Abstract
Bile salts such as sodium taurocholate (NaTC) are routinely used to induce the excystation of Cryptosporidium oocysts. Here we show that NaTC significantly enhanced the invasion of several cultured cell lines by freshly excysted Cryptosporidium parvum and Cryptosporidium hominis sporozoites. A variety of purified bile salts or total bile from bovine also enhanced the invasion of cultured cells by C. parvum. Further studies demonstrated that NaTC increased protein secretion and gliding motility of sporozoites, the key processes for successful invasion. These observations may lead to improved Cryptosporidium infectivity of cultured cells and help future studies on the host-parasite interaction.
As members of the phylum Apicomplexa, the enteric protozoa Cryptosporidium species share a common apical secreting apparatus that mediates locomotion during cellular invasion (5, 21). Cryptosporidium hominis and Cryptosporidium parvum, whose genomes were sequenced recently (1, 28), are the species most frequently linked with human cryptosporidiosis (21, 27). Infection begins with the oral uptake of Cryptosporidium oocysts, the environmentally resistant form, which then pass through the acidic stomach before entering the small intestine, where they presumably excyst. Although the molecular and biochemical mechanisms involved in excystation are poorly understood, it is believed that host environmental factors, such as temperature, pH, proteases, bile salts, and possibly other unknown factors, trigger the excystation of ingested oocysts (19). The newly excysted sporozoites secrete adhesive molecules and other signaling proteins which have been demonstrated to play a key role in the initial attachment and cellular invasion (4, 6).
The in vitro cell culture system, while limited to the asexual phase, has been a useful tool for investigating early parasite attachment and invasion and has been applied to measure Cryptosporidium infectivity and for screening of compounds for inhibitory activity (3, 17). Several cell lines, including human ileocecal adenocarcinoma (HCT-8), human colonic adenocarcinoma (Caco-2), and Madin-Darby bovine kidney (MDBK) cell lines, are widely used for Cryptosporidium in vitro studies (12, 22). To initiate in vitro infection, purified Cryptosporidium oocysts are often treated with sodium hypochlorite either alone or followed by sodium taurocholate (NaTC)-trypsin treatment before infecting cell monolayers. Several publications have dealt with assessment of the conditions and materials for optimal oocyst excystation and tissue culture infection by the excysted sporozoites (15, 16, 23, 24). Upton et al. (24) investigated the optimization of infection of oocysts treated with bleach without prior excystation. Gold et al. showed that the presence of NaTC in the culture medium enhanced infection when oocysts were directly added to cell culture monolayers (11). However, the nature of this enhancement was not fully investigated, and it was assumed that the NaTC facilitated the excystation of oocysts in the culture medium (11). In this study, we have investigated the impact of NaTC and some other bile salts on the invasiveness of freshly excysted sporozoites. We have shown that NaTC treatment increases the secretion of organelles and the gliding motility of sporozoites, thus facilitating their initial attachment and invasion of cells. These observations should lead to improved efficiency of infectivity of cells, as well as shed light on the nature of the host-pathogen interaction.
MATERIALS AND METHODS
Cell lines.
The human ileocecal adenocarcinoma HCT-8 cell line (ATCC CCL-224), human colonic adenocarcinoma Caco-2 cell line (ATCC HTB-37), and MDBK cells (ATCC CCL-22) were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin G, and 50 μg/ml streptomycin sulfate. Cells were transferred to six-well plates and grown to 80% confluent monolayers in a 5% CO2 humidified incubator prior to infection.
Parasites and infection.
The Iowa isolate of C. parvum was purchased from Bunch Grass Farm, Deary, Ind. C. hominis TU502 (2) and C. parvum MD isolates were propagated at our laboratory. For excystation, oocysts were treated with 10% sodium hypochlorite for 7 min on ice. After two washes, oocysts were suspended in phosphate-buffered saline (PBS) with 0.8% NaTC and incubated at 37°C for 30 min. The sporozoites from oocysts with an excystation rate of >80% were used for infection. Approximate 106 excysted oocysts were added to monolayers on six-well plates. An equal number of heat-killed parasites, generated by incubating excysted oocysts at 65°C for 30 min, was added to cells as a mock infection. Bile salts sodium glycochenodeoxycholate, glycocholic acid hydrate, sodium glycocholic, sodium taurochenodeoxycholate, sodium taurodeoxycholate, NaTC, and sodium deoxycholate and total bile from bovine were purchased from Sigma (St. Louis, MO). Bile salts were freshly dissolved in PBS and added to cells immediately.
CFSE labeling.
Oocysts were suspended in 0.8% sodium taurocholate in PBS and incubated at 37°C for 15 min and then for another 15 min in 10 μM of carboxyfluorescein diacetate succinimidyl esters (CFSE; Invitrogen, Carlsbad, CA). Sporozoites were washed three times with ice-cold PBS prior to inoculation.
Flow cytometry.
A flow cytometry assay was performed using a FACSCalibur apparatus, and the data were analyzed with CellQuest software (BD Biosciences, Mountain View, CA). HCT-8 or MDBK monolayers were infected with freshly excysted sporozoites for the indicated times. The same number of heat-killed parasites was added to cells as a mock infection. Cells were detached by treating them with 0.05% trypsin and 0.02% EDTA solution (Invitrogen) at 37°C in the CO2 incubator for 5 to 10 min. Cells were collected, washed twice with PBS, and fixed with 4% formaldehyde in PBS for 20 min before analysis by flow cytometry.
SDS-PAGE, silver staining, and immunoblotting.
The methods for immunoblotting and silver staining have been described previously (9, 10). Briefly, the supernatants and pellets of sporozoites were boiled for 10 min in 1× NuPage LDS sample buffer (Invitrogen) before loading onto a gradient (4-to-20%) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (Bio-Rad, Hercules, CA). After electrophoresis, the gels were silver stained using the SilverQuest staining kit (Invitrogen) according to the manufacturer's instructions. For immunoblotting, Cryptosporidium-specific rabbit serum (generated in our laboratory) and horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (IgG) (Amersham Biosciences, Piscataway, NJ) were used as the primary and secondary antibodies, respectively, and the results were visualized in an enhanced chemiluminescence assay (Amersham Biosciences).
Gliding motility.
C. parvum Iowa oocysts were excysted and passed through a 2-μm filter to remove unexcysted oocysts or empty shells. Filtration recovered up to 85% to 90% pure sporozoites. After centrifugation, the sporozoites were suspended in either PBS or PBS containing 0.05% NaTC and then dropped (10 μl) on a poly-l-lysine-coated slide. The slides were incubated in a 37°C humidified incubator for 8 min before fixation with 4% paraformaldehyde. The gliding trails were visualized under an epifluorescence microscope after staining with anti-C. parvum monoclonal antibody (MAb 4C1; mouse IgM isotype; generated in our laboratory) as the primary antibody and AlexaFluor 488-labeled anti-mouse IgM (Molecular Probes, Oregon) as the secondary antibody. Approximately 10 to 15 high-power fields (100×) were randomly selected to count total sporozoites and sporozoites with gliding trails.
RESULTS
NaTC enhances the initial invasion of cultured cells by C. parvum or C. hominis.
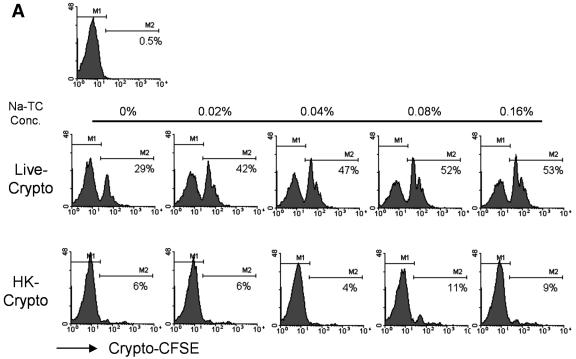
In order to test whether NaTC enhances the initial invasion of C. parvum, newly excysted sporozoites from the C. parvum Iowa isolate were labeled with CFSE and added to MDBK monolayers with or without NaTC in the medium. CFSE labeling does not appear to affect infectivity of Cryptosporidium (unpublished data). After 2 h of incubation, 29% of cells became CFSE positive in live sporozoites, while only about 6% of cells appeared to be CFSE positive in the mock infection (Fig. 1A). Thus, ∼23% more MDBK cells were associated with invasion by C. parvum sporozoites in the absence of NaTC. The presence of 0.16% NaTC in the medium during the infection nearly doubled the percentage of cell invasion by the parasite (44% versus 23%) (Fig. 1A). The enhancement of infection by NaTC was dose dependent, since a decrease in NaTC concentration reduced the percentage of CFSE-positive cells, with 52%, 47%, and 42% cell invasion with 0.08%, 0.04%, and 0.02% NaTC, respectively (Fig. 1A). The presence of NaTC in the medium did not impact the degree of parasite binding to cells, as shown in mock infections (Fig. 1A), suggesting that NaTC enhanced the parasite invasion. Epifluorescence microscopy analysis also showed similar findings (data not shown). The presence of NaTC for 2 h in the culture medium did not cause noticeable cell death as determined by trypan blue exclusion and a thiazolyl blue tetrazolium bromide assay (data not shown).
FIG. 1.
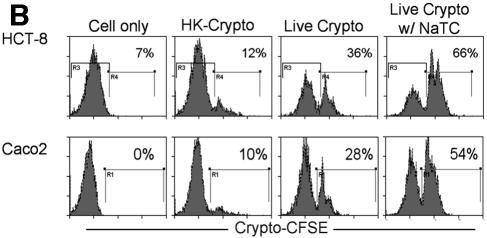
NaTC enhances C. parvum sporozoite invasion of several host cells. (A) MDBK cells were infected with CFSE-labeled C. parvum sporozoites (Iowa isolate) for 2 h with or without the indicated concentrations of NaTC. Control cells were mock infected with an equal number of heat-killed sporozoites. Cells were harvested, and the percentages of CFSE-positive cells were determined by fluorescence-activated cell sorter analysis. M1 and M2 indicate the CFSE-negative and -positive population, respectively. (B) HCT-8 or Caco-2 cells were infected with CFSE-labeled C. parvum for 2 h with or without 0.05% NaTC. Control cells were mock infected with heat-killed sporozoites. Cells were harvested, and the percentages of CFSE-positive cells were determined by fluorescence-activated cell sorter analysis. R4 and R1 indicate the CFSE-positive populations. There are statistically significant differences between the presence and absence of NaTC during the infections in both HCT-8 and Caco-2 cells. Data reflect the representative results from five experiments.
To determine whether NaTC also enhances parasite invasion of other cell lines by C. parvum, HCT-8 and Caco-2 cells were infected with freshly excysted sporozoites with or without NaTC in the medium. NaTC significantly increased sporozoite invasion of both cell lines by C. parvum. The percentages of CFSE-positive HCT-8 or Caco-2 cells were 66% or 54%, respectively, when NaTC was present in the medium during the infection, while only 36% or 28% of cells were CFSE positive in the absence of NaTC (Fig. 1B). These data indicated that the enhancement of invasion was not cell line specific.
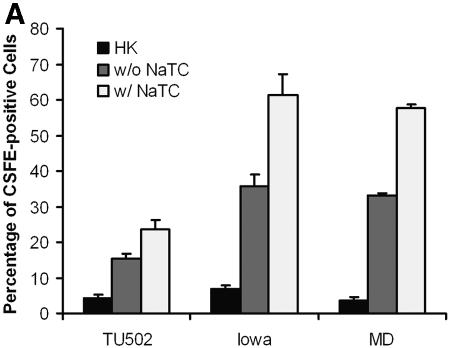
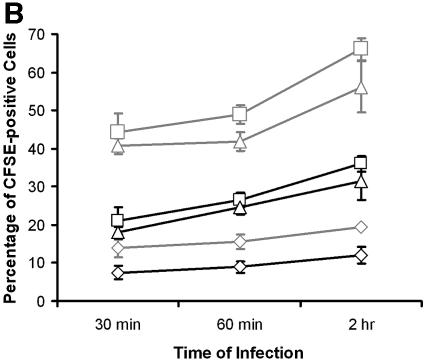
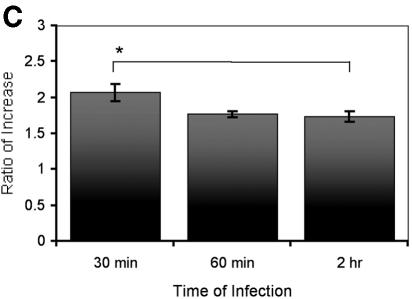
We next examined whether NaTC enhanced the invasion of cells by other isolates of C. parvum or C. hominis. The presence of NaTC in the medium during the infection significantly increased the infection rate (P < 0.01) for all three isolates compared with the same isolates in the absence of NaTC (Fig. 2A). The infection rate for C. hominis TU502 was significantly lower than for C. parvum isolates (Fig. 2A), which is consistent with our earlier observations (unpublished data). Kinetic studies showed that NaTC enhanced parasite invasion within 30 min of inoculation and up to at least 2 h thereafter (Fig. 2B). The ratio of increased infectivity within 30 min of inoculation was significantly higher than after 2 h, with values of 2.1 versus 1.7, respectively (P = 0.02) (Fig. 2C). The differences in the ratios of increased infectivity were not significant between 30 min and 1 h after inoculation, nor between 1 h and 2 h of infection. These results indicate that NaTC is more effective in enhancing infectivity during the early phase of infection.
FIG. 2.
NaTC enhances sporozoite invasion of host cells. (A) HCT-8 cells were inoculated with CFSE-labeled C. hominis TU502 or C. parvum Iowa or MD isolate for 2 h in the absence or presence of 0.05% NaTC. Control cells were mock infected with an equal number of heat-killed strain Iowa sporozoites. (B) MDBK cells were infected with CFSE-labeled C. parvum Iowa (□), MD (Δ), or C. hominis TU502 (⋄) in the absence (black lines) or presence (light gray lines) of 0.05% NaTC for the indicated times. The rates of infection were quantitated by fluorescence-activated cell sorter analysis. (C) The ratios (presence to absence of NaTC) of infectivity were compared at the indicated time points. There was a statistically significant difference between the increase after 30 min of infection and 2 h after inoculation (P = 0.02), but not between 30 min and 1 h after inoculation. These results reflect pooled data from three independent experiments.
NaTC treatment increases sporozoite secretion of soluble proteins.
A successful invasion of host cells by Cryptosporidium involves an effective signaling and interaction with the host cell. To determine whether NaTC enhances parasite invasion through modifying host cell function, HCT-8 cells were treated with NaTC for 30 min prior to infection. NaTC pretreatment of cells did not alter the degree of parasite invasion of HCT-8 monolayers (Fig. 3), suggesting that NaTC treatment enhances the invasiveness of parasites. To explore possible mechanisms, freshly excysted Iowa sporozoites were incubated at 37°C for 30 min with or without NaTC. After centrifugation, the total proteins in the supernatants and pellets were resolved using SDS-PAGE. Silver staining and immunoblotting analysis showed that the total proteins from sporozoites incubated at 37°C were higher in the supernatant but lower in the pellets than those incubated at 4°C (Fig. 4A and B), suggesting that the higher temperature enhanced protein secretion from sporozoites. This result is consistent with a previous report that an increase in incubation temperature induces C. parvum apical organelle discharge (6). In the presence of NaTC (lane 2), the total soluble proteins in the supernatant were higher than without NaTC (lane 3), as revealed by both silver staining and immunoblotting (Fig. 4A and B). As expected, there was less total pellet proteins in lane 5 compared to lane 6 (Fig. 4A and B). These results indicated that NaTC enhanced the protein secretion by sporozoites. Protein secretion or discharge from apical organelles of C. parvum or other Apicomplexa parasites has been shown to play a key role in initiating invasion (6, 18). Therefore, NaTC may enhance invasiveness of C. parvum in part through enhancing protein secretion.
FIG. 3.
Pretreatment of cells with NaTC does not enhance sporozoite invasion. HCT-8 cells were untreated (graphs 1, 2, and 3) or treated with 0.05% NaTC (graphs 4 and 5) for 30 min, after which the medium was either replaced (5) or not (4) with fresh medium. Cells were inoculated with either heat-killed (HK) (1) or live sporozoites (2 to 5). In group 3, 0.05% NaTC was added together with parasites. Cells were harvested after 2 h of incubation, and the percentages of CFSE-positive cells were quantitated by fluorescence-activated cell sorter analysis. The differences between cells with and without NaTC pretreatment are not significant. Data reflect the representative results from three experiments.
FIG. 4.
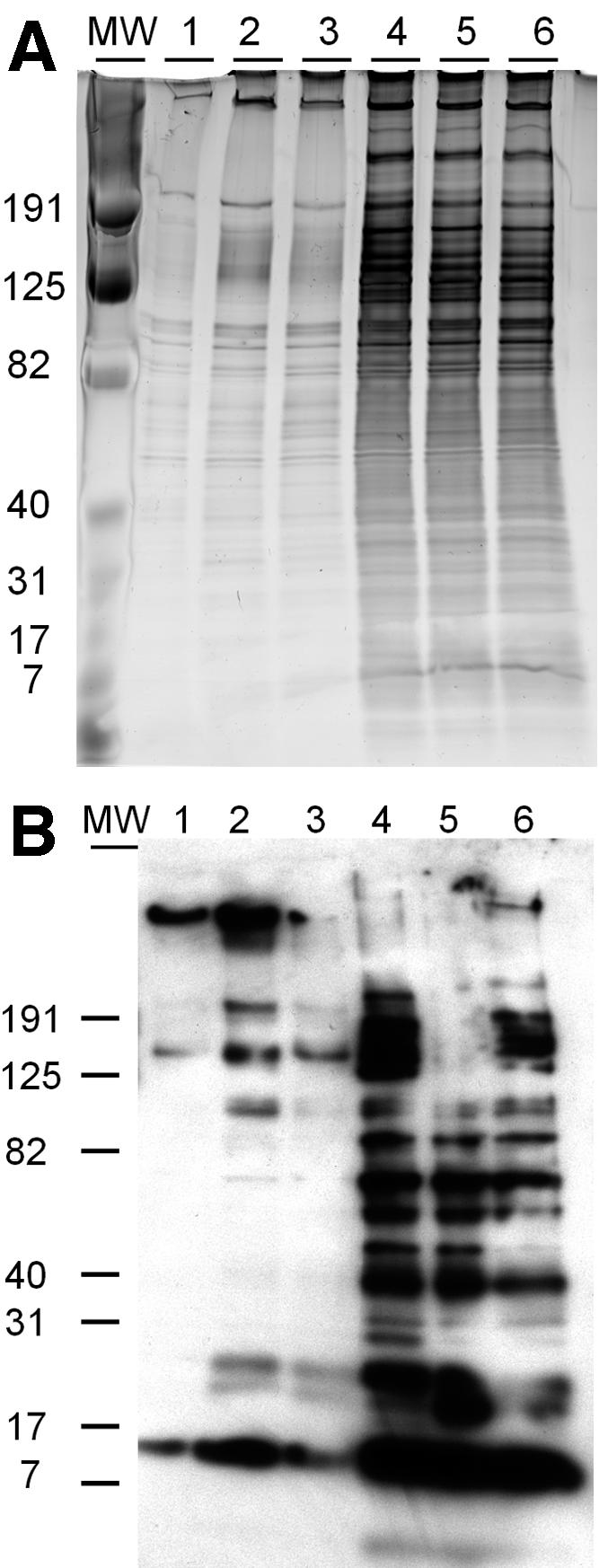
NaTC increases the protein secretion of newly excysted sporozoites. Newly excysted sporozoites were incubated on ice (lanes 1 and 4) or at 37°C with (lanes 2 and 5) or without (lanes 3 and 6) NaTC (0.05%) for 30 min. Proteins in supernatants (lanes 1, 2, and 3) and sporozoite pellets (lanes 4, 5, and 6) were resolved by SDS-PAGE followed by silver staining (A) or immunoblotting (B) using Cryptosporidium-specific rabbit polyclonal antibody.
NaTC enhances the gliding motility of C. parvum sporozoites.
Gliding motility is a prerequisite for the invasive stage of most Apicomplexa (18, 20) and is associated with apical organelle discharge of C. parvum (6). We next investigated whether NaTC enhanced the gliding motility of C. parvum sporozoites. Newly excysted sporozoites were separated from unexcysted oocysts and empty oocyst shells by filtration. The gliding trails of immunofluorescent-labeled sporozoites on glass slides were visualized under a fluorescence microscope. In 10 high-power fields randomly picked, 147 of 239 (62%) sporozoites counted showed gliding trails when NaTC was present during the incubation, compared with only 19 of 104 (18%) sporozoites showing trails in the absence of NaTC.
Various components from bile salts also enhance invasion of cells by C. parvum.
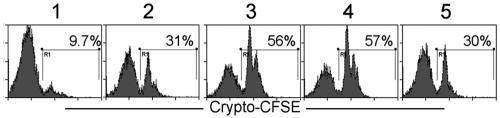
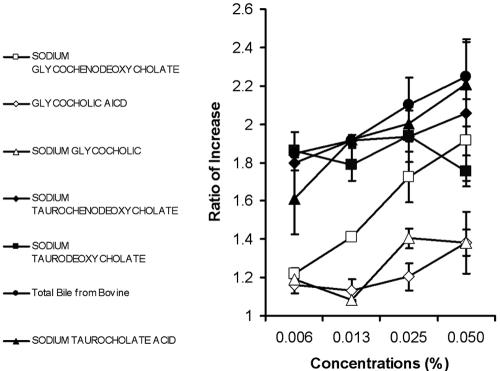
C. parvum or C. hominis excystation and intestinal infection occur in an environment rich in a variety of bile salts, including NaTC. To test whether other components of bile salts also enhance the invasion, HCT-8 cells were infected with C. parvum sporozoites in the presence of several components of bile acids. All tested bile salts enhanced the invasion of C. parvum to various degrees (Fig. 5). The enhancement was dose dependent, with higher doses yielding greater enhancement (Fig. 5). In general, the tauro conjugates had a greater ability to enhance invasion than glyco conjugates at similar concentrations (Fig. 5). Unconjugated bile acids, such as sodium deoxycholate, also enhanced invasion of HCT-8 cells by C. parvum (data not shown).
FIG. 5.
Bile salts enhance sporozoite invasion of HCT-8 cells. HCT-8 cells were inoculated with CFSE-labeled C. parvum sporozoites for 2 h in the presence of the indicated concentrations of sodium glycochenodeoxycholate, glycocholic acid hydrate, sodium glycocholic, sodium taurochenodeoxycholate, sodium taurodeoxycholate, sodium taurocholate acid, and total bile from bovine. Cells were harvested, and the percentage of infected cells was quantitated by fluorescence-activated cell sorter analysis. Data show the higher rate of infection in the presence of bile salts. The results reflect pooled data from three independent experiments.
DISCUSSION
Although Cryptosporidium growth in cell culture is limited to the asexual phase, the system has been useful for evaluating drug therapies against the pathogen and for investigating host-parasite interactions (3, 17). Due to the low infectivity of C. parvum to cultured cells, many attempts have been made to improve infectivity, including optimization of conditions for excystation (11, 15, 16, 23, 24), improvement of nutrients in culture medium (14, 23), and other methods, such as centrifugation (26). In this investigation, we have demonstrated that the presence of NaTC or other bile salts, which are normally excreted in the gut during parasite infection, significantly enhanced the initial invasion of cultured cells by C. parvum and C. hominis. The transient or continuous presence of a low dose of NaTC did not affect the cell viability of HCT-8 or MDBK cells (data not shown) but enhanced the infectivity of C. parvum and C. hominis.
The mechanism by which bile salts enhanced the initial invasion of C. parvum is not fully understood. However, the effect of NaTC does not appear to be on the host cell but rather on the parasite by modifying its secretory pathway and gliding motility. The genus Cryptosporidium belongs to the phylum Apicomplexa, a group which shares a common apical secretory apparatus mediating locomotion and tissue or cellular invasion (21). The apical organelle discharge is likely essential for Cryptosporidium to initiate invasion (6). We have shown that the presence of NaTC in the solution containing freshly excysted sporozoites increased the soluble protein content, which may be due to enhanced apical organelle discharge. Apical organelle discharge of C. parvum is calcium dependent (6), and the induction of calcium signaling by bile salts has been well-documented in mammalian cells (7, 13, 25). Whether or not bile salts induce calcium signaling in C. parvum or other Apicomplexa remains to be determined. The apical organelle discharge of C. parvum sporozoites also affects the gliding motility (6), which is essential for the initial invasion stage of many Apicomplexa (18, 20). The presence of NaTC or other bile salts during inoculation increased the protein secretion and gliding motility of sporozoites, which presumably enhanced the rate of cell invasion.
We have found that NaTC enhanced the invasiveness of newly excysted sporozoites of all Cryptosporidium species tested, which is not surprising given how closely C. parvum and C. hominis are. We have also confirmed here an earlier observation that C. hominis has a much slower rate of infection and infectivity of cultured cells than C. parvum (8; unpublished data). We believe these observations should lead to improving in vitro cultivation by utilizing NaTC and other bile salts.
Acknowledgments
This work was supported by NIH awards NO1 AI25466, K01 RR017383, and RO1 AI05471.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abrahamsen, M. S., T. J. Templeton, S. Enomoto, J. E. Abrahante, G. Zhu, C. A. Lancto, M. Deng, C. Liu, G. Widmer, S. Tzipori, G. A. Buck, P. Xu, A. T. Bankier, P. H. Dear, B. A. Konfortov, H. F. Spriggs, L. Iyer, V. Anantharaman, L. Aravind, and V. Kapur. 2004. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science 304:441-445. [DOI] [PubMed] [Google Scholar]
- 2.Akiyoshi, D. E., X. Feng, M. A. Buckholt, G. Widmer, and S. Tzipori. 2002. Genetic analysis of a Cryptosporidium parvum human genotype 1 isolate passaged through different host species. Infect. Immun. 70:5670-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrowood, M. J. 2002. In vitro cultivation of cryptosporidium species. Clin. Microbiol. Rev. 15:390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackman, M. J., and L. H. Bannister. 2001. Apical organelles of Apicomplexa: biology and isolation by subcellular fractionation. Mol. Biochem. Parasitol. 117:11-25. [DOI] [PubMed] [Google Scholar]
- 5.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 6.Chen, X. M., S. P. O'Hara, B. Q. Huang, J. B. Nelson, J. J. Lin, G. Zhu, H. D. Ward, and N. F. LaRusso. 2004. Apical organelle discharge by Cryptosporidium parvum is temperature, cytoskeleton, and intracellular calcium dependent and required for host cell invasion. Infect. Immun. 72:6806-6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devor, D. C., M. C. Sekar, R. A. Frizzell, and M. E. Duffey. 1993. Taurodeoxycholate activates potassium and chloride conductances via an IP3-mediated release of calcium from intracellular stores in a colonic cell line (T84). J. Clin. Investig. 92:2173-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng, H., W. Nie, R. Bonilla, G. Widmer, A. Sheoran, and S. Tzipori. Quantitative tracking of Cryptosporidium infection in cell culture with CFSE. J. Parasitol., in press. [DOI] [PubMed]
- 9.Feng, H., Y. Zeng, L. Whitesell, and E. Katsanis. 2001. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood 97:3505-3512. [DOI] [PubMed] [Google Scholar]
- 10.Feng, H., D. Zhang, D. Palliser, P. Zhu, S. Cai, A. Schlesinger, L. Maliszewski, and J. Lieberman. 2005. Listeria-infected myeloid dendritic cells produce IFN-beta, priming T cell activation. J. Immunol. 175:421-432. [DOI] [PubMed] [Google Scholar]
- 11.Gold, D., B. Stein, and S. Tzipori. 2001. The utilization of sodium taurocholate in excystation of Cryptosporidium parvum and infection of tissue culture. J. Parasitol. 87:997-1000. [DOI] [PubMed] [Google Scholar]
- 12.Griffiths, J. K., R. Balakrishnan, G. Widmer, and S. Tzipori. 1998. Paromomycin and geneticin inhibit intracellular Cryptosporidium parvum without trafficking through the host cell cytoplasm: implications for drug delivery. Infect. Immun. 66:3874-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau, B. W., M. Colella, W. C. Ruder, M. Ranieri, S. Curci, and A. M. Hofer. 2005. Deoxycholic acid activates protein kinase C and phospholipase C via increased Ca2+ entry at plasma membrane. Gastroenterology 128:695-707. [DOI] [PubMed] [Google Scholar]
- 14.Lawton, P., C. Hejl, R. Mancassola, M. Naciri, and A. F. Petavy. 2003. Effects of purine nucleosides on the in vitro growth of Cryptosporidium parvum. FEMS Microbiol. Lett. 226:39-43. [DOI] [PubMed] [Google Scholar]
- 15.Reduker, D. W., and C. A. Speer. 1985. Factors influencing excystation in Cryptosporidium oocysts from cattle. J. Parasitol. 71:112-115. [PubMed] [Google Scholar]
- 16.Robertson, L. J., A. T. Campbell, and H. V. Smith. 1993. In vitro excystation of Cryptosporidium parvum. Parasitology 106:13-19. [DOI] [PubMed] [Google Scholar]
- 17.Rochelle, P. A., M. M. Marshall, J. R. Mead, A. M. Johnson, D. G. Korich, J. S. Rosen, and R. De Leon. 2002. Comparison of in vitro cell culture and a mouse assay for measuring infectivity of Cryptosporidium parvum. Appl. Environ. Microbiol. 68:3809-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sibley, L. D. 2004. Intracellular parasite invasion strategies. Science 304:248-253. [DOI] [PubMed] [Google Scholar]
- 19.Smith, H. V., R. A. Nichols, and A. M. Grimason. 2005. Cryptosporidium excystation and invasion: getting to the guts of the matter. Trends Parasitol. 21:133-142. [DOI] [PubMed] [Google Scholar]
- 20.Soldati, D., B. J. Foth, and A. F. Cowman. 2004. Molecular and functional aspects of parasite invasion. Trends Parasitol. 20:567-574. [DOI] [PubMed] [Google Scholar]
- 21.Tzipori, S., and H. Ward. 2002. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect. 4:1047-1058. [DOI] [PubMed] [Google Scholar]
- 22.Upton, S. J., M. Tilley, and D. B. Brillhart. 1994. Comparative development of Cryptosporidium parvum (Apicomplexa) in 11 continuous host cell lines. FEMS Microbiol. Lett. 118:233-236. [DOI] [PubMed] [Google Scholar]
- 23.Upton, S. J., M. Tilley, and D. B. Brillhart. 1995. Effects of select medium supplements on in vitro development of Cryptosporidium parvum in HCT-8 cells. J. Clin. Microbiol. 33:371-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upton, S. J., M. Tilley, M. V. Nesterenko, and D. B. Brillhart. 1994. A simple and reliable method of producing in vitro infections of Cryptosporidium parvum (Apicomplexa). FEMS Microbiol. Lett. 118:45-49. [DOI] [PubMed] [Google Scholar]
- 25.Voronina, S., R. Longbottom, R. Sutton, O. H. Petersen, and A. Tepikin. 2002. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J. Physiol. 540:49-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir, S. C., N. J. Pokorny, R. A. Carreno, J. T. Trevors, and H. Lee. 2001. Improving the rate of infectivity of Cryptosporidium parvum oocysts in cell culture using centrifugation. J. Parasitol. 87:1502-1504. [DOI] [PubMed] [Google Scholar]
- 27.Xiao, L., and U. M. Ryan. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17:483-490. [DOI] [PubMed] [Google Scholar]
- 28.Xu, P., G. Widmer, Y. Wang, L. S. Ozaki, J. M. Alves, M. G. Serrano, D. Puiu, P. Manque, D. Akiyoshi, A. J. Mackey, W. R. Pearson, P. H. Dear, A. T. Bankier, D. L. Peterson, M. S. Abrahamsen, V. Kapur, S. Tzipori, and G. A. Buck. 2004. The genome of Cryptosporidium hominis. Nature 431:1107-1112. [DOI] [PubMed] [Google Scholar]