Abstract
Endocytic trafficking plays an important role in the regulation of the epidermal growth factor receptor (EGFR). To address if cellular kinases regulate EGFR internalization, we used anisomycin, a potent activator of kinase cascades in mammalian cells, especially the stress-activated mitogen-activated protein (MAP) kinase subtypes. Here, we report that activation of p38 MAP kinase by anisomycin is sufficient to induce internalization of EGFR. Anisomycin and EGF employ different mechanisms to promote EGFR endocytosis as anisomycin-induced internalization does not require tyrosine kinase activity or ubiquitination of the receptor. In addition, anisomycin treatment did not result in delivery and degradation of EGFR at lysosomes. Incubation with a specific inhibitor of p38, or depletion of endogenous p38 by small interfering RNAs, abolished anisomycin-induced internalization of EGFR while having no effect on transferrin endocytosis, indicating that the effect of p38 activation on EGFR endocytosis is specific. Interestingly, inhibition of p38 activation also abolished endocytosis of EGFR induced by UV radiation. Our results reveal a novel role for p38 in the regulation of EGFR endocytosis and suggest that stimulation of EGFR internalization by p38 might represent a general mechanism to prevent generation of proliferative or anti-apoptotic signals under stress conditions.
Keywords: anisomycin, EGFR, endocytosis, p38, UV
Growth factors and their transmembrane receptor tyrosine kinases (RTK) play important roles during embryonic development and in the regulation of several cellular processes including proliferation, survival, migration and differentiation (1, 2). Binding of growth factors to their receptors activates a myriad of signaling pathways that permit cells to respond to changes in the environment (3). In many cases, the termination of these signaling events is mediated by receptor internalization and degradation (4). The epidermal growth factor receptor (EGFR) is considered the prototypical member of the RTK family, and its activation and trafficking have been exhaustively characterized. Ligand binding results in receptor dimerization (5), activation and autophosphorylation of tyrosine residues in the cytosolic tail (6). Phosphotyrosines serve then as docking sites for the formation of protein networks that mediate signaling as well as receptor endocytosis and degradation (7). For example, autophosphorylation of specific tyrosines (Y992, Y1045, Y1068 and Y1086) is known to recruit specific adaptors such as phospholipase Cγ, Casitas B-lineage lymphoma (Cbl) or growth factor receptor binding protein 2 (Grb2).
Monoubiquitination of EGFR at multiple sites also seems to play an important role in receptor down-regulation (8). At the plasma membrane, receptor activation promotes the recruitment of Cbl, an ubiquitin ligase that mediates the ubiquitination of EGFR (9). Cbl can also interact with the endocytic machinery, thus ensuring EGFR internalization (10). At the endosomes, ubiquitin acts as a targeting signal for degradation through interaction with the multivesicular body-sorting machinery (11, 12).
However, there are still several aspects of EGFR trafficking that remain controversial. One is whether receptor ubiquitination is required for internalization or if it just plays a role in the delivery of the receptor to lysosomes. It has been shown that chimerae consisting of the extracellular and transmembrane domains of EGFR fused to a single cytosolic exposed ubiquitin are constitutively internalized and degraded (8, 12). However, other investigators have reported that receptor ubiquitination is not sufficient for EGFR endocytosis and that the role of Cbl is to link EGFR to coated pits through the interaction of Cbl with CIN85/endophilin complex (10, 13). Recently, it has been suggested that ubiquitin could determine the route of EGFR internalization so that non-ubiquitinated EGFR exclusively follows a clathrin-dependent pathway, while ubiquitinated EGFR can enter into the cell through both clathrin-coated pits and caveolae (14, 15).
Another open question to be resolved is the role of kinases in RTK internalization. As mentioned previously, EGF stimulation activates different protein kinases that participate in the transmission of many proliferative and differentiative signals. These protein kinases include the extracellular signal-regulated protein kinases (ERK) and two mitogen-activated protein (MAP) kinases, the c-Jun N-terminal kinase (JNK) (16) and the p38 MAP kinase (p38) (17). There is growing evidence showing that kinase activation does not only happen at the plasma membrane but also at endosomes. Several EGFR downstream signaling factors such as Grb2, Shc and mSOS localize to endosomes shortly after EGFR internalization (18, 19). In addition, robust MAP kinase activation requires the presence of active EGFR at endosomes (20, 21) and seems to promote cell survival (22, 23). Therefore, trafficking of EGFR regulates signal propagation and amplification (24, 25). Conversely, it has been suggested that signaling can regulate EGFR internalization, as different proteins implicated in signal transduction have been shown to interact with the endocytosis sorting machinery (26, 27). Recently, Zerial's group carried out high-throughput RNA interference analysis to address the role of kinases in endocytosis and showed that a large number of kinases previously implicated in proliferation, growth and cell adhesion also have a role in endocytosis (28).
Anisomycin is an antibiotic isolated from Streptomyces griseolus that inhibits protein synthesis by blocking peptidyl transferase activity in eukaryote ribosomes (29). Anisomycin is a very useful tool because it selectively activates kinase cascades in mammalian cells, especially the MAP kinases (30, 31). In this study, we used anisomycin to activate MAP kinases in the absence of ligand and analyzed the effect of this activation on EGFR internalization. Interestingly, we observed that anisomycin treatment induced EGFR endocytosis and that this process was independent of tyrosine phosphorylation or ubiquitination. Moreover, preincubation of the cells with SB203580, a highly specific inhibitor of p38 (32, 33), or depletion of endogenous p38 by small interfering RNAs (siRNAs) treatment, abolished the anisomycin-induced EGFR internalization suggesting that this MAP kinase plays an important role in the regulation of EGFR trafficking.
Results
Anisomycin induces EGFR internalization
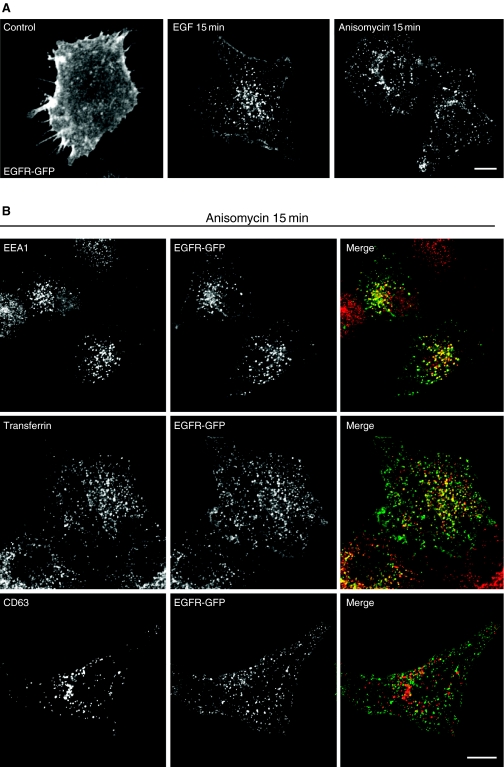
To address if the activation of MAP kinases induced by anisomycin has any effect on EGFR internalization, we made use of a chimera in which green fluorescent protein (GFP) has been attached to the carboxyl terminus of human EGFR (EGFR-GFP). This construct allowed us to easily visualize EGFR trafficking by immunofluorescence. It has been previously described that EGFR-GFP biochemical and cellular properties do not differ from EGFR-wt (34). Figure 1A shows that at stationary state, most of EGFR-GFP localized at the plasma membrane confirming that the presence of the GFP did not alter the normal distribution of the protein. Addition of EGF caused a rapid internalization of the receptor to endosomal structures as previously described (35). Interestingly, treatment with anisomycin for short periods of time also induced endocytosis of EGFR-GFP.
Figure 1. Anisomycin induces internalization of epidermal growth factor receptor-green fluorescent protein (EGFR-GFP).
(A) HeLa cells were transfected with a plasmid encoding EGFR-GFP. Twenty-four hours after transfection, unstimulated (control) cells or cells treated with EGF (100 ng/mL) or anisomycin (60 µm) for 15 min were fixed and analyzed by confocal microscopy. (B) Cells expressing EGFR-GFP were treated with anisomycin for 15 min, fixed and stained with the indicated antibodies. For transferrin staining, cells were incubated with rhodamine transferrin for 15 min at 37 °C. In the merge image, EGFR-GFP is in green; EEA1, transferrin and CD63 are in red and yellow indicates co-localization. Scale bar represents 10 µm.
In order to characterize the route followed by EGFR-GFP after anisomycin treatment, we analyzed the co-localization of the receptor with different markers. As shown in Figure 1B, we found extensive co-localization of EGFR-GFP with early endosomal markers, such as EEA1 or internalized transferrin, after incubation with the drug for 15 min. In contrast, no co-localization with the late endosomal/lysosomal marker CD63 was observed. Incubation with EGF for 15 min also caused redistribution of EGFR-GFP from the plasma membrane to early endosomes (see Supplementary Material, Figure S1) indicating that both compounds promote trafficking of EGFR-GFP to the same compartments.
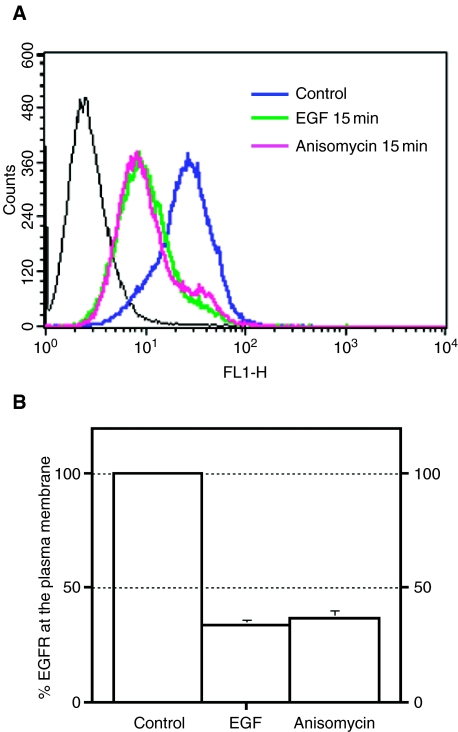
Next, we analyzed if anisomycin had the same effect on endogenous EGFR. Control cells or cells treated with anisomycin or EGF for 15 min were fixed, stained with an antibody that recognizes the extracellular domain of EGFR and analyzed by flow cytometry. As shown in Figure 2, treatment with either anisomycin or EGF caused a reduction of approximately 70% in the amount of endogenous EGFR present at the plasma membrane. This indicated that the effect observed by using EGFR-GFP was not a consequence of protein over-expression or mistargeting of the receptor due to the presence of GFP at the cytosolic tail, and validates the use of this chimera.
Figure 2. Anisomycin promotes endocytosis of endogenous epidermal growth factor receptor (EGFR).
(A) Control HeLa cells (blue line) or cells treated with EGF (green) or anisomycin (pink) for 15 min were fixed, and the amount of EGFR present at the plasma membrane was measured by flow cytometry. (B) Diagram representing the mean + SD from three independent experiments. Percentage of EGFR at the plasma membrane refers to the fractional reduction of surface receptor in response to 15-min agonist exposure. For all flow cytometry experiments, a minimum of 10 000 cells were counted.
To address if anisomycin causes the internalization of all cell-surface receptors, we analyzed the effect of the drug on the distribution of Tac, a cell-surface type 1 transmembrane glycoprotein that localizes at the plasma membrane, and TGFβRII, a cell-surface receptor kinase that requires ligand for internalization. As seen in Figure S2 (Supplementary Material), incubation with anisomycin for 20 min caused a robust internalization of EGFR while having no effect on the cell-surface distribution of Tac and TGFβRII. These data show the specifity of anisomycin for EGFR trafficking.
Anisomycin promotes endocytosis of EGFR through clathrin-coated pits
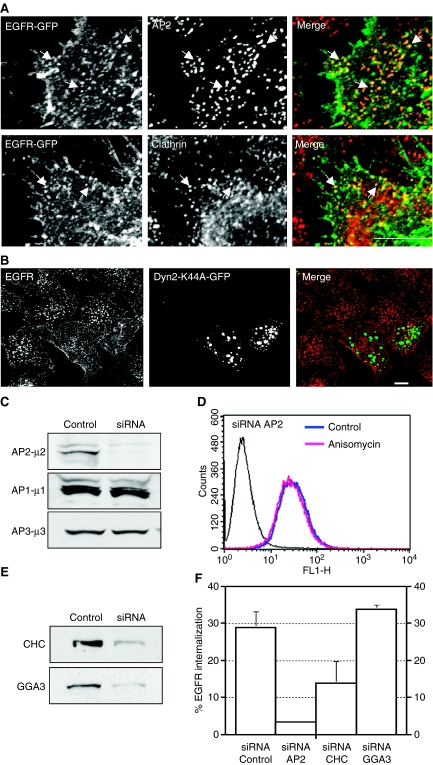
Treatment with anisomycin for short periods of time revealed that there were no apparent changes in the distribution of EGFR-GFP throughout the first 5 min of incubation. However, the receptor appeared at punctate structures following approximately 7 or 8 min of incubation. Many of these punctate structures contained clathrin and the clathrin adaptor AP2 (Figure 3A) suggesting that anisomycin induces EGFR internalization through a clathrin-dependent pathway. To test this possibility, we examined the effect of over-expressing a dominant-negative mutant of dynamin2 (Dyn2-K44A-GFP), a GTPase that is required for the clathrin-dependent internalization of endocytic receptors. As seen in Figure 3B, over-expression of Dyn2-K44A-GFP clearly blocked anisomicyn-induced internalization of EGFR. To further corroborate these data, we reduced the expression of endogenous AP2μ subunit (μ2) by using specific siRNA oligonucleotides. Immunoblot analysis revealed a 92% reduction in the levels of μ2 when compared with cells transfected with control (non-silencing) siRNA. Levels of μ1 and μ3 were monitored as control for the specificity of the antisense (Figure 3C). HeLa cells depleted of µ2 showed very little internalization of transferrin-rhodamine when compared with control cells, indicating that AP2-dependent endocytosis is impaired under our experimental conditions (data not shown). As expected, depletion of µ2 abolished anisomycin-induced internalization of endogenous EGFR indicating that the endocytosis is AP2 dependent (Figure 3D,F). Finally, we analyzed the effect of clathrin siRNA. Figure 3E,F shows that reduction in the levels of clathrin caused a clear decrease in the amount of EGFR that was internalized in response to anisomycin. In contrast, depletion of GGA3, a clathrin adaptor that regulates sorting of proteins at trans Golgi network and endosomes, or treatment with non-silencing siRNA, did not affect EGFR internalization. All together, these data indicate that anisomycin induces endocytosis of EGFR through clathrin-coated pits.
Figure 3. Anisomycin promotes endocytosis of epidermal growth factor receptor (EGFR) through clathrin-coated pits.
(A) HeLa cells expressing EGFR-green fluorescent protein (GFP) were treated with anisomycin (60 µm) for 8 min, fixed, stained with the indicated antibodies and analyzed by confocal microscopy. Bar represents 5 µm. (B) HeLa cells transfected with a plasmid encoding the GFP-tagged form of dynamin 2 K44A mutant (Dyn2-K44A-GFP) were incubated for 1 h on ice with a monoclonal antibody to the extracellular domain of EGFR, washed and allowed to internalize for 20 min at 37 °C in the presence of anisomycin (60 µm). Cells were then fixed, stained with a Cy3-conjugated donkey anti-mouse immunoglobulin G and analyzed by confocal microscopy. Bar represents 10 µm. (C) HeLa cells were transfected with small interfering RNA (siRNA) targeted to control (non-silencing) or to the µ2 subunit of the AP2 complex. Seventy-two hours after the second round of transfection, equivalent amounts of homogenate from control and AP2 siRNA-treated cells were subjected to SDS–PAGE and immunoblotted using an antibody to the µ subunit of AP2. To test the specificity of knockdown, lysates were also blotted to the µ subunits of AP1 and AP3. (D) Cells treated with AP2 siRNA were left untreated or stimulated with anisomycin for 20 min, and cell-surface EGFR was quantified by flow cytometry. (E) Western blot of total cellular levels of CHC or GGA3 in HeLa cells following transfection with either control (non-silencing) siRNA or the specific siRNA. (F) HeLa cells transfected with either non-targeting siRNA, siRNA-µ2, siRNA-CHC or siRNA-GGA3 were incubated with anisomycin for 20 min after which the amount of endogenous EGFR internalized was quantified by flow cytometry.
Anisomycin-induced internalization of EGFR does not require tyrosine phosphorylation or ubiquitination
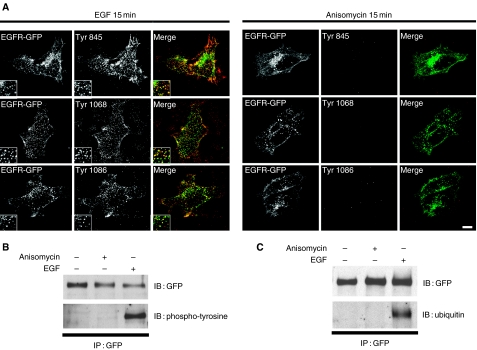
EGF binding induces EGFR dimerization, activation, autophosphorylation of specific tyrosine residues and monoubiquitination of the cytosolic tail. It has been proposed that the recruitment of the endocytic machinery, through interactions with both phosphotyrosines and ubiquitin, plays a role in the regulation of the receptor endocytosis. In Figure 4, we used antibodies against specific phosphotyrosines to follow the activation of the EGFR-GFP after EGF or anisomycin treatment. As mentioned previously, the addition of EGF for 15 min induced a clear redistribution of EGFR-GFP to intracellular vesicles. The majority of these structures was also labeled with antibodies against phophotyrosines 845, 1068 and 1086 indicating that EGFR-GFP is activated. In contrast, incubation with anisomycin caused receptor internalization, but no activation as no staining for phosphorylated tyrosines 845, 1068 or 1086 was observed (Figure 4A). We also generated several mutants in which different residues that are phosphorylated after receptor activation were mutated to alanines, including Y845, Y974, Y1045, Y1068, Y1086 and the double mutant S1046/S1047. Figure S3, Supplementary Material, shows that none of these mutations affected the normal distribution of the EGFR-GFP or its response to anisomycin. In addition, EGFR-GFP immunoprecipitates from HeLa cells treated with EGF or anisomycin were immunoblotted with the anti-phosphotyrosine antibody 4G10 confirming that EGF, but not anisomycin, induced tyrosine phosphorylation of the receptor (Figure 4B). Anisomycin also failed to stimulate ubiquitination of EGFR-GFP as observed by immunoprecipitation followed by immunoblotting with FK2 antibody (Figure 4C). These results indicate that neither phosphotyrosines nor ubiquitin seems to be required for anisomycin-induced EGFR internalization.
Figure 4. Anisomycin does not induce an increase in tyrosine phosphorylation nor ubiquitination of the epidermal growth factor receptor (EGFR).
(A) Cells transfected with EGFR-green fluorescent protein (GFP) (green) were stimulated with EGF or anisomycin for 15 min, fixed and stained with specific antibodies against phosphotyrosine 845, 1068 and 1086 (red). Insets show twofold-magnified views of peripheral cytoplasmic regions. Scale bar represents 10 µm. (B) (C) Whole-cell lysates of HeLa cells expressing EGFR-GFP prepared from unstimulated cells or cells exposed to EGF or anisomycin for 15 min were immunoprecipitated (IP) with GFP antibodies and blotted (IB) with anti-phosphotyrosine (4G10) (B) or anti-ubiquitin (FK2) (C) antibodies.
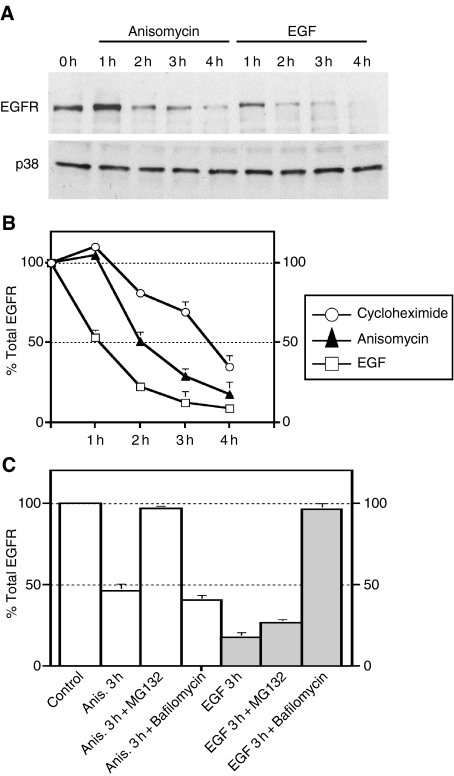
Anisomycin induces EGFR degradation
Next, we followed the fate of internalized EGFR after anisomycin or EGF treatment. HeLa cells were treated with cycloheximide alone, anisomycin or EGF plus cycloheximide for different periods of time. Western blotting showed that there was a marked down-regulation of EGFR after 2 h of incubation with EGF. In contrast, anisomycin caused a much slower degradation of EGFR (Figure 5A). Quantification of several independent experiments revealed that the differences in the kinetics of EGFR degradation were more evident after short incubation times. For example, EGFR levels dropped a 50% after 1 h of incubation with EGF but showed little change with anisomycin treatment (Figure 5B). Interestingly, treatment with proteosomal inhibitors such as MG132 almost completely abolished anisomycin-induced EGFR degradation but had little effect on EGF-mediated degradation. Conversely, lysosomal inhibitors blocked EGF but not anisomycin-mediated degradation (Figure 5C). These results suggest that anisomycin does not induce delivery of EGFR to lysosomes. Instead, the receptor could cycle between plasma membrane and endosomes until it is degraded most probably through the proteosome, although alternative mechanisms like caspase activation cannot be ruled out (36). Consistent with this idea, we observed a good co-localization between EGFR-GFP and transferrin up to 1 h following EGFR-GFP internalization (data not shown).
Figure 5. Anisomycin induces down-regulation of epidermal growth factor receptor (EGFR).
(A) HeLa cells were starved for 8 h in DMEM containing 0.1% BSA and incubated with cycloheximide alone, EGF plus cycloheximide or anisomycin for the indicated times. Cells were then lysed, subjected to SDS–PAGE and immunoblotted with antibodies against EGFR. Lysates were also blotted for p38 as loading control. (B) Quantification of the optical densities of the corresponding EGFR bands from three independent experiments. (C) Cells were preincubated with MG132 (10 µm) or bafilomycin (0.25 µm) and then treated with EGF or anisomycin. Three hours after the treatment, cells were harvested and immunoblotted with anti-EGFR.
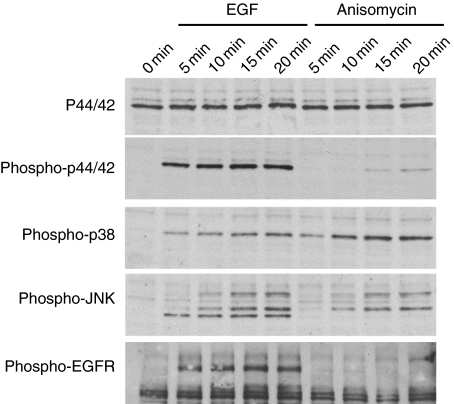
Anisomycin stimulates early p38 kinase activation
It has been reported that anisomycin is a selective activator of the MAP kinases (37, 38). We examined if this activation takes place under our experimental conditions and if it happens quickly enough to justify the early endocytosis observed for EGFR. Total lysates of HeLa cells stimulated with either EGF or anisomycin at different times were analyzed by SDS–PAGE and immunobloted with antibodies against activated p44/42, p38 and JNK. Total p44/42 was monitored as loading control. As previously reported, EGF stimulation induced a rapid activation of three members of the MAP kinase family (Figure 6). Anisomycin was also able to induce phosphorylation of both p38 and JNK. In contrast, anisomycin failed to increase p44/42 activation at early time-points while inducing a very low increase in the levels of phosphorylated p44/42 after 15 min of incubation. Total levels of p38 and JNK protein were not altered by anisomycin treatment (data not shown).
Figure 6. Time–course analysis of the effect of anisomycin on mitogen-activated protein kinase activity.
After treatment with epidermal growth factor (EGF) or anisomycin, cell lysates were prepared and subjected to SDS–PAGE followed by Western blotting analysis with the indicated antibodies.
Pharmacologic or genetic inhibition of p38 blocks anisomycin-induced internalization of EGFR
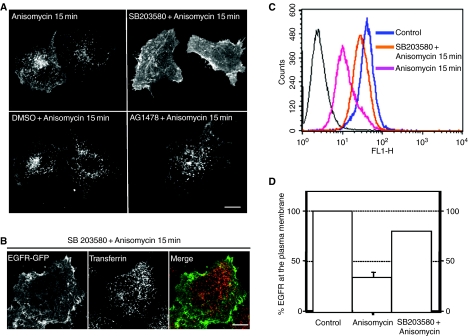
As previously stated, EGFR-GFP appeared in punctated structures that co-localize with clathrin at approximately 7 min after anisomycin addition. This suggests that the protein responsible for the endocytosis of EGFR-GFP is probably activated prior to EGFR-GFP redistribution. The early activation of p38 induced by anisomycin (Figure 6) makes this protein an excellent candidate to play a role in EGFR-GFR internalization. In order to test this possibility, we used a specific inhibitor for p38. Pyridinyl imidazol, SB203580, is a highly specific, cell-permeable inhibitor of p38 (32, 33). This compound has been previously used to determine a role for p38 in several biological processes including UV- and anisomycin-induced c-jun and c-fos expression (37, 39). SB203580 selectively inhibits p38 while having no significant effect on other related kinases such as ERK and JNK. To block the activity of p38, cells were treated with 10 µm SB203580 for 30 min at 37 °C before being exposed to anisomycin for 15 min. As shown in Figure 7A, treatment with SB203580 dramatically decreased the internalization of EGFR-GFP induced by anisomycin, suggesting that p38 activation is necessary for this process. Interestingly, treatment with AG1478, a selective inhibitor of EGFR tyrosine kinase, had no effect on EGFR-GFP endocytosis, further confirming that phosphorylation of EGFR tyrosines is not required for anisomycin-induced EGFR internalization.
Figure 7. Inactivation of p38 by SB203580 inhibits anisomycin-induced epidermal growth factor receptor (EGFR) internalization.
(A) HeLa cells expressing EGFR-green fluorescent protein (GFP) were left untreated or preincubated with SB203580, dimethyl sulphoxide or AG1748 for 30 min prior to stimulation with anisomycin for 15 min. (B) After 30 min preincubation with SB203580, HeLa cells expressing EGFR-GFP were stimulated with anisomycin for 15 min in the presence of rhodamine transferrin, fixed and analyzed by confocal microscopy. (C) Control cells or cells preincubated with SB203580 for 30 min were treated with anisomycin for 15 min, fixed and the cell surface EGFR measured by flow cytometry. (D) Graphics represents the mean + SD values obtained from two independent experiments. Scale bar represents 10 µm.
Next, we determined if inhibition of p38 by SB203580 had a non-specific effect on clathrin-mediated endocytosis. Figure 7B shows that preincubation with SB203580 blocked anisomycin-induced internalization of EGFR-GFP without affecting transferrin endocytosis. This result indicated that activation of p38 does not play a general role in clathrin-mediated internalization but it is required for down-regulation of specific receptors.
The requirement of p38 MAPK activation for anisomycin-dependent EGFR internalization was further analyzed by flow cytometry. FACS analysis of the EGFR surface expression indicated that preincubation with SB203580 caused a 2.5 fold reduction in the amount of endogenous EGFR internalized after 15 min of anisomycin stimulation (Figure 7C,D). Treatment with dimethyl sulphoxide (DMSO) had no effect on EGFR endocytosis (data not shown).
To corroborate that p38 is required for anisomycin-induced internalization of EGFR, we employed genetic means to selectively deplete endogenous p38. To do so, HeLa cells were transfected either with a pool of siRNAs targeting p38α (siRNA-p38α), p38β (siRNA-p38β) or a combination of both (siRNA-p38α + β). As seen in Figure 8, expression of p38α, but not that of p38β, was abrogated in siRNA-p38α cells, while an inhibition in the expression of p38β, but not p38α, was seen in siRNA-p38β cells. Transfection of siRNA-p38α +β caused a significant reduction in the levels of both p38α and p38β, while treatment with a control non-targeting siRNA did not affect the expression of either of the two isoforms. Interestingly, abrogation of expression of either p38α or p38β reduced the efficiency of EGFR internalization after anisomycin treatment (Figure 8B). This reduction was much more robust in siRNA-p38α + β cells, where only a 10% EGFR internalization was observed after incubation with anisomycin for 15 min. All together, these results indicate that both p38α and p38β might act synergistically in the regulation of EGFR trafficking.
Figure 8. Effect of silencing of p38α and p38β on anisomycin-induced epidermal growth factor receptor (EGFR) internalization.
(A) HeLa cells were treated with small interfering RNA (siRNA) targeted to p38α (siRNA-p38α), p38β (siRNA-p38β) or both (siRNA-p38α + β). After two consecutive transfections with siRNA, the expression of the two isoforms was assessed by Western blotting. (B) HeLa cells transfected with either non-targeting siRNA, siRNA-p38α, siRNA-p38β or siRNA-p38α + β were incubated with anisomycin for 15 min after which the amount of endogenous EGFR remained at the plasma membrane was quantified by flow cytometry.
p38 activation is also required for endocytosis of EGFR induced by UV radiation
It has been reported that UV radiation can induce internalization of EGFR independent of its phosphorylation or RTK activation. The mechanism that mediates this process has not been characterized although it has been suggested that direct absorption of UV energy might induce a conformational change of EGFR (40, 41). The fact that p38 can be activated by a variety of stress signals, including UV radiation, suggested that this protein could be involved in the UV-induced internalization of EGFR. Figure 9 shows that after UV radiation (200 J/m2) an extensive EGFR-GFP internalization, comparable with that seen after EGF or anisomycin stimulation, was observed. As expected, addition of SB203580 to the cells 30 min prior to UV radiation inhibited EGFR-GFP internalization which confirmed our hypothesis that p38 plays an important role in UV-induced endocytosis of EGFR.
Figure 9. SB203580 inhibits UV-mediated internalization of epidermal growth factor receptor (EGFR).
Control cells or cells preincubated with SB203580 for 30 min were irradiated with UV light (200 J/m2) and chased at 37 °C for 30 min. Cells were fixed and the distribution of EGFR-green fluorescent protein was analyzed by confocal microscopy. Scale bar represents 10 µm.
Discussion
We have shown that activation of the stress-induced p38 MAP kinase by anisomycin induces a rapid internalization of EGFR. Interestingly, EGFR endocytosis was independent of RTK activity and monoubiquitination of the cytosolic tail indicating that EGF and anisomycin employ different mechanisms to promote internalization. The lack of ubiquitination prevented the delivery of EGFR to lysosomes for degradation. This observation is in agreement with recent reports that found that ubiquitination of EGFR is required for interaction with the mutivesicular bodies-sorting machinery (42, 43).
p38 was originally identified as a kinase phosphorylated in response to endocytic lipopolysacharides (44, 45). Later, it was found that p38 activity is also up-regulated when cells are exposed to a variety of stimuli including certain growth factors, proinflammatory cytokines (46–48) and different forms of environmental stress such as UV light (35, 49), heat (32) and osmotic shock (32, 45, 46). To date, four members of the p38 group of MAP kinases have been characterized, including p38α (50), p38β (51), p38γ (52, 53) and p38δ (51). SB203580 is a selective inhibitor for both, p38α and β, but has no effect on the activity of the γ and δ isoforms. The inhibition of the anisomycin-induced EGFR internalization observed in the presence of SB203580 strongly suggests the involvement of p38α and/or p38β in this process. The additive effect of p38α and p38β silencing on EGFR internalization after anisomycin treatment corroborate that both isoforms likely play an important role in the regulation of EGFR trafficking. It is important to note that, while inhibition of p38 activation clearly prevented EGFR entry into the cells, no effect on transferrin internalization was observed, indicating that SB203580 does not cause a general block of clathrin-mediated endocytosis.
Recently, it has been shown that exposure of cells to UV radiation also induces internalization of EGFR (40), but the precise mechanism that controls this process remains unknown. Significantly, there are several similarities between UV-induced and anisomycin-induced EGFR endocytosis. For example, in both cases, EGFR internalization is processed by clathrin-coated pits, is independent of RTK activity or autophosphorylation, does not require receptor ubiquitination and does not result in lysosomal degradation of the receptor (41, 54, 36). In this paper, we show that activation of p38 is involved in the UV-induced internalization of EGFR, suggesting that the regulation of EGFR endocytosis by p38 can occur under physiological conditions. It has been shown that EGFR is involved in cell proliferation, survival and tumorogenesis and that preventing EGFR down-regulation facilitates cell transformation. Therefore, external stimuli that cause p38 activation could control the accessibility of EGFR at the plasma membrane preventing that way cell proliferation and survival under stress conditions.
How does p38 promote the internalization of EGFR? There is growing evidence showing that p38 plays an important role in numerous biological processes including inflammation (55, 56), development (57, 58), cell death (59, 60), cell cycle (61), cardiomyocyte hypertrophy (62), cell differentiation (63, 64), senescence (65) and tumorogenesis (66). Recently, it has been suggested that p38 might also participate in the regulation of endocytic trafficking. Cavalli et al. (2001) (67) have reported that p38 can phosphorylate and activate guanyl-nucleotide dissociation inhibitor (GDI), a key regulator of the Rab cycle. Rab proteins are small monomeric GTPases with molecular masses in the 20–30 kDa range (68). Multiple Rabs have been shown to participate in the formation, fusion and movement of vesicular traffic intermediaries between different membrane compartments of the cell. Rabs function as molecular switches by cycling between two interconvertible forms, a cytosolic GDP-bound (inactive) form and a membrane associated GTP-bound (active) form. In addition, Rabs also cycle between membrane-bound and cytosolic states, and this cycling is regulated by GDI (69).
Rab 5 in particular seems to play an important role in the trafficking of EGFR. Several studies have established that activated EGFR modulates the GTPase activity of Rab5 by targeting either GTPase-activating proteins, such as RN-Tre (70), or GTP exchange factors, such as RIN1 (71). The activation of Rab5 through EGFR or the expression of constitutively active mutants of Rab5 stimulates both the internalization and degradation of activated EGFR. In contrast, dominant negative Rab5 blocks EGF-stimulated receptor-mediated and fluid-phase endocytosis (72). Therefore, p38 could promote EGFR endocytosis by stimulating the activity of GDI in extracting Rab5 from the endosomal membranes and forming cytosolic GDI-Rab5 complex. Interestingly, GDI-Rab5 has been identified as a component of the machinery controlling clathrin-coated endocytic vesicle formation (73). In addition, GDI might facilitate the delivery of Rab5 to specific regions of the plasma membrane facilitating the interaction with cytoskeleton or specific effectors. In agreement with this idea, Huang et al. (74) have shown that the activation of p38 induced by metabotropic glutamate receptor agonists accelerates loss of surface amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors by stimulating the formation of GDI-Rab5 complexes.
We cannot discard the possibility that activation of p38 could also affect EGFR recycling. Recent evidence suggests that p38 can be recruited to endosomes (28) and regulates endosomal Rab5 effectors such as EEA1 (75). p38 also seems to play a negative regulatory role in Mycobacterium tuberculosis infection by modulating the recruitment of EEA1 to phagosomes and inhibiting that way phagosome maturation (76). This involvement may reflect a more general role for p38 in the regulation of intracellular trafficking. While further studies will be required to assess the specific mechanisms used by p38 to regulate EGFR internalization, our results strengthen the idea that there is a clear intercommunication between signaling and intracellular trafficking and suggest that p38 is a key player in this process.
Materials and Methods
Antibodies and reagents
The following commercial antibodies were used: mouse monoclonal anti-ubiquitin (FK2; Affiniti, Devon, UK), mouse monoclonal anti-EEA1, mouse monoclonal anti-CHC and mouse monoclonal anti-GGA3 (BD Transduction Laboratories, San Jose, CA, USA), mouse monoclonal anti-human TGFβRII (R&D Systems Inc., Minneapolis, MN, USA), mouse monoclonal to α-adaptin (AP-6; Affinity Bioreagents, Golden, CO, USA), mouse monoclonal anti-CD63 (H5C6; BD Pharmigen, San Jose, CA, USA), mouse phosphotyrosine antibody (clone 4G10; Upstate Biotechnology, Lake Placid, NY, USA), rabbit anti-GFP (MBL International, Woburn, MA, USA), mouse monoclonal and rat monoclonal anti-EGF receptor (ab3103 and ICR10; Abcam, Cambridge, MA, USA). Rabbit polyclonal antibodies against p38, phospho-p38, p44/42, phospho-p44/42, phospho-JNK, EGF-receptor, phospho-EGF receptor (Tyr845), phospho-EGF receptor (Tyr1068) and phospho-EGF receptor (Tyr1086) were obtained from Cell Signaling Technology (Beverly, MA, USA). Rabbit polyclonal antibodies to µ1, µ2 and µ3 were the kind gift of JS. Bonifacino (NIH, Bethesda, MD, USA).
Anisomycin, EGF, SB203580, AG1478, MG132, bafilomycin A1, ubiquitin aldehyde, mammalian phosphatase inhibitor cocktail and NP40 were obtained from Sigma (St. Louis, MO, USA). Rhodamine-transferrin was supplied by Molecular Probes (Eugene, OR, USA).
Plasmids
Cloning and characterization of the EGFR-GFP chimera has been previously described (34). Mutations of residues in the cytosolic tail of EGFR-GFP were introduced using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). The GFP-tagged dynamin2 K44A construct was a gift from M. McNiven (Mayo Clinic, Rochester, MN, USA).
siRNA
RNAi of CHC, GGA3 and the µ subunit of the AP2 complex was performed using siRNAs to the following human target sequences: UAAUCCAAUUCGAAGACCAAUU for CHC, GUGGAUGCCUUUCGGGUCA for µ2 and AAAAACGGCUUCCGCAUCCUC for GGA3 (Dharmacon Research, Lafayette, CO). Heterogeneous duplex siRNA pool against p38 MAP kinase and p38 MAP kinase β were obtained from Cell Signaling Technology and Dharmacon, respectively. For each siRNA, two consecutive transfections every 72 h were carried out in HeLa cells according to the manufacturer's instructions.
Transfection and immunofluorescence microscopy
HeLa cells were transfected with a plasmid encoding EGFR-GFP by using FuGENE-6 (Roche Diagnostics, Indianapolis, IN, USA). For immunofluorescence, transfected HeLa cells were grown on coverslips. Twenty-four hours after transfection, the cells were maintained in serum-free medium for 6–8 h, stimulated with EGF (100 ng/mL) or anisomycin (60 µm) for 15 min and fixed in methanol/acetone (1:1, v/v) for 10 min at −20 °C. Coverslips were mounted onto glass slides with Fluoromount G (Southern Biotechnology Associates, Birmingham, AL, USA). Fluorescence images were acquired on an LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY, USA).
To examine the co-localization of EGFR-GFP with endosomal markers, incubation with primary antibodies diluted in PBS, 0.1% (wt/vol) saponin and 0.1% BSA, was carried out for 1 h at room temperature. Unbound antibodies were removed by rinsing with PBS for 5 min, and cells were subsequently incubated with a secondary antibody (Cy3-conjugated donkey anti-rabbit or anti-mouse Ig) diluted in PBS, 0.1% (wt/vol) saponin and 0.1% BSA, for 30–60 min at room temperature. After a final rinse with PBS, coverslips were mounted onto glass slides with Fluoromount G and analyzed by confocal microscopy.
Treatments with SB203580 or AG1478 were carried out by incubation of the cells with the inhibitors for 30 min prior to anisomycin stimulation. Cells were exposed to UV radiation in a UV Stratalinker 2400 (Stratagene).
Immunoprecipitation and immunoblot
HeLa cells were starved for 8 h in serum-free medium and then stimulated with EGF (100 ng/mL) or anisomycin (60 µm) for 15 min. The cells were then washed with ice-cold PBS and lysed for 15 min in lysis buffer (25 mm Tris–Cl, pH 7.4, 150 mm NaCl, 5 mm ethylenediaminetetraacetic acid, 20 mm NaF, 0.5% NP40) supplemented with protease, tyrosine phosphatase and ubiquitin hydrolase inhibitors. The lysate was precleared by centrifugation and incubated with 5 µL of anti-GFP or a non-specific antibody and protein G-Sepharose (Amersham Biosciences, Piscataway, NJ). Immunoprecipitates were collected, washed six times with 1 mL of lysis buffer supplemented with phosphatase inhibitors and analyzed by SDS–PAGE under reducing conditions.
Immunoblotting was carried out according to standard procedures. Proteins were separated by SDS–PAGE and transferred to nitrocellulose. The membrane was then blocked with PBS/0.05% Tween-20/10% BSA and incubated with the various antibodies. Enhanced chemiluminescence reagent (Amersham Biosciences) was used for detection.
Degradation and MAP kinase activation assays
HeLa cells were serum starved for 8 h and stimulated with EGF (100 ng/mL) or anisomycin (60 µm) for the indicated times. For degradation assays, total cell lysates were subjected to immunoblotting with antibodies against EGFR. The amounts of EGFR were quantified at each time-point by using the public domain NIH Image program (1.6–2) and are represented as the percentage of remaining EGFR in comparison with unstimulated cells in the same experiment. The activation of p44/42, JNK and p38 was determined by Western blotting with antibodies specific for phosphorylated, activated forms of these kinases.
Quantitative internalization assay
HeLa cells were grown to 90% confluence. Following serum starvation for 4–5 h, the cells were harvested in Cellstripper™ solution (Mediatech, Inc., Herdon, VA), washed once with cold PBS and incubated with DMSO or SB203580 (10 μM) at 4 °C for 30 min. The cells were then incubated with EGF (100 ng/mL) or anisomycin (10 µm) for the indicated times at 37 °C, and then placed on ice to stop internalization. The cells were fixed with 2% paraformaldehyde, incubated with the FITC-conjugated anti-EGFR antibody for 45 min on ice, washed and analyzed by the FACS-Calibur using cellquest software (BD Bioscience, San Jose, CA). Amounts of EGFR remaining on the cell surface were defined as the specific fluorescence value, which was calculated after subtracting background (fluorescence of non-stained cells). Percent internalization was calculated where the specific mean fluorescence value of cells incubated at 37 °C with SB203580 was treated as 100%.
Acknowledgments
We thank C. Mullins for critical reading of the manuscript. We also appreciate the editorial assistance of the NCI, CCR Fellows Editorial Board. This project was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute (NHLBI).
Supplementary Material
EGFR-GFP redistributes from the plasma membrane to early endosomes after EGF stimulation. HeLa cells were transfected with a plasmid encoding EGFR-GFP. Transfected cells were incubated with EGF (100 ng/mL) for 15 min, fixed, permeabilized, immunostained with the indicated antibodies and examined by confocal fluorescence microscopy. For transferrin staining, cells were incubated with rhodamine-transferrin for 15 min at 37 °C. EGFR-GFP is in green; EEA1, transferrin and CD63 are in red and yellow indicates co-localization. Scale bar represents 10 µm.
Anisomycin has no effect on the distribution of Tac and TGFβRII. (A) HeLa cells expressing EGFR-GFP or Tac were left untreated or stimulated with anisomycin for 20 min. Cells were then fixed and analyzed by confocal microscopy. Bar represents 10 µm. (B) Cell surface levels of EGFR or TGFβRII were quantified by flow cytometry in unstimulated cells or cells exposed to anisomycin for 20 min. For detection of TGFβRII, HeLa cells were transiently transfected with a plasmid encoding human TGFβRII.
Anisomycin-induced internalization of EGFR-GFP was not affected by mutation of specific carboxy-terminal tyrosine residues to alanines. Wild-type EGFR-GFP or EGFR-GFP carrying the indicated mutations were transiently expressed in HeLa cells. Twenty-four hours after transfection, cells were either left untreated, or incubated with anisomycin for 15 min, fixed and analyzed by confocal microscopy. Scale bar represents 10 µm.
References
- 1.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T, Gish GD, Nash P. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 2001;11:504–511. doi: 10.1016/s0962-8924(01)02154-7. [DOI] [PubMed] [Google Scholar]
- 3.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 4.Waterman H, Yarden Y. Mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 5.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 6.Hackel PO, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 7.Pawson T, Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993;3:434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- 8.Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278:21323–21326. doi: 10.1074/jbc.C300096200. [DOI] [PubMed] [Google Scholar]
- 9.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 10.Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–187. doi: 10.1038/416183a. [DOI] [PubMed] [Google Scholar]
- 11.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 12.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 14.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 17.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 18.Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohba Y, Kurokawa K, Matsuda M. Mechanism of the spatio-temporal regulation of Ras and Rap1. EMBO J. 2003;22:859–869. doi: 10.1093/emboj/cdg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 21.Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ. Essential role for G-protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 22.Pennock S, Wang Z. Stimulation of cell proliferation by endosomal epidermal growth factor receptor as revealed through two distinct phases of signaling. Mol Cell Biol. 2003;23:5803–5815. doi: 10.1128/MCB.23.16.5803-5815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002a;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mc Pherson PS, Kay BK, Hussain NK. Signaling on the endocytic pathway. Traffic. 2001;2:375–384. doi: 10.1034/j.1600-0854.2001.002006375.x. [DOI] [PubMed] [Google Scholar]
- 25.Yarden Y, iwkowski MX. Untang1ing the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 26.Mohney RP, Das M, Bivona TG, Hanes R, Adams AG, Philips MR, O'Bryan JP. Intersectin activates Ras but stimulates transcription through an independent pathway involving JNK. J Biol Chem. 2003;278:47038–47045. doi: 10.1074/jbc.M303895200. [DOI] [PubMed] [Google Scholar]
- 27.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 28.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 29.Sobin BA, Tanner FW., Jr Anisomycin, a new antiprotozoan antibiotic. J Am Chem Soc. 1954;76:4053. [Google Scholar]
- 30.Cano E, Hazzalin CA, Mahadevan LC. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol Cell Biol. 1994;14:7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinck R, Cahill MA, Kracht M, Sachsenmaier C, Hipskind RA, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin MM, Kumar S, McDonnell PC, Van Horn S, Lee JC, Livi GP, Young PR. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 34.Carter RE, Sorkin A. Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J Biol Chem. 1998;273:35000–35007. doi: 10.1074/jbc.273.52.35000. [DOI] [PubMed] [Google Scholar]
- 35.Cohen S, Fava RA. Internalization of functional epidermal growth factor: receptor kinase complexes in A-431 cells. J Biol Chem. 1985;260:12351–12358. [PubMed] [Google Scholar]
- 36.He YY, Huang JL, Gentry JB, Chignell CF. Epidermal growth factor receptor down-regulation induced by UVA in human keratinocytes does not require the receptor kinase activity. J Biol Chem. 2003;278:42457–42465. doi: 10.1074/jbc.M303376200. [DOI] [PubMed] [Google Scholar]
- 37.Hazzalin CA, Cano E, Cuenda A, Barratt MJ, Cohen P, Mahadevan LC. p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient. Curr Biol. 1996;6:1028–1031. doi: 10.1016/s0960-9822(02)00649-8. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38b) J Biol Chem. 1996;271:17920–17926. doi: 10.1074/jbc.271.30.17920. [DOI] [PubMed] [Google Scholar]
- 39.Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 40.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 41.Oksvold MP, Thien CB, Widerberg J, Chantry A, Huitfeldt HS, Langdon WY. UV-radiation-induced internalization of the epidermal growth factor receptor requires distinct serine and tyrosine residues in the cytoplasmic carboxy-terminal domain. Radiat Res. 2004;16:685–691. doi: 10.1667/rr3185. [DOI] [PubMed] [Google Scholar]
- 42.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Sanghera JS, Weinstein SL, Aluwalia M, Girn J, Pelech SL. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 45.Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 46.Moriguchi T, Toyoshima F, Gotoh Y, Iwamatsu A, Irie K, Mori E, Kuroyanagi N, Hagiwara M, Matsumoto K, Nishida E. Purification and identification of a major activator for p38 from osmotically shocked cells: activation of mitogen-activated protein kinase kinase 6 by osmotic shock, tumor necrosis factor-alpha, and HO. J Biol Chem. 1996;271:26981–26988. doi: 10.1074/jbc.271.43.26981. [DOI] [PubMed] [Google Scholar]
- 47.Geng Y, Valbracht J, Lotz M. Selective activation of the mitogen-activated protein kinase subgroups c-Jun NH2 terminal kinase and p38 by IL-1 and TNF in human articular chondrocytes. J Clin Invest. 1996;98:2425–2430. doi: 10.1172/JCI119056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitmarsh AJ, Yang SH, Su MS, Sharrocks AD, Davis RJ. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price MA, Cruzalegui FH, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 50.Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–25014. [PubMed] [Google Scholar]
- 51.Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J. Characterization of the structure and function of the fourth member of p38 group mitogenactivated protein kinases, p38delta. J Biol Chem. 1997;272:30122–30128. doi: 10.1074/jbc.272.48.30122. [DOI] [PubMed] [Google Scholar]
- 52.Lechner C, Zahalka MA, Giot JF, Moler NPH, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38gamma: a new member of p38 group of MAP kinase. Biochem Biophys Res Commun. 1996;228:334–340. doi: 10.1006/bbrc.1996.1662. [DOI] [PubMed] [Google Scholar]
- 54.Oksvold MP, Huitfeldt HS, Østvold AC, Skarpen E. UV induces tyrosine kinase-independent internalisation and endosome arrest of the EGF receptor. J Cell Sci. 2002;115:793–803. doi: 10.1242/jcs.115.4.793. [DOI] [PubMed] [Google Scholar]
- 55.Perregaux DG, Dean D, Cronan M, Connelly P, Gabel CA. Inhibition of interleukin-1 beta production by SKF86002: evidence of two sites of in vitro activity and of a time and system dependence. Mol Pharmacol. 1995;48:433–442. [PubMed] [Google Scholar]
- 56.Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;13:1550–1552. doi: 10.1096/fj.04-1642fje. [DOI] [PubMed] [Google Scholar]
- 57.Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 58.Natale DR, Paliga AJ, Beier F, D'Souza SJ, Watson AJ. p38 MAPK signaling during murine preimplantation development. Dev Biol. 2004;268:76–88. doi: 10.1016/j.ydbio.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 60.Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, Biemann HP, Blenis J. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/ CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- 62.Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38 alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 63.Engelman JA, Lisanti MP, Scherer PE. Specific inhibitorsof p3-8 mitogen-activated protein kinase b1ock 3T3-L1 adipogenesis. J Bio1 Chem. 1998;273:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- 64.Nagata Y, Takahashi N, Davis RJ, Todokoro K. Activation of p3-8 MAP kinase and JNK but not ERK is required for erythropoietin-induced erythroid differentiation. B1ood. 1998;92:1859–1869. [PubMed] [Google Scholar]
- 65.Wang W, Chen JX, Liao R, Deng Q, Zhou JJ, Huang S, Sun P. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol Cell Biol. 2002b;22:3389–3403. doi: 10.1128/MCB.22.10.3389-3403.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brancho D, Tanaka N, Jaeschke A, Ventura JJ, Kelkar N, Tanaka Y, Kyuuma M, Takeshita T, Flavell RA, Davis RJ. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–1978. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, Arkinstall S, Gruenberg J. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol Cell. 2001;7:421–432. doi: 10.1016/s1097-2765(01)00189-7. [DOI] [PubMed] [Google Scholar]
- 68.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 69.Pfeffer S, Aivazian D. Targeting Rab GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 70.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 71.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 72.Barbieri MA, Roberts RL, Gumusboga A, Highfield H, Alvarez-Dominguez C, Wells A, Stahl PD. Epidermal growth factor and membrane trafficking. EGF receptor activation of endocytosis requires Rab5a. J Cell Biol. 2000;151:539–550. doi: 10.1083/jcb.151.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLauchlan H, Newell J, Morrice N, Osborne A, West M, Smythe EA. Novel role for Rab5-GDI in ligand sequestration into clathrin-coated pits. Curr Biol. 1998;8:34–45. doi: 10.1016/s0960-9822(98)70018-1. [DOI] [PubMed] [Google Scholar]
- 74.Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem. 2004;279:12286–12292. doi: 10.1074/jbc.M312868200. [DOI] [PubMed] [Google Scholar]
- 75.Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fratti RA, Chua J, Deretic V. Induction of p38 mitogen-activated protein kinase reduces early endosome autoantigen 1 (EEA1) recruitment to phagosomal membranes. J Biol Chem. 2003;278:46961–46967. doi: 10.1074/jbc.M305225200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
EGFR-GFP redistributes from the plasma membrane to early endosomes after EGF stimulation. HeLa cells were transfected with a plasmid encoding EGFR-GFP. Transfected cells were incubated with EGF (100 ng/mL) for 15 min, fixed, permeabilized, immunostained with the indicated antibodies and examined by confocal fluorescence microscopy. For transferrin staining, cells were incubated with rhodamine-transferrin for 15 min at 37 °C. EGFR-GFP is in green; EEA1, transferrin and CD63 are in red and yellow indicates co-localization. Scale bar represents 10 µm.
Anisomycin has no effect on the distribution of Tac and TGFβRII. (A) HeLa cells expressing EGFR-GFP or Tac were left untreated or stimulated with anisomycin for 20 min. Cells were then fixed and analyzed by confocal microscopy. Bar represents 10 µm. (B) Cell surface levels of EGFR or TGFβRII were quantified by flow cytometry in unstimulated cells or cells exposed to anisomycin for 20 min. For detection of TGFβRII, HeLa cells were transiently transfected with a plasmid encoding human TGFβRII.
Anisomycin-induced internalization of EGFR-GFP was not affected by mutation of specific carboxy-terminal tyrosine residues to alanines. Wild-type EGFR-GFP or EGFR-GFP carrying the indicated mutations were transiently expressed in HeLa cells. Twenty-four hours after transfection, cells were either left untreated, or incubated with anisomycin for 15 min, fixed and analyzed by confocal microscopy. Scale bar represents 10 µm.