Abstract
We sequenced the adhesin-cell wall-anchoring domain of the atlE gene of 49 invasive and commensal Staphylococcus epidermidis strains. We identified 22 alleles, which could be separated into two main groups: group 1 (alleles 1 and 6 to 16, 32/49 strains) and group 2 (alleles 2 to 5 and 17 to 22, 17/49 strains). Allele 1 (the type strain sequence) was by far the most prevalent (21 of 49 strains). Multilocus sequence typing showed a clear relationship between the atlE allele and the sequence type (ST), with the “nosocomial” ST27 clone and closely related STs expressing group 1 alleles.
Staphylococcus epidermidis is a ubiquitous commensal organism of human skin and mucous membranes and is one of the main bacterial agents involved in infections related to the presence of indwelling or implanted foreign bodies (10). AtlE, encoded by the atlE gene (8), is thought to play a key role in the pathogenicity of this bacterium, enabling it to adhere to the surface of implanted medical devices (19). AtlE is a bifunctional autolysin with an N-terminal alanine amidase domain, a central cell wall-anchoring (CWA) domain consisting of three repeats (R1 to R3), each about 165 amino acids long, and a C-terminal glucosaminidase domain. It is secreted as a proenzyme and is cleaved between R2 and R3 at the surface of the bacterium, generating two separate enzymes: a 60-kDa alanine amidase containing R1 and R2 at its C terminus and a 52-kDa glucosaminidase containing R3 at its N terminus. The products of AtlE processing have been shown to have adhesive properties (8), probably involving domains R1, R2, and R3 (9).
Other autolysins from gram-positive bacteria able to mediate bacterial adhesion via their CWA domains have been described. These adhesins-autolysins include Aas from Staphylococcus saprophyticus (9), AtlC from Staphylococcus caprae (1, 2), and Ami from Listeria monocytogenes (14). The staphylococcal adhesins-autolysins AtlE, Aas, and AtlC appear to have different adhesion patterns (1, 8, 9). For example, unlike Aas (9), AtlE mediates binding to vitronectin but not to fibronectin (8). Thus, the adhesion patterns mediated by adhesins-autolysins clearly differ between species within the genus Staphylococcus, probably because of differences in the CWA domains of these molecules. We recently showed that the structure and function of the CWA domain of the adhesin-autolysin Ami vary within L. monocytogenes (15). Thus, adhesins-autolysins may also display intraspecific polymorphism, resulting in differences in binding capacity between strains of the same species. To date, atlE has been sequenced in only a few strains (6, 8, 25). It is therefore unclear whether the CWA domain of AtlE displays polymorphism in invasive and commensal strains. We investigated this possibility by sequencing the DNA region encoding the CWA domain of AtlE (atlEcwa) in a large panel of S. epidermidis strains.
We studied a total of 49 S. epidermidis strains, including 27 clinical strains from 27 individuals (RPC99P332, RPC99P469, RPC99P756, RPC99P1232, RPC99P1478, RPC99P1794, RPC00P332, RPC00P865, RPC00C0506, RPC01C0086, RPC01C0113, RPC01C0123, RPC01C0186, RPC01C0209, RPC01C0326, RPC01C0382, RPC01C0944, RPC01C1020, RPC01C1231, RPC01C1366, RPC02C0294, RPC02C0280, RPC02C0598, RPC02C1560, RPC02C1720, RPC02C1854, and RPC02G4270 [this study]) and 22 commensal strains from the cutaneous flora of 20 individuals (RPC02E1b, RPC02E4a, RPC02E9a, RPC02E19a, RPC02E21a, RPC02L9b, RPC02L10a, RPC02L11, RPC02V6c, RPC02V36a, RPC02V42a, RPC02V47b, RPC02V48b, RPC02V50a, RPC02V51b [this study], Hcc1, Hcc2, Hff7, Hmm1, Htt4, Hww1, and Hww2 [5]). Type strain CIP 81.55T (= ATCC 14990T) and reference strain CIP 105777 (= ATCC 35984) were also included in this study. We studied only one strain per individual unless genotyping clearly demonstrated that strains were unrelated (strains Hcc1 and Hcc2 and strains Hww1 and Hww2 [5]). Strains were identified by gram staining, catalase tests, slide tests, tube coagulase tests, and the ID 32 Staph system. All strains were assigned to a species on the basis of partial sodA sequencing (17) as previously described (21). Bacteria were stored in vials of beads covered with cryogenic preservative solution (Cryobank, Mast Group Ltd., Bootle, Merseyside, United Kingdom) at −80°C.
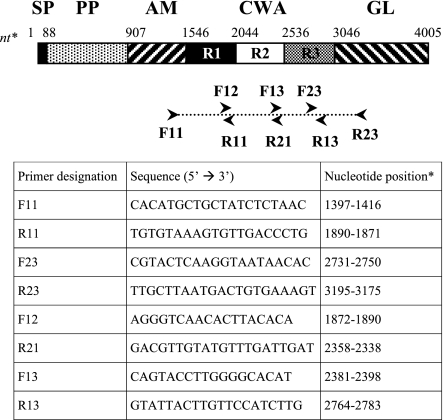
DNA was prepared from bacteria grown on sheep blood agar at 37°C for 18 h under aerobic conditions. Colonies were scraped from the plates and resuspended in sterile distilled water (McFarland 5). DNA was extracted by boiling or with the DNeasy tissue kit (QIAGEN SA, Courtabœuf, France) as recently described (22). We used the F11 and R23 (Fig. 1) primers to amplify a 1,799-bp fragment encompassing atlEcwa. The reaction mixture (50 μl) contained 5 μl of template, 200 μM each deoxyribonucleoside triphosphate, 0.25 μM each primer, and 2.5 U of Taq DNA polymerase in 1× buffer containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, and 1.5 mM MgCl2 (Sigma, St. Louis, Missouri). Amplification reactions were performed in a PCR Express thermal cycler (Thermo Hybaid, Ashford, Middlesex, United Kingdom) as follows: initial denaturation for 5 min at 95°C; 35 cycles of 30 s at 94°C, 60 s at 49°C, and 60 s at 72°C; followed by 10 min at 72°C. PCR products were purified by ExoSAP-IT (USB Corporation, Cleveland, Ohio) digestion for 30 min at 37°C (2 μl of enzyme mixture per 5 μl of PCR product), followed by enzyme inactivation for 15 min at 80°C. Dideoxy sequencing was performed with the DYEnamic ET Terminator Cycle sequencing kit (Amersham Biosciences Corp., Piscataway, NJ) and primers F11, F12, F13, F23, R11, R13, R21, and R23 (this study; Fig. 1). All primers were hybridized at 49°C. Dideoxy sequencing products were purified by gel filtration (Sephadex G25; Amersham Biosciences) and run on a Megabace fluorescence analyzer (Amersham Biosciences). Sequences were analyzed with BioEdit software (7) and aligned with ClustalW (23) software. Phylogenetic trees were constructed by the neighbor-joining method as implemented in MEGA version 2.1 (13) with Kimura's two-parameter model. Bootstrap values for the trees were calculated from 1,000 iterations.
FIG. 1.
Primers used in this study. Abbreviations: SP, signal peptide; PP, propeptide; AM, amidase domain, catalytic center; GL, glucosaminidase domain, catalytic center; R1, R2, and R3, repeats 1, 2, and 3 of the CWA domain. Nucleotide (nt) positions are given relative to the sequence with GenBank accession number U71377, region 2620 to 6627 coding sequence.
All of the strains studied yielded a PCR fragment of the expected size (∼1,800 kb) with primers F11 and R23. This rules out the possibility of large deletions or duplications involving the CWA domain repeats, as observed in Aas (9) and Ami (15). Our results also confirm that atlE is ubiquitous in S. epidermidis, as suggested by a previous Southern blotting study (4). Previous PCR studies have provided conflicting results concerning the possible presence of atlE in S. epidermidis, with 52 to 100% positive results (3, 11, 16, 18). These discrepancies may be related to the choice of primers, the quality of the DNA extracted, and possibly the origin of strains or their misidentification as S. epidermidis. Thus, the available data suggest that atlE is probably ubiquitous in this species, consistent with the important role that its product is thought to play in bacterial physiology.
Analysis of the nucleotide sequence of the atlEcwa domain (nucleotides 1546 to 3045) showed few differences between strains; the most distantly related sequences differed by only 42 nucleotides over a length of about 1,500 nucleotides (maximal interstrain divergence, 2.8%). We identified 22 alleles (alleles 1 to 22), with the sequence of the S. epidermidis type strain (CIP 81.55T; this study) arbitrarily designated allele 1 (Table 1). These alleles could be separated into two main groups (Fig. 2), group 1 (alleles 1 and 6 to 16; 97.8% nucleotide identity; 32/49 strains, 65%) and group 2 (alleles 2 to 5 and 17 to 22; 98.8% nucleotide identity; 17/49 strains, 35%). Allele 1 predominated in group 1 (21/32, 65.6%), and allele 2 predominated in group 2 (5/17, 29.4%). About 50% (13/27) of the clinical strains and more than 85% (19/22) of the commensal strains expressed group 1 alleles. Allele 1 was the predominant group 1 allele in both clinical (8/13) and commensal (13/19) strains.
TABLE 1.
atlEcwa alleles identified in this study
| Allelea | Prototype strain | GenBank accession no. |
|---|---|---|
| 1 | CIP 81.55T | AJ887965 |
| 2 | Hww1 | AJ887966 |
| 3 | RPC99P756 | AJ887967 |
| 4 | RPC02C1560 | AJ887968 |
| 5 | RPC02C0280 | AJ887969 |
| 6 | RPC02C1720 | AJ887970 |
| 7 | RPC01C1231 | AJ887971 |
| 8 | RPC02C1854 | AJ887972 |
| 9 | RPC02V51b | AJ887973 |
| 10 | RPC99P469 | AJ887974 |
| 11 | RPC02E4a | AJ887975 |
| 12 | RPC02C0598 | AJ887976 |
| 13 | RPC02V36a | AJ887977 |
| 14 | RPC02E9a | AJ887978 |
| 15 | Hcc1 | AJ887979 |
| 16 | Hmm1 | AJ887980 |
| 17 | RPC00P865 | AJ887981 |
| 18 | RPC99P1478 | AJ887982 |
| 19 | RPC01C0326 | AJ887983 |
| 20 | RPC00C0506 | AJ887984 |
| 21 | Hcc2 | AJ887985 |
| 22 | RPC01C0382 | AJ887986 |
These alleles were distributed among the other strains as follows: allele 1, strains CIP 105777, RPC02E19a, RPC02E1b, Hff7, Htt4, Hww2, RPC02L10a, RPC02L11, RPC02L9b, RPC02V42a, RPC02V47b, RPC02V48b, RPC02V50a, RPC02V6c, RPC01C0186, RPC01C0944, RPC00P332, RPC01C1020, RPC01C0086, RPC01C0123, RPC02C0294, and RPC02G4270; allele 2, strains RPC02E21a, RPC01C0113, RPC99P332, and RPC01C0209; allele 3, strain RPC99P1794; allele 4, strain RPC99P1232; allele 19, strain RPC01C1366.
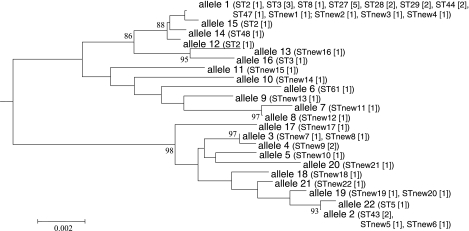
FIG. 2.
Relationship between atlEcwa alleles and STs. The atlEcwa phylogenetic tree was based on the nucleotide sequences of the 22 alleles identified in this study. The tree was constructed by the neighbor-joining method, rooted on the S. aureus strain RN450 atl gene (GenBank accession no. D17366). Bootstrap values are shown only when they exceed 80%. The scale bar above the tree indicates sequence divergence. STs identified from the mlst.net S. epidermidis database are in parentheses, and the number of strains for each ST is in brackets. The 22 new allelic profiles identified in this study are also indicated (STnew1 to -22).
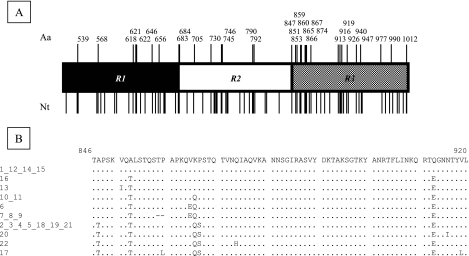
Allelic variations were not evenly distributed among or within R1, R2, and R3 (Fig. 3A). R3 was the most polymorphic in terms of its nucleotide sequence (38 variable nucleotides versus 25 each for R1 and R2). Furthermore, about 60% (22/38) of the variations within R3 led to amino acid changes versus only 36% (9/25) for R1 and 33% (8/25) for R2. Overall, almost 55% (18/33) of the amino acid differences between alleles concerned R3. The N-terminal region of R3 was particularly polymorphic, with 8 amino acids over a 21-amino-acid stretch (positions 847 to 867) displaying variation (Fig. 3B). Furthermore, some of the allelic changes within R3 were clearly not isofunctional (see positions 865 [V↔ E], 866 [K↔ Q], and 913 [Q↔ E] in Fig. 3B). Interestingly, alignment of AtlE sequences with the sequences of Staphylococcus aureus Atl (GenBank accession number D17366) and S. caprae AtlC (GenBank accession number AF244123) showed that the N-terminal part of R3 was also weakly conserved in all three molecules (data not shown).
FIG. 3.
Allelic variations in the CWA domain of AtlE. (A) Positions of nucleotide (Nt) and amino acid (Aa) variations. R1, R2, and R3, repeats 1, 2, and 3 of the CWA domain. (B) Amino acid variations in the N-terminal part of R3. The numbers on the left correspond to atlEcwa alleles (see Table 1). Amino acid positions are given relative to the sequence with GenBank accession number AAB63571.
Multilocus sequence typing (MLST) was used to examine the relationship between the atlEcwa allele and the sequence type (ST). MLST was performed as previously described (12). Partial nucleotide sequences from seven housekeeping genes (for carbamate kinase [arcC], shikimate 5-dehydrogenase [aroE], glycerol kinase [glpK], guanylate kinase [gmk], phosphate acetyltransferase [pta], triosephosphate isomerase [tpiA], and acetyl coenzyme A acetyltransferase [yqi]) were compared with the sequences of the known alleles for each locus in the MLST database (http://www.mlst.net). The resulting seven-digit profiles, defining STs, were used to search the database for matches.
Of the 48 strains studied (one strain did not grow from the frozen aliquot), 25 (52%) belonged to known allelic profiles listed in the mlst.net S. epidermidis database and 23 (48%) did not (new STs). The most frequent STs were ST27 (five strains), ST3 (four strains), and ST2 (three strains), followed by ST28, ST29, ST43, ST44 (two strains each), ST5, ST8, ST47, ST48, and ST61 (one strain each). All but two (ST5 and ST43) of the STs were found in group 1 of the atlEcwa alleles, with all five ST27 strains studied expressing atlEcwa allele 1 (Fig. 2). We identified 22 distinct new allelic profiles. These new STs were distributed similarly within each of the two groups of atlEcwa alleles (Fig. 2). MLST has recently been used to study evolutionary relationships between S. epidermidis strains (12, 24). These studies have shown that most human strains are closely related, forming a single clonal complex around ST2 (12). Our results are consistent with these data and show a clear relationship between atlEcwa and ST polymorphisms. All strains of the ST clonal complex derived from ST2 belong to atlEcwa group 1. The two exceptions, ST5 and ST43, are closely related STs that may have diverged from ST2 (12).
This is the first study of AtlE CWA domain polymorphism in a large panel of human strains of S. epidermidis. It shows that there are two main groups of alleles, differing primarily in the encoded N-terminal part of R3 close to the cleavage site separating the 60-kDa alanine amidase and the 52-kDa glucosaminidase (8). Variations in the CWA domain of Ami were recently shown to be correlated with teichoic acid structures in L. monocytogenes (15). Accordingly, variations in the AtlE CWA domain may be related to variations in the structure of teichoic acids or other structures involved in the targeting of AtlE to the staphylococcal surface. The structure of the cell wall teichoic acids of S. epidermidis RP62A (= CIP 105777), an allele 1 strain, was recently determined (20). This work should be extended to strains expressing other group 1 and group 2 alleles.
It remains unclear whether this allelic polymorphism affects the adhesion and/or autolytic properties of AtlE and has implications for pathogenicity. Recent studies have shown that ST27 and a few other STs close to ST2 occur preferentially in hospitals, and this selectivity has been linked to the presence of genes playing a major role in biofilm formation (i.e., the icaADBC operon) and antibiotic resistance (i.e., SCCmec cassettes) (12). We found that all these hospital-associated STs expressed allele 1 or related alleles of the CWA domain of AtlE. It is tempting to speculate that this trait may constitute a major colonization factor mediating the adhesion of bacteria to the surface of the skin, medical devices, or both. If this is the case, then AtlE alleles may constitute interesting pathogenicity markers. We are currently investigating this possibility in a large prospective study.
Nucleotide sequence accession numbers.
The sequences defining the 22 alleles identified in this study have been submitted to GenBank and assigned accession numbers AJ887965 to AJ887986 (Table 1).
Acknowledgments
We thank M. Le Moal, S. Chaverot, and V. Avettand for technical assistance and I. Senegas for help with the manuscript.
REFERENCES
- 1.Allignet, J., S. Aubert, K. G. Dyke, and N. El Solh. 2001. Staphylococcus caprae strains carry determinants known to be involved in pathogenicity: a gene encoding an autolysin-binding fibronectin and the ica operon involved in biofilm formation. Infect. Immun. 69:712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allignet, J., P. England, I. Old, and N. El Solh. 2002. Several regions of the repeat domain of the Staphylococcus caprae autolysin, AtlC, are involved in fibronectin binding. FEMS Microbiol. Lett. 213:193-197. [DOI] [PubMed] [Google Scholar]
- 3.Frebourg, N. B., S. Lefebvre, S. Baert, and J. F. Lemeland. 2000. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J. Clin. Microbiol. 38:877-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galdbart, J. O., J. Allignet, H. S. Tung, C. Ryden, and N. El Solh. 2000. Screening for Staphylococcus epidermidis markers discriminating between skin-flora strains and those responsible for infections of joint prostheses. J. Infect. Dis. 182:351-355. [DOI] [PubMed] [Google Scholar]
- 5.Galdbart, J. O., A. Morvan, N. Desplaces, and N. el Solh. 1999. Phenotypic and genomic variation among Staphylococcus epidermidis strains infecting joint prostheses. J. Clin. Microbiol. 37:1306-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill, S. R., D. E. Fouts, G. L. Archer, E. F. Mongodin, R. T. Deboy, J. Ravel, I. T. Paulsen, J. F. Kolonay, L. Brinkac, M. Beanan, R. J. Dodson, S. C. Daugherty, R. Madupu, S. V. Angiuoli, A. S. Durkin, D. H. Haft, J. Vamathevan, H. Khouri, T. Utterback, C. Lee, G. Dimitrov, L. Jiang, H. Qin, J. Weidman, K. Tran, K. Kang, I. R. Hance, K. E. Nelson, and C. M. Fraser. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 187:2426-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 8.Heilmann, C., M. Hussain, G. Peters, and F. Gotz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 9.Hell, W., H. G. Meyer, and S. G. Gatermann. 1998. Cloning of aas, a gene encoding a Staphylococcus saprophyticus surface protein with adhesive and autolytic properties. Mol. Microbiol. 29:871-881. [DOI] [PubMed] [Google Scholar]
- 10.Kloos, W. E., and T. L. Bannerman. 1994. Update on clinical significance of coagulase-negative staphylococci. Clin. Microbiol. Rev. 7:117-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klug, D., F. Wallet, S. Kacet, and R. J. Courcol. 2003. Involvement of adherence and adhesion Staphylococcus epidermidis genes in pacemaker lead-associated infections. J. Clin. Microbiol. 41:3348-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozitskaya, S., M. E. Olson, P. D. Fey, W. Witte, K. Ohlsen, and W. Ziebuhr. 2005. Clonal analysis of Staphylococcus epidermidis isolates carrying or lacking biofilm-mediating genes by multilocus sequence typing. J. Clin. Microbiol. 43:4751-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 14.Milohanic, E., R. Jonquieres, P. Cossart, P. Berche, and J. L. Gaillard. 2001. The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol. Microbiol. 39:1212-1224. [DOI] [PubMed] [Google Scholar]
- 15.Milohanic, E., R. Jonquières, P. Glaser, P. Dehoux, P. Jacquet, P. Berche, P. Cossart, and J.-L. Gaillard. 2004. Sequence and binding activity of the autolysin-adhesin Ami from epidemic Listeria monocytogenes 4b. Infect. Immun. 72:4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto, H., K. Imamura, A. Kojima, H. Takenaka, N. Hara, A. Ikenouchi, T. Tanabe, and H. Taniguchi. 2003. Survey of nasal colonization by, and assessment of a novel multiplex PCR method for detection of biofilm-forming methicillin-resistant staphylococci in healthy medical students. J. Hosp. Infect. 53:215-223. [DOI] [PubMed] [Google Scholar]
- 17.Poyart, C., G. Quesne, C. Boumaila, and P. Trieu-Cuot. 2001. Rapid and accurate species-level identification of coagulase-negative staphylococci by using the sodA gene as a target. J. Clin. Microbiol. 39:4296-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohde, H., M. Kalitzky, N. Kroger, S. Scherpe, M. A. Horstkotte, J. K. Knobloch, A. R. Zander, and D. Mack. 2004. Detection of virulence-associated genes not useful for discriminating between invasive and commensal Staphylococcus epidermidis strains from a bone marrow transplant unit. J. Clin. Microbiol. 42:5614-5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rupp, M. E., P. D. Fey, C. Heilmann, and F. Gotz. 2001. Characterization of the importance of Staphylococcus epidermidis autolysin and polysaccharide intercellular adhesin in the pathogenesis of intravascular catheter-associated infection in a rat model. J. Infect. Dis. 183:1038-1042. [DOI] [PubMed] [Google Scholar]
- 20.Sadovskaya, I., E. Vinogradov, J. Li, and S. Jabbouri. 2004. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr. Res. 339:1467-1473. [DOI] [PubMed] [Google Scholar]
- 21.Sivadon, V., M. Rottman, S. Chaverot, J. Quincampoix, V. Avettand, P. de Mazancourt, L. Bernard, P. Trieu-Cuot, J. Feron, A. Lortat-Jacob, P. Piriou, T. Judet, and J. Gaillard. 2005. Use of genotypic identification by sodA sequencing in a prospective study to examine the distribution of coagulase-negative Staphylococcus species among strains recovered during septic orthopedic surgery and evaluate their significance. J. Clin. Microbiol. 43:2952-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivadon, V., M. Rottman, J. C. Quincampoix, V. Avettand, S. Chaverot, P. de Mazancourt, P. Trieu-Cuot, and J.-L. Gaillard. 2004. Use of sodA sequencing for the identification of clinical isolates of coagulase-negative staphylococci. Clin. Microbiol. Infect. 10:939-942. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wisplinghoff, H., A. E. Rosato, M. C. Enright, M. Noto, W. Craig, and G. L. Archer. 2003. Related clones containing SCCmec type IV predominate among clinically significant Staphylococcus epidermidis isolates. Antimicrob. Agents Chemother. 47:3574-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, Y. Q., S. X. Ren, H. L. Li, Y. X. Wang, G. Fu, J. Yang, Z. Q. Qin, Y. G. Miao, W. Y. Wang, R. S. Chen, Y. Shen, Z. Chen, Z. H. Yuan, G. P. Zhao, D. Qu, A. Danchin, and Y. M. Wen. 2003. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49:1577-1593. [DOI] [PubMed] [Google Scholar]