Abstract
Objective: The purpose of this study was to determine whether the levator plate is (1) horizontal in women with normal support, (2) different between women with and without prolapse, (3) related to levator hiatus and perineal body descent.
Study design: Cohorts of cases with prolapse at least 1 cm below the hymen and normal controls with all points 1 cm or more above the hymen were prospectively enrolled in a study of pelvic organ support to be of similar age, race, and parity. Subjects underwent supine midsagittal dynamic magnetic resonance imaging (MRI) during Valsalva. Levator plate angle (LPA) was measured relative to a horizontal reference line. Levator hiatus length (LH) and perineal body location (PB) were also measured. Student t tests and Pearson correlation coefficients (r) were performed.
Results: Sixty-eight controls and 74 cases were analyzed. During Valsalva, controls had a mean LPA of 44.3(. Cases, compared to controls, had 9.1((21%) more caudally directed LPA (53.4(vs 44.3(, P<.01), 15% larger LH length (7.8 cm vs 6.8 cm, P<.01), and 24% more caudal PB location (6.8 cm vs 5.5 cm, P<.01). Increases in LPA were correlated with increased LH length (r = 0.42, P<.0001) and PB location (r =.51, P<.0001).
Conclusion: The measured levator plate angle in women with normal support is 44.3(.During Valsalva, women with prolapse have a modest (9.1() though statistically greater levator plate angle compared to controls. This larger angle showed moderate correlation with larger levator hiatus length and greater displacement of the perineal body in women with prolapse compared to controls.
Keywords: Pelvic organ prolapse, Dynamic MRI, Levator plate, Levator hiatus, Levator ani muscle
The levator ani muscles are important to pelvic organ support. Halban and Tandler coined the term levator plate as being “.in the posterior segment where (the levator muscles) unite with the similar muscle of the other side, intertwined with each other. Thereby the posterior part of the diaphragm in back of the rectum is considerably strengthened. We want to designate this part as the levator plate.”1 In 1953, based on radiographic levator myography studies, Berglas and Rubin suggested that the levator plate in women with normal support was “horizontal.”2 Their observations of women with prolapse during straining showed the levator plate tips more vertically, a change felt to be associated with an enlarged levator hiatus and increased perineal body descent. This has become a commonly accepted theory expressed in standard texts.3
With the advent of cine dynamic magnetic resonance (MR) imaging, it is possible to study the interaction between the pelvic floor musculature and the pelvic organs during Valsalva without injecting contrast into the muscles. These ultrafast sequences allow the acquisition of multiple images at the same location at a rate of 1 frame per second, thereby capturing the motion of the pelvic floor as it deforms under increasing intra-abdominal pressure. On midsagittal dynamic images, a flat region is seen between the anus and coccyx where the levator ani muscles join in the midline (iliococcygeal raphé), corresponding to the “levator plate.” Quantitative comparison of the levator plate using a large sample size of women with normal and abnormal pelvic organ support has not been done.
It is the aim of this study to answer the following questions: Is the levator plate horizontal in women with normal pelvic support? Is there a difference in the levator plate angle between women with prolapse and those with normal support? Are changes in levator hiatus length and perineal body location related to changes in the levator plate angle?
Material and methods
Dynamic MR imaging studies were obtained from an ongoing Institutional Review Board approved study of pelvic organ support. The parent study recruited women with pelvic organ prolapse where at least 1 vaginal wall (anterior, posterior, or apical) point or cervix were 1 cm or more beyond the hymen. A group of women with normal support where all vaginal points were 1 cm or more above the hymen was recruited to be of similar age, parity, and race. All subjects underwent clinical Pelvic Organ Prolapse Quantification (POP-Q) examination. Cases were recruited through the Urogynecologic clinic at the University of Michigan. Controls were recruited through newspaper advertisements and the Women’s Health Registry, a list of women in Michigan who were willing to be contacted for women’s health studies. Patients were excluded if they had previous surgery for prolapse or pelvic floor dysfunction, had any findings which may distort pelvic anatomy such as a pelvic mass or history of pelvic radiation, or were unable to complete the clinic exam or MR imaging study. Women who had undergone hysterectomy were eligible if the surgery had been done at least 1 year before enrollment and if the indication for surgery did not include pelvic floor dysfunction (eg, pelvic organ prolapse, urinary incontinence, or fecal incontinence).
MR imaging technique
MR imaging was performed on a 1.5 Tesla system (Signa, General Electric, Milwaukee, WI) using a 4-channel torso phased array coil with the subject in the supine position. Before starting the examination, the patient was instructed in regards to the straining maneuvers to be performed during the examination starting from minimal to maximal straining. Standard imaging for detailed anatomic evaluation of the pelvic floor muscles was performed using an axial T1-weighted spin-echo (SE) sequence (TR/TE: 500/17 ms, slice thickness/gap: 4/1 mm, matrix: 256×128, field of view: 30 cm, 1 excitation), and proton density-weighted sequences acquired in the axial, coronal, and sagittal planes (TR/TE: 4000/14 ms, slice thickness/gap: 4/1 mm, matrix: 256×256, field of view 18-20 cm, 2 excitations). For dynamic imaging, a multiphase, single level image of the pelvis in the midsagittal plane was obtained approximately every second for 23 to 27 seconds using a T2-weighted single-shot fast spin-echo (SSFSE) sequence (TR: 1300 ms, TE: 60 ms, slice thickness: 6 mm, field of view 32-36 cm, matrix: 256×160, 1 excitation and half-Fourier acquisition). The time needed to acquire each of the images was determined by the patient’s weight and was just over a second. A set of 20 successive images were acquired in 23 to 27 seconds during rest and graded Valsalva effort as follows: the operator instructed the patient to hold their breath in inspiration and initiated the scan; and after 5 seconds of imaging during rest, the operator instructed the patient to strain minimally for 5 seconds, moderately for 5 seconds, and maximally for 5 seconds, then to breath normally and relax for another 5 to 7 seconds before ending the acquisition. This was done so that we could obtain a graded Valsalva response as opposed to an all or none effect. Usually, 3 images were acquired at rest during suspended inspiration, 12 images during the graded Valsalva effort, and 5 images during post Valsalva relaxation and normal breathing. These images were viewed either individually and used to obtain pelvic floor measurements, or placed in a cine-loop for display as a movie clip to evaluate the dynamics of prolapse.
There were 199 dynamic MR imaging studies available for review. The images acquired at rest as well as the images at peak Valsalva effort were used to obtain the required measurements. Seventeen MR exams were excluded because Valsalva was not performed properly. An additional 15 exams were excluded as the dynamic MR images were either not midsagittal or if the image quality did not allow accurate landmark identification. Twenty-five exams were excluded as only some of the structures (ie, levator plate, levator hiatus, and perineal body) were visible. This left 68 controls and 74 cases for analysis.
All images were analyzed by the same examiner, who was blinded to group status. On both the static and the Valsalva images, a best fit line was placed for the levator plate at the initial take off portion of the iliococcygeus from the coccyx. The angle between this and a horizontal reference line was measured as the levator plate angle (LPA) (Figure 1). A point was placed behind the levator hiatus and a line was drawn to the inferior portion of the pubic bone as a measurement of levator hiatus length (LH). A point was also placed for the perineal body in the region of the perianal skin immediately anterior to the external anal sphincter, and a vertical line drawn from the sacro-coccygeal inferior pubic point (SCIPP) line4 was used as the measure of perineal body location (PB). Figure 2 shows an MR image of a woman with normal pelvic support from our study in which the measurements have been done. In order to compare distance between women of different stature and pelvis sizes, we scaled all pelvises to the same size by normalizing a woman’s pelvis to a SCIPP line of 11.5 cm, the average distance from the inferior margin of the pubis to the sacral tip.5 For example, in a woman with a larger pelvis where the SCIPP line is 12.0 cm, the LH and PB values were multiplied by 11.5/12.0, or 0.958.
Figure 1.
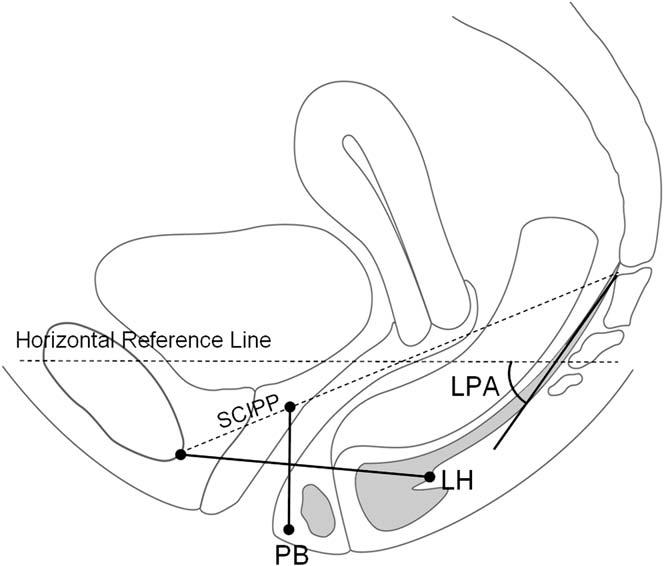
Schematic of pelvic floor measurements: Grey structures representing the levator plate as well as the external anal sphincter. LPA, Levator plate angle; LH, levator hiatus length; PB, perineal body location; SCIPP, sacro-coccygeal inferior pubic point line.
Figure 2.
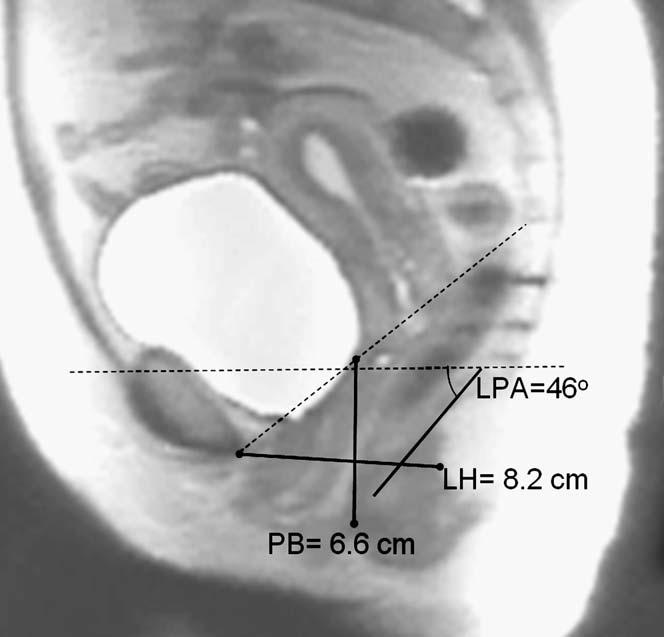
MR example of measurements. LPA was measured relative to the horizontal reference line. The LH and PB measurements are normalized using the SCIPP line length.
Two-sample t tests were calculated between groups on the resting as well as the Valsalva images for each structure. Pearson correlations were calculated for LH and LPA as well as PB and LPA. Variability of patient positioning in the MR scanner was also examined by measuring the SCIPP line angle relative to the scanner axis during peak Valsalva. As a secondary analysis, all images were analyzed independently by another investigator, who was also blinded to group status. Inter-rater reliability was calculated by use of Pearson correlations.
Results
Demographics and patient characteristics
Demographic data between controls and cases are compared in Table I. There were no statistical differences for mean age, body mass index (BMI), or number of vaginal deliveries. Pelvic organ support is compared between groups in Table II by mean POP-Q points. There was a statistically significant difference between groups for all points with cases showing greater descent compared to controls.
Table I.
Demographic data
| Controls (n = 68) | Cases (n = 74) | P value | |
|---|---|---|---|
| Age (y) | 55.8 ± 12.4 | 55.4 ± 11.7 | .83 |
| BMI (kg/m2) | 26.8 ± 4.6 | 25.8 ± 4.1 | .19 |
| Vaginal parity | 2.5 ± 1.7 | 2.9 ± 2.0 | .24 |
All values are mean ± SD.
Table II.
Comparison of pelvic support with mean POP-Q points
| Controls (n = 68) | Cases (n = 74) | P value | |
|---|---|---|---|
| Aa (cm) | -1.6 ± .7 | 0.6 ± 1.3 | < .0001 |
| Ba (cm) | -1.6 ± .7 | 1.7 ± 1.7 | < .0001 |
| C (cm) | -6.4 ± 1.6 | -1.3 ± 3.8 | < .0001 |
| Ap (cm) | -1.9 ± .8 | -0.7 ± 1.7 | < .0001 |
| Bp (cm) | -1.9 ± .8 | -0.4 ± 2.0 | < .0001 |
| GH (cm) | 3.6 ± 1.0 | 5.4 ± 1.3 | < .0001 |
| TVL (cm) | 10.6 ± 1.4 | 10.0 ± 1.5 | .03 |
Mean ± SD. Negative values express distance above hymen, positive values distance below hymen.
Valsalva images
Table III shows results during peak Valsalva. With loading, the LPA in women with normal support is not horizontal as previously assumed but has a mean angle of 44.3(relative to the horizontal. Women with prolapse have a 9.1((21%) more caudally inclined levator plate. Women with prolapse also have a 15% larger LH and a PB that is 24% more displaced. A visual display of the mean values during peak Valsalva is shown in Figure 3. Both LH (R = .42) and PB (R = .51) were moderately correlated to LPA (Figure 4).
Table III.
Levator plate angle (LPA), levator hiatus (LH), and perineal body location (PB) between controls and cases during peak Valsalva and rest
| LPA (degrees) |
LH (cm) |
PB (cm) |
||||
|---|---|---|---|---|---|---|
| Controls (n = 68) | Cases (n = 74) | Controls (n = 68) | Cases (n = 74) | Controls (n = 68) | Cases (n = 74) | |
| Valsalva | 44.3 ± 15.2 | 53.4 ± 16.0 | 6.8 ± 1.4 | 7.8 ± 1.5 | 5.5 ± 1.6 | 6.8 ± 1.4 |
| P value | < .0008 | < .0001 | < .0001 | |||
| Rest | 36.2 ± 12.3 | 45.8 ± 12.4 | 6.3 ± 1.1 | 6.7 ± 1.3 | 4.5 ± 1.2 | 5.4 ± 1.3 |
| P value | < .0001 | .031 | < .0001 | |||
Mean ± SD.
Figure 3.
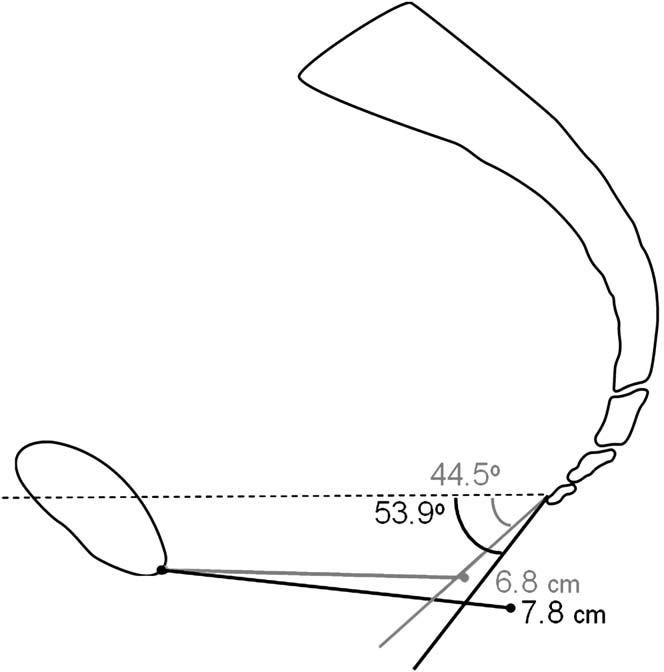
Visual representation of results. All values shown are means. Cases, black; controls, grey.
Figure 4.
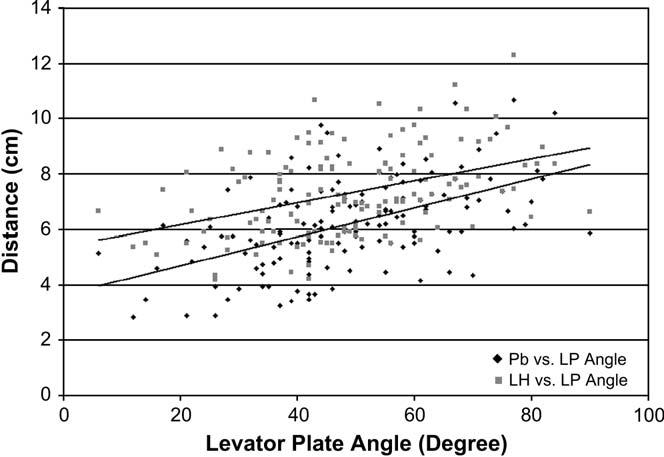
Correlation between perineal body (PB) versus levator plate angle (LPA) and levator hiatus (LH) versus levator plate angle. LH, R = .42; PB, R = .51.
Resting images
During rest or the unloaded state, cases had statistically larger levator plate angles, levator hiatus lengths, and more perineal body displacement compared to controls (Table III).
Variability of patient positions
To assess whether bias might be introduced by the pelvis of women with prolapse being at a different angle to the horizontal than normal women, the angle between the SCIPP line and the MR scanner was recorded for all the resting and the Valsalva images. This is a measurement of patient positioning in the scanner since the SCIPP line is based on bony landmarks. The mean SCIPP angle at rest for controls was 57.9(G 5.7(and for cases, 56.9(G 5.7((P = .32). For Valsalva controls were 60.7(G 7.3(, and cases = 61.3(G 7.9((P = .63).
Inter-rater reliability
Comparison of results from the 2 independent examiners showed high correlation for LPA (r = 0.90), LH (r = 0.97), and PB (r = 0.93). There was no bias between the second and first examiner for LPA (P = .75). The second examiner measured slightly larger LH length (difference = 0.14 cm, P<.0001) and PB distance (difference = .08 cm, P<.001) compared to the first examiner.
Comment
The levator plate in women with normal support has a mean angle of 44.3(relative to a horizontal reference line during Valsalva. This is almost exactly halfway between horizontal and vertical, not horizontal as previously described.2 Women with prolapse have a 9.1(more vertically oriented LPA, which is statistically different. Women with prolapse also have larger levator hiatus lengths and more caudal perineal body displacements, both of which correlated with the displacement of the levator plate. The interaction between the levator plate angle, levator hiatus, and perineal body position reflect the fact that they are all related as a single structural unit. Although the pelvic floor measurements of women with normal support and those with prolapse differed at rest, the differences during Valsalva are the most meaningful as that is when the levator ani muscles and pelvic floor are subjected to load (force) from an increase in intra-abdominal pressure. Loading is necessary to accurately assess levator ani function. These data provide a quantitative assessment of topographic changes in the pelvic floor in a large sample of well selected patients. This technique can also be easily taught and is repeatable between examiners.
When visually represented, a 9.1(difference in the mean levator plate angle of women with prolapse compared to controls does not appear to be a large difference. If prolapse was primarily caused by a vertically tipped levator plate angle, we would expect the difference to be more striking. It is our belief that the levator plate angle is an indicator of damage to the levator ani muscle. The lack of a more direct relationship between prolapse and levator plate angle may be explained by the fact that the levator plate is a part of the iliococcygeal muscle (the iliococcygeal raphé). This portion of the levator has different origin and insertion than the pubovisceral and puborectal portions of the levator ani muscle that close the hiatus.6 Levator damage occurs primarily in the pubovisceral portion of the muscle and iliococcygeal damage is only seen in about 10% of women with levator damage.7 If there is damage to the pubovisceral muscle it may result in increased loads on the iliococcygeal muscle and raphé, resulting in downward displacement, but not to the same degree as if the iliococcygeal muscle itself is damaged. In addition, there will be women with intact muscles and a normal levator plate angle that have prolapse because of pure connective tissue failure. These issues deserve further investigation.
The use of dynamic MR imaging of the pelvic floor to depict pelvic organ movement was first introduced by Yang et al in 1991.8 Multiple studies have shown that dynamic MRI is just as good if not better for studying pelvic structure motion as compared to traditional modalities such as fluoroscopic cystocolpoproctography, bead-chain cystourethography, or other fluoroscopic studies.9-9 Defects in the levator ani muscles have been observed during straining dynamic MR imaging studies. Kaufman12 found levator ani herniations in 3 of 22 patients with prolapse studied by dynamic MR imaging, while Gearhart13 found women with prolapse to have a 15% rate of hernias in the iliococcygeus portion of the levator ani muscles.
There are only a few studies in the literature that characterize the biomechanical movement of the pelvic floor during pelvic loading. The first was a descriptive study by Berglas and Rubin, who used levator myography to demonstrate that with straining, women with prolapse have a greater inclination of the levator plate.2 Using the improved visualization of soft tissue structures with MR imaging, investigators are better able to study the levator plate in detail. Singh found evidence of “flattening: of the domes in the iliococcygeal muscles seen in coronal section with straining though could not identify a uniform pattern of change.14,14 Hjartardottir also describes the levator ani muscles descending and becoming more basin-shaped with bearing down but the authors did not attempt quantitative analysis.16 Goodrich quantified the difference in the levator plate angle of 10 normal volunteers and 5 prolapse patients before and after surgery.17 Interestingly, they found that women who had surgical repair of prolapse had 10(more vertically oriented levator plate angle as well as a larger levator hiatus during straining even without recurrence of prolapse. This led the authors to conclude that the levator plate was not the sole support of the pelvic organs. In a study that also used midsagittal images and best fit line for the levator plate, Ozasa compared the levator plates of 14 women with prolapse and 19 women without prolapse.18 They found that a best fit line through the levator plate always crossed the pubic bone in women with normal support but never crossed the pubic bone in those with prolapse, an alternative way to assess inclination.
Many MR imaging sequences have been used to dynamically image pelvic organ prolapse. These sequences have been performed as multislice or single-slice acquisition during relaxation and straining and include several gradient-recalled echo and FSE sequences such as: true fast imaging with steady state (true FISP, Siemens, Erlangen, Germany), or gradient recalled acquisition in a steady state (GRASS, General Electric, Milwaukee, WI)8,9,11; spoiled gradient-recalled echo (SPGR, General Electric, or fast low angle shot (FLASH, Siemens), or fast field echo (FFE, Philips, Best, the Netherlands)16; single-shot fast spin-echo (SSFSE, General Electric, or half-fourier acquisition single-shot turbo SE (HASTE, Siemens and Philips)10,12,13; and inner-volume modified FSE.19 All these sequence are characterized by having acceptable spatial resolution, good to excellent contrast resolution, and being fast, usually each slice acquired within seconds. The latter feature is essential because it enables imaging the pelvic organs during straining with only minimal degradation of image quality by motion artifact, as most women cannot sustain the straining effort for more than a few seconds at a time. Some of the sequences, however, especially the gradient-recalled echo sequences, are more prone to susceptibility artifact from air within bowel.
We elected to use a SSFSE sequence for dynamic imaging of the pelvic floor because of its excellent contrast resolution, resistance to motion and susceptibility artifact, and brief imaging time, allowing for its use while the patient is straining and relaxing. The excellent contrast difference between fat, fluid, and soft tissues, as well as the minimal susceptibility artifact from gas in bowel, allows clear delineation of the bladder, vagina, small and large bowel loops without the need to opacify any of these organs. This sequence is, however, limited by relatively low signal-to-noise ratio (SNR) and blurring,20 which limits its use for detailed anatomic evaluation of the pelvic floor musculature. We attempted to maximize the SNR yet keep the contrast resolution by using a moderately high TE value of 60 ms. We also applied a higher receiver bandwidth (62 MHz) to reduce the inter-echo spacing and minimize image blurring. Our sequence parameters provided a good balance between the contrast resolution of the various pelvic organs and the image spatial resolution. Moreover, the dynamic SSFSE sequence was only 1 element of our comprehensive pelvic imaging examination. Anatomic details of the pelvic floor musculature are mostly provided by the T1-weighted and proton-density weighted sequences, whereas kinetics of the pelvic organs/tissue are visualized with excellent fidelity via the dynamic SSFSE approach.
This type of research has certain limitations. Scans are obtained in the supine position. When the effects of gravity are removed, there is concern that it is not possible to show maximal pelvic load in the supine position. In the past, researchers have compared supine dynamic MR imaging with those performed in a sitting position in an open configuration scanner. Although sitting MR imaging studies led to greater degrees of descent, they found supine scans during straining to be comparable in documenting pelvic floor movement.19,19 One study comparing sitting colpocystodefecography and supine dynamic MR imaging found that the latter method had lower sensitivity in the anterior and middle compartments.22 Because of the study design, supine scans would not introduce bias as both cases and controls were tested in the same manner. In order to conduct more meaningful biomechanical studies of the pelvic floor, it is our group’s goal in the future to develop the ability to obtain simultaneous pressure readings during the dynamic MR imaging studies.
This study adds to the important and growing literature regarding the biomechanical changes of pelvic organ prolapse. Dynamic MR imaging is unique in its ability to show and quantify movement of the levator ani muscles in relation to the pelvic viscera. Dynamic MR imaging should not be limited to grading the severity of prolapse or for preoperative assessments because the high cost of the study limits its routine use for these purposes. In addition, MR correlation with clinical findings is unlikely to add useful information. Using dynamic MR imaging we have demonstrated the ability to quantify the levator plate angle, the size of the levator hiatus, and perineal descent in symptomatic and asymptomatic women in a reliable manner. We hope to employ the quantified differences between women with prolapse and normal support in biomechnical models to determine which of these factors is important in the occurrence of prolapse.
Footnotes
Funded by National Institute of Child Health and Human Development R01 HD 38665.
References
- 1.Halban J, Tandler J. Anatomie und atiologie der genitalprolapse beim weibe. Wilhelm Braumüller; Wien: 1907. [Google Scholar]
- 2.Berglas B, Rubin IC. Study of the supportive structures of the uterus by levator myography. Surg Gynecol Obstet. 1953;97:677–92. [PubMed] [Google Scholar]
- 3.Nichols DH, Randall CL. Vaginal surgery. 4th ed Williams and Wilkins; Baltimore: 1996. [Google Scholar]
- 4.Noll LE, Hutch JA. The SCIPP lined an aid in interpreting the voiding lateral cystourethrogram. Obstet Gynecol. 1969;33:680–9. [PubMed] [Google Scholar]
- 5.Miller NF, Evans TN, Haas RL. Human parturition: normal and abnormal labor. Williams & Wilkins; Baltimore: 1958. p. 18. [Google Scholar]
- 6.Kearney R, Sawhney R, DeLancey JO. Levator ani muscle anatomy evaluated by origin-insertion pairs. Obstet Gynecol. 2004;104:168–73. doi: 10.1097/01.AOG.0000128906.61529.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang A, Mostwin JL, Rosenshein NB, Zerhouni EA. Pelvic floor descent in women: dynamic evaluation with fast MR imaging and cinematic display. Radiology. 1991;179:25–33. doi: 10.1148/radiology.179.1.2006286. [DOI] [PubMed] [Google Scholar]
- 9.Lienemann A, Anthuber C, Baron A, Kohz P, Reiser M. Dynamic MR colpocystorectography assessing pelvic-floor descent. Eur Radiol. 1997;7:1309–17. doi: 10.1007/s003300050294. [DOI] [PubMed] [Google Scholar]
- 10.Gufler H, Laubenberger J, DeGregorio G, Dohnicht S, Langer M. Pelvic floor descent: dynamic MR imaging using a half-Fourier RARE sequence. J Magn Reson Imaging. 1999;9:378–83. doi: 10.1002/(sici)1522-2586(199903)9:3<378::aid-jmri3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Kelvin FM, Maglinte DD, Hale DS, Benson JT. Female pelvic organ prolapse: a comparison of triphasic dynamic MR imaging and triphasic fluoroscopic cystocolpoproctography. Am J Roentgenol. 2000;174:81–8. doi: 10.2214/ajr.174.1.1740081. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman HS, Buller JL, Thompson JR, Pannu HK, DeMeester SL, Genadry RR, et al. Dynamic pelvic magnetic resonance imaging and cystocolpoproctography alter surgical management of pelvic floor disorders. Dis Colon Rectum. 2001;44:1575–83. doi: 10.1007/BF02234374. discussion 1583-4. [DOI] [PubMed] [Google Scholar]
- 13.Gearhart SL, Pannu HK, Cundiff GW, Buller JL, Bluemke DA, Kaufman HS. Perineal descent and levator ani hernia: a dynamic magnetic resonance imaging study. Dis Colon Rectum. 2004;47:1298–304. doi: 10.1007/s10350-004-0585-0. [DOI] [PubMed] [Google Scholar]
- 14.Singh K, Reid WM, Berger LA. Assessment and grading of pelvic organ prolapse by use of dynamic magnetic resonance imaging. Am J Obstet Gynecol. 2001;185:71–7. doi: 10.1067/mob.2001.113876. [DOI] [PubMed] [Google Scholar]
- 15.Singh K, Reid WM, Berger LA. Magnetic resonance imaging of normal levator ani anatomy and function. Obstet Gynecol. 2002;99:433–8. doi: 10.1016/s0029-7844(01)01743-4. [DOI] [PubMed] [Google Scholar]
- 16.Hjartardottir S, Nilsson J, Petersen C, Lingman G. The female pelvic floor: a domed not a basin. Acta Obstet Gynecol Scand. 1997;76:567–71. doi: 10.3109/00016349709024586. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich MA, Webb MJ, King BF, Bampton AE, Campeau NG, Riederer SJ. Magnetic resonance imaging of pelvic floor relaxation: dynamic analysis and evaluation of patients before and after surgical repair. Obstet Gynecol. 1993;82:883–91. [PubMed] [Google Scholar]
- 18.Ozasa H, Mori T, Togashi K. Study of uterine prolapse by magnetic resonance imaging: topographical changes involving the levator ani muscle and the vagina. Gynecol Obstet Invest. 1992;34:43–8. doi: 10.1159/000292723. [DOI] [PubMed] [Google Scholar]
- 19.Fielding JR, Griffths DJ, Versi E, Mulkern RV, Lee MLT, Jolesz FA. MR imaging of pelvic floor continence mechanisms in the supine and sitting positions. Am J Roentgenol. 1998;171:1607–10. doi: 10.2214/ajr.171.6.9843296. [DOI] [PubMed] [Google Scholar]
- 20.Leyendecker JR, Brown JJ. Abdominal and pelvic protocol basics. In: Leyendecker JR, Brown JJ, editors. Practical guide to abdominal and pelvic MRI. 1st ed Lippincott Williams and Wilkins; Philadelphia, (PA): 2004. p. 40. [Google Scholar]
- 21.Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223:501–8. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 22.Vanbeckevoort D, Van Hoe L, Oyen R, Ponette E, De Ridder D, Deprest J. Pelvic floor descent in females: comparative study of colpocystodefecography and dynamic fast MR imaging. J Magn Reson Imaging. 1999;9:373–7. doi: 10.1002/(sici)1522-2586(199903)9:3<373::aid-jmri2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]


