Abstract
We isolated a human monoclonal antibody against diphtheria toxin (DT). It bound to fragment B with a binding activity (Kd) of 3.01 nM. The neutralizing activity assayed by the rabbit skin test was estimated to be 73,600 IU/g. This could be used as a therapeutic drug against DT in place of the traditional equine sera.
Antisera prepared from hyperimmune horse blood are still used as drugs against diphtheria toxin (DT) in emergency situations. Since equine antisera could induce serious side effects such as serum sickness, there is a strong need to develop a human monoclonal antibody (Ab) against DT. DT excreted by Corynebacterium diphtheriae has been well characterized (12). It is a single polypeptide chain (Mr, 58,000) composed of two structurally distinct regions with three functional domains and contains a protease-sensitive site. The nicked toxin produced upon limited proteolysis consists of two polypeptides that are held together by a disulfide bond. The NH2-terminal region, fragment A, catalyzes the transfer of the ADP-ribose moiety from NAD to elongation factor 2 and thus blocks protein synthesis (4). The COOH-terminal region, fragment B, binds to a specific receptor on the cell surface and mediates transfer of fragment A to the cytoplasm (6, 11, 14). DT is lethal for susceptible animals, including humans, in doses of 100 ng/kg or less (12). Mass immunization of children has been performed on a worldwide scale since the 1940s. The degree of immunity to DT in the serum of each person should be critical for determination of susceptibility to diphtheria. There is a good correlation between clinical protection and the presence of serum antitoxin, whether this results from disease or immunization. According to internationally accepted definitions, an antitoxin concentration of less than 0.01 IU/ml indicates susceptibility, 0.01 to 0.09 IU/ml indicates basic protection, and >0.1 IU/ml indicates full protection (2). Once the symptoms of this disease start to appear, the antiserum should be given to the patient as soon as possible. The amount of Abs required for curing is much larger than that required to prevent infection. It ranges from 5,000 to 50,000 IU, depending on the degree of disease progress (2).
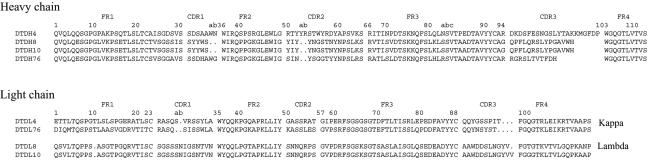
A human Ab library was screened with DT and diphtheria toxoid (DTD) as the antigen (Ag) by the panning method (3, 5). DT and DTD were kindly given to us by Kunio Ohkuma (Kaketsuken, Kumamoto, Japan). DTD is inactivated toxin that is used for vaccination. It has been prepared by treatment with formaldehyde (13). The Abs were initially prepared in the form of an Ab fused with truncated cp3 (Fab-cp3) and converted to immunoglobulin G1 (IgG1) (3). In this paper, we report data obtained with IgG. Fifty-five different clones were isolated. Four of them, DTD4, DTD8, DTD10, and DTD76, distinctively showed neutralizing activities. The amino acid sequences of these four clones are shown in Fig. 1. Western blotting with separated fragments A and B indicated that DTD4 and DTD76 bound to fragment B and DTD8 and DTD10 bound to fragment A. The rate constants, and thus the binding constants, of these four clones against DTD and DT were measured with the BIAcore instrument (5) (Table 1). Abs were coupled to the sensor chip, and Ags were injected to avoid the influence of divalency. Clones DTD4, DTD8, and DTD10 bound to DT more strongly than to DTD, whereas DTD76 bound to DTD more strongly than to DT.
FIG. 1.
Amino acid sequences of variable regions of the heavy and light chains of Abs that exhibited neutralizing activities against DT. The numbering of amino acid positions is according to the definition of Kabat et al. (8).
TABLE 1.
Rate constants (ka, kd) and dissociation constant (Kd) of IgG form of Abs with DTD and DT assayed by the BIAcore instrument
| Clone | Anti-DTD
|
Anti-DT
|
||||
|---|---|---|---|---|---|---|
| ka (104 M−1 s−1) | kd (10−4 s−1) | Kd (10−9 M) | ka (104 M−1 s−1) | kd (10−4 s−1) | Kd (10−9 M) | |
| DTD4 | 4.14 | 8.70 | 21.2 | 10.6 | 3.19 | 3.01 |
| DTD8 | 4.99 | 3.82 | 7.66 | 16.2 | 0.831 | 0.513 |
| DTD10 | 8.89 | 3.76 | 4.22 | 8.52 | 0.243 | 0.285 |
| DTD76 | 11.9 | 2.29 | 1.93 | 7.8 | 4.83 | 6.19 |
In vitro DT-neutralizing activities were estimated by the pH color change method (9, 10). When the cells were metabolically active, the color of the medium changed to yellow. When cellular metabolism was stopped by toxin action, it remained red. Thus, the titration endpoint for anti-DT neutralizing activity was taken at the highest dilution of anti-DT Ab to be tested in the well in which the color of the medium was orange. The results are indicated in the left column of values in Table 2. The antitoxin titers are expressed in international units by comparison with the result obtained with equine sera. The in vivo neutralizing activities of Abs against DT were determined by the rabbit skin test as described previously (1, 7). In brief, DT mixed with serially diluted Abs was injected into rabbit back skin. The diameter of local erythema was measured at the site of injection at 48 h postinjection. The results are shown in the rightmost column of Table 2. The antitoxin titers are expressed in international units as relative potency with respect to the result obtained with the standard antitoxin. The neutralizing activities of Abs assayed by the pH color change method and by the rabbit skin test were similar to each other in four cases, indicating a good correlation between the two systems (10).
TABLE 2.
Neutralizing activity against DT shown by IgG form of Abs
| Clone | Neutralizing activitya
|
|
|---|---|---|
| In vitro assayb | In vivo assayc | |
| DTD4 | 52,000 | 73,600 |
| DTD8 | 4,800 | 3,360 |
| DTD10 | 2,300 | 1,612 |
| DTD76 | 215 | 372 |
In international units per gram.
Measured by the pH color change method.
Measured by the rabbit skin test.
In the case of DTD4, which showed the strongest neutralizing activity of the four clones, it was estimated to be 73,600 IU/g by the in vivo assay. The binding activity (Kd) with DT was 3.01 nM. On the other hand, while DTD76 bound to DTD with strong affinity, it showed very weak neutralizing activity. Although both clones bound to fragment B, they should recognize completely different epitopes. It is possible that clone DTD4 corresponded to the Ab that had matured in vivo by immunization with vaccine against DT. We propose that DTD4 be clinically tested for therapeutic use.
Nucleotide sequence accession numbers.
The nucleotide sequences of the eight genes described in Fig. 1 have been registered in GenBank under accession numbers AB063724 (DTDH4), AB063723 (DTDH8), AB063729 (DTDH10), AB063743 (DTDH76), AB063937 (DTDL4), AB064049 (DTDL76), AB063977 (DTDL8), and AB064205 (DTDL10).
Acknowledgments
We thank Kunio Ohkuma and Eisuke Mekada for providing materials. We also thank Atsuko Suzuoki for preparation of the manuscript.
This study was supported in part by a grant for Research on Pharmaceutical and Medical Safety from the Ministry of Health, Labor, and Welfare of Japan.
Editor: J. B. Bliska
REFERENCES
- 1.Barile, M. F., R. W. Kolb, and M. Pittman. 1971. United States standard diphtheria toxin for the Schick test and the erythema potency assay for the Schick test dose. Infect. Immun. 4:295-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galazka, A. M. 1993. Expanded programme on immunization: the immunological basis for immunization. Module 2: diphtheria. WHO document WHO/EPI/GEN/93.12. World Health Organization, Geneva, Switzerland.
- 3.Higo-Moriguchi, K., Y. Akahori, Y. Iba, Y. Kurosawa, and K. Taniguchi. 2004. Isolation of human monoclonal antibodies that neutralize human rotavirus. J. Virol. 78:3325-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honjo, T., Y. Nishizuka, O. Hayaishi, and I. Kato. 1968. Diphtheria toxin-dependent adenosine diphosphate ribosylation of aminoacyl transferase II and inhibition of protein synthesis. J. Biol. Chem. 243:3553-3555. [PubMed] [Google Scholar]
- 5.Iba, Y., W. Ito, and Y. Kurosawa. 1997. Expression vectors for the introduction of highly diverged sequences into the six complementarity-determining regions of an antibody. Gene 194:35-46. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto, R., S. Higashiyama, T. Mitamura, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1994. Heparine-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivities. EMBO J. 13:2322-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jerne, N. K. 1951. Study of avidity based on rabbit skin responses to diphtheria toxin-antitoxin mixtures. Acta Pathol. Microbiol. Scand. Suppl. 87:3-183. [PubMed] [Google Scholar]
- 8.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest. National Institutes of Health, Bethesda, Md.
- 9.Miyamura, K., S. Nishio, A. Ito, R. Murata, and R. Kono. 1974. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using VERO cells. I. Studies on factors affecting the toxin and antitoxin titration. J. Biol. Stand. 2:189-201. [DOI] [PubMed] [Google Scholar]
- 10.Miyamura, K., E. Tajiri, A. Ito, R. Murata, and R. Kono. 1974. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using VERO cells. II. Comparison with the rabbit skin method and practical application for sero-epidemiological studies. J. Biol. Stand. 2:203-209. [DOI] [PubMed] [Google Scholar]
- 11.Naglich, J. G., J. E. Metherall, D. W. Russell, and L. Eidels. 1992. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell 69:1051-1061. [DOI] [PubMed] [Google Scholar]
- 12.Pappenheimer, A. M. 1977. Diphtheria toxin. Annu. Rev. Biochem. 46:69-94. [DOI] [PubMed] [Google Scholar]
- 13.Pappenheimer, A. M., T. Uchida, and A. A. Harper. 1972. An immunological study of the diphtheria toxin molecule. Immunochemistry 9:891-906. [DOI] [PubMed] [Google Scholar]
- 14.Rolf, J. M., H. M. Gaudin, and L. Eidels. 1990. Localization of diphtheria toxin receptor-binding domain to the carboxyl-terminal M∼6000 region of the toxin. J. Biol. Chem. 265:7331-7337. [PubMed] [Google Scholar]