Abstract
Early identification of Acanthamoeba in cerebrospinal fluid is mandatory to prevent fatal granulomatous amebic encephalitis. In the case presented here amebic trophozoites were detected in a routine cerebrospinal fluid sample. The antibiotic treatment and the apparently low virulence of this isolate were responsible for the benign progression of the infection.
CASE REPORT
A 64-year-old immunocompetent woman presented in February 2004 with periorbital headache and nausea without abnormal findings on neurological examination. She was being treated for hypertonus, hyperlipidemia, and diabetes mellitus. A coronary bypass operation had been performed in 1996. She reported a midfacial fracture 20 years ago. A computer tomography revealed pneumatocele, which was confirmed by cranial magnetic resonance imaging, without evidence of meningitis or encephalitis. No physical connection between the pneumatocele and the paranasal sinuses was found. Therefore, liquor rhinorrhea was ruled out at that time. A control cranial computer tomography (CCT) in July 2004 showed no pneumatocele. In December 2004 she presented herself once more with the same symptoms as in February. Using CCT and magnetic resonance imaging a new pneumatocele was detected. Radionuclide cisternography was performed, confirming cerebrospinal fluid (CSF) rhinorrhea; however, as no definite morphological defect could be detected, no neurosurgery was performed. The initial CSF sample contained 23 leukocytes/μl (predominantly granulocytes and monocytes) and 300 erythrocytes/μl, probably due to blood contamination. CSF glucose and lactate were normal. CSF protein was slightly elevated (73.7 mg/dl), with mild impairment of the blood-CSF barrier (albumin ratio, 10.5; age-specific normal value, <9). The peripheral white cell count was 11 to 16/nl. Her body temperature was normal.
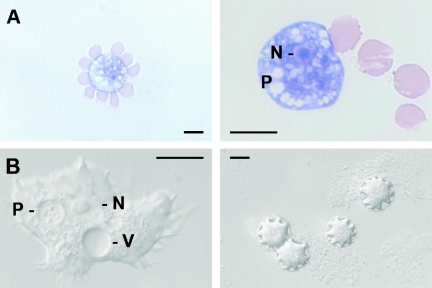
Routine CSF cytology (cytocentrifugation and subsequent May-Grunwald-Giemsa staining) led to the detection of four cells with morphological features resembling amebic trophozoites. Two are shown in Fig. 1A. Each was characterized by a small nucleus with prominent central nucleolus and digestive vacuoles, some of which were filled with bacteria (not shown). Upon superficial scrutiny, the cells may have been misclassified as macrophages. To confirm the diagnosis of free-living amebae (FLA), a second CSF sample was drawn. This sample was taken 3 days after the initial one and contained 1 leukocyte/μl and 17 erythrocytes/μl. One trophozoite was directly detected by differential interference microscopy.
FIG. 1.
(A) Two cells with morphological features resembling amebic trophozoites in CSF stained with May-Grunwald-Giemsa. N, nucleus with central nucleolus; P, digestive vacuoles. (B) Trophozoite and cysts of in vitro cultured Acanthamoeba (differential interference contrast microscopy). Note the pseudopodia (acanthopodia) of the trophozoite. V, contractile vacuole. Scale bars: 10 μm.
Furthermore, cultures were initiated on media that supported growth of different genera of FLA. After 4 days, trophozoites were detected on sea salt agar seeded with a Phyllobacterium species (strain Mü), which served as feeder bacteria. The organism was classified as Acanthamoeba morphological group II on the basis of morphometric characteristics of the cysts (Fig. 1B). Further propagation of the isolate was achieved on nonnutrient Page's saline agar seeded with Escherichia coli C600 (1). Despite numerous attempts we were not able to establish axenic cultures of this isolate. In order to obtain a uniform genetic population for DNA sequencing, the isolate was cloned by transferring a single cyst onto a fresh plate using a micromanipulator. The 18S rRNA gene was amplified by PCR from chromosomal DNA extracted from trophozoites using the SSU1 and SSU2 primers (4). Multiple sequence alignment was performed by pairwise alignment using the CLUSTAL X application (11). For cluster analyses the PHYLIP package was used (3). Sequence analysis of the 18S rRNA gene identified this isolate as Acanthamoeba sequence type T4 (10).
After confirmation of the presence of Acanthamoeba in the CSF sample by culture, treatment was initiated with a combination of parenteral fluconazole (400 mg), rifampin (600 mg), metronidazole (500 mg three times a day), and oral sulfadiazine (1,000 mg four times a day) for 14 days. Cultures from CSF samples taken during treatment and 4 weeks after termination of treatment were negative. The patient was discharged from the hospital after complete disappearance of the initial neurological symptoms.
Species of three genera of FLA have been repetitively associated with infections of the central nervous system. Of these, Naegleria fowleri causes acute fulminant meningitis, also called primary amebic meningoencephalitis. Balamuthia mandrillaris and Acanthamoeba spp. cause a more chronic but eventually fatal disease termed granulomatous amebic encephalitis (GAE) (7). In addition to this, species of the genus Acanthamoeba frequently cause amebic keratitis and also skin infection (5). Acanthamoeba spp. are known to serve as natural hosts and vehicles of various microorganisms such as Legionella spp. and other pathogens (6). As infections with FLA are rare, clinicians, pathologists, and clinical microbiologists are unfamiliar with these diseases. Therefore, most cases of GAE reported in the literature have been diagnosed postmortem. A recent report emphasizes the importance of early diagnosis (2). The images presented hopefully help physicians to identify this unusual pathogen in CSF, because early diagnosis is mandatory to prevent fatal outcomes of GAE.
For the diagnosis of Acanthamoeba in CSF direct microscopy of wet mounts or sediments of CSF after gentle centrifugation of samples is recommended (5). However, trophozoites may be unrecognized because they resemble macrophages. Fixed samples and samples stained with hematoxylin and eosin or trichrome will show characteristic features of trophozoites such as a prominent nucleolus and contractile and digestive vacuoles. This can be done only if amebic infection is suspected in the first place. In the case reported here no signs of meningitis or encephalitis were apparent and therefore a standard Gram stain and CSF culture for bacteria and fungi were not performed. The CSF was processed by routine cytology including May-Grunwald Giemsa staining. The occurrence of an unusual cell type in the first CSF sample suggested trophozoites of FLA, which prompted us to confirm our suspicion by in vitro culture techniques for FLA.
The outcomes of most reported cases of GAE have been uniformly fatal despite various treatment regimens with few exceptions (7). Treatment with fluconazole and sulfadiazine was reportedly successful in an AIDS patient (8), and two immunocompetent children with GAE survived with a combination therapy of trimethoprim-sulfamethoxazole, rifampin, and ketoconazole (9). In the case reported here, there were no signs of encephalitis and the amebae appeared to be confined to the CSF. The organisms may have never been able to invade the central nervous system had they not accidentally gained entry via a fistula from the nasopharynx to the CSF. As no axenic cultures could be established, pathogenicity of this isolate could not be formally tested in vitro by a cytotoxicity assay. However, in the past we found (R. Michel, unpublished results) that isolates that could not be propagated in axenic cultures were apathogenic in a mouse model, whereas pathogenic strains could be propagated in axenic cultures easily. On the other hand this isolate was clearly identified as genotype T4, which is the most common genotype isolated from cases of acanthamebic keratitis and GAE. We assume that two factors may have been responsible for the benign progression of the infection. First, the early start of the quadruple antimicrobial treatment and second, the apparently low virulence of this particular isolate.
Nucleotide sequence accession number.
The nucleotide sequence data were deposited at GenBank under accession number DQ103890.
REFERENCES
- 1.Balows, A., W. J. J. Hausler, K. L. Herrmann, H. D. Isenberg, and H. J. Shadomy (ed.). 1999. Manual of clinical microbiology. ASM Press, Washington, D.C.
- 2.Bloch, K. C., and F. L. Schuster. 2005. Inability to make a premortem diagnosis of Acanthamoeba species infection in a patient with fatal granulomatous amebic encephalitis. J. Clin. Microbiol. 43:3003-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felsenstein, J. 1989. PHYLIP—phylogeny inference package, vers. 3.2. Cladistics 5:164-166. [Google Scholar]
- 4.Gast, R. J., D. R. Ledee, P. A. Fuerst, and T. J. Byers. 1996. Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J. Eukaryot. Microbiol. 43:498-504. [DOI] [PubMed] [Google Scholar]
- 5.Marciano-Cabral, F., and G. Cabral. 2003. Acanthamoeba spp. as agents of disease in humans. Clin. Microbiol. Rev. 16:273-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrie, T. J., D. Raoult, B. La Scola, R. J. Birtles, E. de Carolis, and the Canadian Community-Acquired Pneumonia Study Group. 2001. Legionella-like and other amoebal pathogens as agents of community-acquired pneumonia. Emerg. Infect. Dis. 7:1026-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuster, F. L., and G. S. Visvesvara. 2004. Opportunistic amoebae: challenge in prophylaxis and treatment. Drug Resist. Updates 7:41-51. [DOI] [PubMed] [Google Scholar]
- 8.Seijo Martinez, M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez De Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singhal, T., A. Bajpai, V. Kalra, S. K. Kabra, J. C. Samantaray, G. Satpathy, and A. K. Gupta. 2001. Successful treatment of Acanthamoeba meningitis with combination oral antimicrobials. Pediatr. Infect. Dis. J. 20:623-627. [DOI] [PubMed] [Google Scholar]
- 10.Stothard, D. R., J. M. Schroeder-Diedrich, M. H. Awwad, R. J. Gast, D. R. Ledee, S. Rodriguez-Zaragoza, C. L. Dean, P. A. Fuerst, and T. J. Byers. 1998. The evolutionary history of the genus Acanthamoeba and the identification of eight new 18S rRNA gene sequence types. J. Eukaryot. Microbiol. 45:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. Higgins. 1997. The Clustal_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]