patients with cutaneous diphtheria to be coinfected with streptococci and/or staphylococci. Interestingly, Arcanobacterium haemolyticum was also recovered from a number of wounds in our series.
In this study, nearly all of the patients with C. diphtheriae bacteremia lived in or frequented the impoverished urban core of Vancouver. Similar to the case for outbreaks of diphtheria in Seattle in the 1980s among urban alcoholic men (27), most of our patients were male (five of seven) and had a history of alcohol abuse (four of seven). Homelessness, injection drug use, diabetes mellitus, and skin colonization/infection with C. diphtheriae all appeared to be predisposing factors for the development of bacteremia. Human immunodeficiency virus positivity did not appear to increase risk for invasive C. diphtheriae disease despite the relatively high prevalence of human immunodeficiency virus infection in Vancouver's indigent population. Many of the patients in this series led itinerant lifestyles and may have acquired the organism in the packed hotels and rooming houses of the inner city. Transmission of C. diphtheriae appears to be facilitated by crowded, unhygienic living conditions. Blankets and other fomites may serve as reservoirs and have been implicated in previous outbreaks (3, 16).
In 1996, a report from Australia described the emergence of an invasive nontoxigenic clone that was responsible for three cases of endocarditis and was isolated from throat cultures of close contacts (18). In 1997, a report from France described 40 strains of nontoxigenic C. diphtheriae isolated from sterile sites, including 34 from blood cultures (22). Almost half of the patients developed endocarditis, and the fatality rate was 36% despite specific antibiotic treatment. Twenty-four of the 34 bloodstream isolates were of the same ribotype, with 19 of the 24 being from the Paris area. In 1998, a report from Switzerland described an outbreak of an invasive clone of nontoxigenic C. diphtheriae in the injection drug user population of Zürich (15). Nontoxigenic C. diphtheriae was recovered from the blood of 13 patients with a histories of injection drug use; of those, 9 developed endocarditis and 4 died. Following molecular studies, it was discovered that all of the isolates that were associated with intravenous drug use were of the same ribotype. In 1999, a report from Germany described the emergence of a nontoxigenic strain of C. diphtheriae in six patients with bone/joint infections (11). The isolates had the same ribotype pattern and appeared to be related to the Swiss clone reported in injection drug users in 1998.
Even in the absence of toxin production, C. diphtheriae appears to have an impressive propensity to cause invasive bloodstream infections. Systemic complications of C. diphtheriae bacteremia are not unusual and include endocarditis, joint infections, and peripheral embolic disease (28). The pathogenesis for nontoxigenic C. diphtheriae infections remains unknown, although the various manifestations of severe disease suggest that the organism has a predilection for endocardial and synovial tissues.
Routine diphtheria toxoid vaccination was introduced in Vancouver in 1929 and has been a part of the routine universal immunization schedule in Canada for many decades. So, it is likely that all seven case patients from this report received diphtheria toxoid vaccine. Given the success of the diphtheria vaccine in preventing toxin-mediated disease, it is possible that selective pressure has forced C. diphtheriae to express or develop other mechanisms for causing disease. At the present time, the details of these mechanisms remain unclear.
This report also highlights the importance of considering all organisms recovered from blood, even so-called “diphtheroids,” as potential pathogens. Positive blood cultures need to be interpreted within the patient's clinical context in order to differentiate skin contaminants from true pathogens. Failure to appreciate this point, especially in patient populations that are known to carry pathogenic and potentially invasive organisms such as C. diphtheriae, may lead to delays in instituting specific antibiotic treatment and appropriate public health measures.
In conclusion, we report the emergence of an invasive clone of nontoxigenic C. diphtheriae, represented by a predominant ribotype, in Vancouver's urban poor population. Underlying factors associated with the development of C. diphtheriae bacteremia included homelessness, abuse of alcohol and injection drugs, and diabetes mellitus. Endocarditis was diagnosed in only one patient. Skin colonization with C. diphtheriae was common, and breaks in skin were the most likely portals of entry for the organism. The pathogenesis of invasive disease caused by nontoxigenic C. diphtheriae is presently not well understood, and further studies at the cellular and molecular levels are warranted.
Acknowledgments
We thank Carol Shaw at the British Columbia Centre for Disease Control for some of the toxin studies and the in vivo guinea pig inoculation test and Deborah Wiebe at the National Microbiology Laboratory of the Public Health Agency of Canada. We also gratefully acknowledge P. A. D. Grimont for allowing the use of the database of C. diphtheriae ribotypes generated by BstEII.
REFERENCES
- 1.Belsey, M. A., M. R. Sinclair, M. Roder, and D. R. Leblanc. 1969. Corynebacterium diphtheriae skin infections in Alabama and Louisana: a factor in the epidemiology of diphtheria. N. Engl. J. Med. 280:135-141. [DOI] [PubMed] [Google Scholar]
- 2.Brosch, R., G. F. Lefèvre, and P. A. D. Grimont. 1996. Taxonomic diversity of pseudomonads revealed by computer-interpretation of ribotyping data. Syst. Appl. Microbiol. 19:541-555. [Google Scholar]
- 3.Cockroft, W., W. Boyko, and D. Allen. 1973. Cutaneous infections due to Corynebacterium diphtheriae. Can. Med. Assoc. J. 108:329-331. [PMC free article] [PubMed] [Google Scholar]
- 4.De Zoysa, A., and A. Efstratiou. 1999. PCR typing of Corynebacterium diphtheriae by random amplification of polymorphic DNA. J. Med. Microbiol. 48:335-340. [DOI] [PubMed] [Google Scholar]
- 5.De Zoysa, A., A. Efstratiou, R. George, M. Jahkola, J. Vuopio-Varkila, S. Deshevoi, G. Tseneva, and Y. Rikushin. 1995. Molecular epidemiology of Corynebacterium diphtheriae from northwestern Russia and surrounding countries studied by using ribotyping and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:1080-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efstratiou, A., K. Engler, C. Dawes, and D. Sesardic. 1998. Comparison of phenotypic and genotypic methods for detection of diphtheria toxin among isolates of pathogenic corynebacteria. J. Clin. Microbiol. 36:3173-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efstratiou, A., K. Engler, I. K. Mazurova, T. Glushkevich, J. Vuopio-Varkila, and T. Popovic. 2000. Current approaches to the laboratory diagnosis of diphtheria. J. Infect. Dis. 181(Suppl.):S138-S145. [DOI] [PubMed] [Google Scholar]
- 8.Efstratiou, A., and P. A. C. Maple. 1994. Manual for the laboratory diagnosis of diphtheria. The Expanded Programme on Immunization in the European Region of WHO. World Health Organization, Copenhagen, Denmark.
- 9.Efstratiou, A., and C. Roure. 2000. The European Laboratory Working Group on Diphtheria: a global microbiologic network. J. Infect. Dis. 181(Suppl.):S146-S151. [DOI] [PubMed] [Google Scholar]
- 10.Engler, K., T. Glushkevich, I. Mazurova, R. C. George, and A. Efstratiou. 1997. A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J. Clin. Microbiol. 35:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funke, G., M. Altwegg, L. Grommelt, and A. von Graevenitz. 1999. Emergence of related nontoxigenic Corynebacterium diphtheriae biotype mitis strains in Western Europe. Emerg. Infect. Dis. 5:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galazka, A., S. Robertson, and G. Oblapenko. 1995. Resurgence of diphtheria. Eur. J. Epidemiol. 11:95-105. [DOI] [PubMed] [Google Scholar]
- 13.Grimont, F., and P. A. D. Grimont. 1995. Determination of rRNA gene restriction patterns, p. 149-164. In J. Howard and D. M. Whitcombe (ed.), Diagnostic bacteriology protocols. Humana Press, Totowa, N.J.
- 14.Grimont, P. A. D., F. Grimont, A. Efstratiou, A. De Zoysa, I. Mazurova, C. Ruckly, M. Lejay-Collin. S. Martin-Delautre, and B. Regnault. 2004. International nomenclature for Corynebacterium diphtheriae ribotypes. Res. Microbiol. 155:162-166. [DOI] [PubMed] [Google Scholar]
- 15.Gubler, J., C. Huber-Schneider, E. Gruner, and M. Altwegg. 1998. An outbreak of nontoxigenic Corynebacterium diphtheriae infection: single bacterial clone causing invasive infection among Swiss drug users. Clin. Infect. Dis. 27:1295-1298. [DOI] [PubMed] [Google Scholar]
- 16.Harnisch, J., E. Tronca, C. Nolan, M. Turck, and K. K. Holmes. 1989. Diphtheria among alcoholic urban adults: a decade of experience in Seattle. Ann. Intern. Med. 111:71-82. [DOI] [PubMed] [Google Scholar]
- 17.Hogg, G., J. Strachan, L. Huayi, S. A. Beaton, P. A. Robinson, and K. Taylor. 1996. Non-toxigenic Corynebacterium diphtheriae biovar. gravis: evidence for an invasive clone in a south-eastern Australian community. Med. J. Aust. 164:72-75. [DOI] [PubMed] [Google Scholar]
- 18.Holthouse, D., B. Power, A. Kermode, and C. Golledge. 1998. Non-toxigenic Corynebacterium diphtheriae: two cases and review of the literature. J. Infect. 37:62-66. [DOI] [PubMed] [Google Scholar]
- 19.Isaac-Renton, J., W. Boyko, R. Chan, and E. Crichton. 1981. Corynebacterium diphtheriae septicemia. Am. J. Clin. Pathol. 75:631-634. [DOI] [PubMed] [Google Scholar]
- 20.Koopman, J., and J. Campbell. 1975. The role of cutaneous diphtheria infections in a diphtheria epidemic. J. Infect. Dis. 131:239-244. [DOI] [PubMed] [Google Scholar]
- 21.Pallen, M., A. Hay, L. Puckey, and A. Efstratiou. 1994. Polymerase chain reaction for screening clinical isolates of corynebacteria for the production of diphtheria toxin. J. Clin. Pathol. 47:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patey, O., F. Bimet, P. Riegel, B. Halioua, J. P. Emond, E. Estrangin, S. Dellion, J. M. Alonso, M. Kiredjian, A. Dublanchet, and C. Lafaix. 1997. Clinical and molecular study of Corynebacterium diphtheriae systemic infections in France. J. Clin. Microbiol. 35:441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 24.Popovic, T., I. Mazurova, A. Efstratiou, J. Vuopio-Varkila, M. W. Reeves, A. De Zoysa, T. Glushkevich, and P. Grimont. 2000. Molecular epidemiology of diphtheria. J. Infect. Dis. 181(Suppl.):S168-S177. [DOI] [PubMed] [Google Scholar]
- 25.Regnault, B., F. Grimont, and P. A. D. Grimont. 1997. Universal ribotyping method using a chemically labelled oligonucleotide probe mixture. Res. Microbiol. 148:649-659. [DOI] [PubMed] [Google Scholar]
- 26.Sirinavin, S., and P. Suthas-Na-Ayuthaya. 1985. Diphtheritic septicaemia and probable endocarditis: a case report and review of the literature. Eur. J. Pediatr. 144:395-398. [DOI] [PubMed] [Google Scholar]
- 27.Sneath, P. H., and R. R. Sokal. 1973. Numerical taxonomy: the principles and practice of numerical classification. W. H. Freeman, San Francisco, Calif.
- 28.Tiley, S., K. Kociuba, L. Heron, and R. Munro. 1993. Infective endocarditis due to nontoxigenic Corynebacterium diphtheriae: report of seven cases and review. Clin. Infect. Dis. 16:271-275. [DOI] [PubMed] [Google Scholar]
- 29.Wilson, A., A. Efstratiou, E. Weaver, E. Allason-Jones, J. Bingham, G. L. Ridgway, A. Robinson, D. Mercey, G. Colman, and B. D. Cookson. 1992. Unusual non-toxigenic Corynebacterium diphtheriae in homosexual men. Lancet 339:998. [DOI] [PubMed] [Google Scholar]
- 30.Wilson, A. 1995. The return of Corynebacterium diphtheriae: the rise of non-toxigenic strains. J. Hosp. Infect. 30(Suppl.):306-312. [DOI] [PubMed] [Google Scholar]