Abstract
Bacterial adherence to mucosal surfaces is an important virulence trait of pathogenic bacteria. Adhesion of enterotoxigenic Escherichia coli (ETEC) to the intestine is mediated by a number of antigenically distinct colonization factors (CFs). One of the most common CFs is CFA/I. This has a fimbrial structure composed of a major repeating subunit, CfaB, and a single tip subunit, CfaE. The potential carbohydrate recognition by CFA/I was investigated by binding CFA/I-fimbriated bacteria and purified CFA/I fimbriae to a large number of variant glycosphingolipids separated on thin-layer chromatograms. For both fimbriated bacteria and purified fimbriae, specific interactions could be identified with a number of nonacid glycosphingolipids. These included glucosylceramide, lactosylceramide with phytosphingosine and/or hydroxy fatty acids, neolactotetraosylceramide, gangliotriaosylceramide, gangliotetraosylceramide, the H5 type 2 pentaglycosylceramide, the Lea-5 glycosphingolipid, the Lex-5 glycosphingolipid, and the Ley-6 glycosphingolipid. These glycosphingolipids were also recognized by recombinant E. coli expressing CFA/I in the absence of tip protein CfaE, as well as by purified fimbriae from the same strain. This demonstrates that the glycosphingolipid-binding capacity of CFA/I resides in the major CfaB subunit.
Enterotoxigenic Escherichia coli (ETEC) infections are a major cause of diarrhea in developing countries, especially among young children (6). ETEC also causes traveler's diarrhea. Infecting bacteria adhere to and colonize the intestinal epithelium and cause diarrhea primarily by the production of heat-labile and/or heat-stable enterotoxin. Adherence is mediated by colonization factors (CFs), which usually are fimbrial structures present on the surface of the bacteria that have affinity for receptors exposed on the luminal surface of epithelial cells. Although more than 20 serologically distinct CFs have been identified, the first to be characterized was colonization factor antigen (CFA/I), which remains one of those most commonly associated with ETEC infection (8, 11). CFA/I belongs to a group of eight ETEC fimbriae with genetic and biochemical similarities (reference 1 and references therein). Other members of this group are coli surface antigen 1 (CS1), CS2, CS4, CS14, CS17, CS19, and PCF071.
Expression of four genes (cfaABCE) in the CFA/I operon is required for assembly of CFA/I fimbriae (17). The same is true for CS1 (cooBACD) and CS2 (cotBACD) (30). cfaB encodes the major pilin subunit (corresponding to cooA and cotA in CS1 and CS2, respectively), whereas cfaE (cooD and cotD) encodes a minor tip protein. The cfaA (cooB and cotB) gene product is a chaperone-like protein, and cfaC (cooC and cotC) encodes a protein involved in transport of fimbriae across the outer membrane.
The adhesion of fimbriated bacteria to host epithelium may be mediated by the major fimbrial subunit as in K99 fimbriae (16) or the minor tip protein as in P fimbriae (21). In the case of CFA/I, it has been proposed that adhesion is via the major subunit (7). However, a point mutation in the tip protein CfaE abolished fimbrially induced hemagglutination (31). A similar result was obtained with CS1 when the corresponding tip protein, CooD, was mutated in the same manner. Furthermore, Fab fragments directed against the N-terminal region of the minor tip protein inhibited both hemagglutination induced by bacteria expressing CFA/I and their ability to bind to Caco-2 cells (1). These results suggest that the minor tip proteins of these fimbriae are involved in adhesion.
Although the genetics and architecture of CFA/I and related fimbriae have been extensively studied, far less is known about their target cell receptors. In the present study, the ability of CFA/I fimbriae, with and without the minor tip protein, to bind to glycosphingolipids from various sources was investigated. Specific interactions could be demonstrated between CFA/I-fimbriated bacteria or purified CFA/I fimbriae and a number of nonacid glycosphingolipids. Interestingly, there appeared to be no difference in binding between CFA/I with or without the minor tip protein, demonstrating that these activities reside with the major CfaB subunit.
MATERIALS AND METHODS
Bacterial strains, culture, and labeling.
Recombinant CFA/I fimbriae and CFA/I fimbriae without the tip protein were recombinantly expressed in E. coli strain Top10 (37; J. Tobias et al., unpublished data). The recombinant strains were designated strain Top10-CFA/I and strain Top10-CFA/I/E−, respectively. Helicobacter pylori strain J99 was described by Aspholm-Hurtig et al. (3).
E. coli recombinant strains were grown in CFA broth containing Casamino Acids (10 g), yeast extract (1.5 g), MgSO4 · 7H2O (102 mg), and MnCl2 · 4H2O (8 mg/liter; pH 7.4) and supplemented with ampicillin (100 μg/ml) at 37°C with shaking. For metabolic labeling, the medium (10 ml) was supplemented with 10 μl of [35S]methionine (400 μCi; Amersham Pharmacia Biotech). After 2 h, 100 μl of isopropyl-β-d-thiogalactopyranoside (100 mM; Saveen Werner AB, Malmö, Sweden) was added and the bacteria were then incubated overnight. Bacteria were harvested, washed three times in phosphate-buffered saline (PBS, pH 7.3), and then resuspended in PBS containing 2% (wt/vol) bovine serum albumin (BSA), 0.1% (wt/vol) NaN3, and 0.1% (wt/vol) Tween 20 (BSA-PBS-Tween) to a density of 108 CFU/ml. The specific radioactivity of bacterial suspensions was approximately 1 cpm/100 bacteria.
Culture and labeling of H. pylori strain J99 were done as previously described (2).
Immunoelectron microscopy.
Aliquots (10 μl) of a bacterial suspension (1010 bacteria/ml in PBS) were applied to Parafilm. Formvar-coated grids were put on the suspensions and left for 2 min with the grids placed 15 cm below a lamp to increase the temperature of the sample. The grids were then washed twice and then incubated for 15 min with 25 μl of monoclonal anti-CFA/I antibody 1:6 (22) diluted to approximately 0.6 μg/ml in PBS-0.1% BSA-0.05% Tween. The grids were washed six times with PBS-1% BSA and then incubated for 15 min with anti-mouse immunoglobulin G-gold conjugate (Amersham) in PBS-0.1% BSA-0.05% Tween. After washing the grids three times with PBS-0.1% BSA and three times with distilled water, negative staining was performed by applying the grids to 25 μl of 1% ammonium molybdate (pH 7.0) for 50 to 60 s, followed by air drying on filter paper for 5 min. The grids were stored at 4°C. The grids were examined with an electron microscope (LEO 912 AB Omega TEM; Carl Zeiss SMT AG, Oberkochen, Germany) operated at 120 kV.
Fimbrial preparations.
Different ETEC fimbriae were isolated as described previously (28). In brief, bacteria grown on CFA agar were homogenized with a blender and centrifuged at 12,000 × g for 20 min. The supernatant was precipitated with ammonium sulfate (at 20 and 40% saturation), and after centrifugation at 12,000 × g and dialysis, the pellet was purified by chromatography on a DEAE-Sephadex column. The purity of the different fimbrial preparations was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie staining (Phastsystem; Pharmacia, Uppsala, Sweden) and by immunoblotting with antisera against whole bacteria expressing the homologous or heterologous fimbriae. The concentration of the fimbriae was determined by inhibition enzyme-linked immunosorbent assay with a highly purified fimbrial preparation as described in reference 22.
125I-labeling.
Aliquots of 100 μg of fimbrial preparations were labeled with 125I by using Na-125I (100 μCi/ml; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), according to the IODO-GEN protocol of the manufacturer (Pierce, Rockford, IL), giving approximately 5 × 103 cpm/μg of protein.
Reference glycosphingolipids.
Total acid and nonacid glycosphingolipid fractions were prepared as described earlier (18). Individual glycosphingolipids were isolated by repeated chromatography on silicic acid columns and by high-performance liquid chromatography and identified by mass spectrometry (32), 1H NMR spectroscopy (20), and degradation studies (33, 38).
The glycosphingolipid nomenclature used in this report follows the recommendations of the IUPAC-IUB Commission on Biochemical Nomenclature (15a). It is assumed that Gal, Glc, GlcNAc, GalNAc, and NeuAc are of the d configuration, Fuc is of the l configuration, and all sugars are present in the pyranose form.
Thin-layer chromatography.
Aluminum- or glass-backed silica gel 60 high-performance thin-layer chromatography plates (Merck, Darmstadt, Germany) were used for thin-layer chromatography and eluted with chloroform-methanol-water (60:35:8, by volume) as the solvent system. The different glycosphingolipids were applied to the plates in quantities of 0.1 to 4 μg of pure glycosphingolipids and 40 μg of glycosphingolipid mixtures. Chemical detection was done with anisaldehyde (34).
Chromatogram binding assays.
Binding of radiolabeled fimbriae and of bacteria to glycosphingolipids on thin-layer chromatograms was done as described previously (15). Dried chromatograms were dipped in diethyl ether-n-hexane (1:5 vol/vol) containing 0.5% (wt/vol) polyisobutylmethacrylate for 1 min, dried, and then blocked with BSA-PBS-Tween for 2 h at room temperature. Thereafter, the plates were incubated with 125I-labeled fimbriae (1 × 106 to 5 × 106 cpm/ml) or 35S-labeled bacteria (1 × 106 to 5 × 106 cpm/ml) diluted in BSA-PBS-Tween for another 2 h at room temperature. After washing six times with PBS and drying, the thin-layer plates were autoradiographed for 12 h with XAR-5 X-ray films (Eastman Kodak, Rochester, NY).
Chromatogram binding assays with monoclonal antibodies directed against the Lea determinant (Signet Laboratories, Inc., Dedham, MA) were done as previously described (13), by using 125I-labeled anti-mouse antibodies for detection.
Competition experiments.
As a test for possible inhibition of binding by soluble sugars, 10 μg of 125I-labeled CFA/I fimbriae in 100 μl of BSA-PBS-Tween was incubated with lactose (Galβ4Glc; J. T. Baker Chemical Co., Phillipsburg, NJ) or Lea pentasaccharide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glc; IsoSep, Tullinge, Sweden] at a final concentration of 1 mg/ml. Incubations were done for 2 h at room temperature, and then the suspensions were diluted 40 times and used in the chromatogram binding assay as described above.
RESULTS
Screening for CFA/I carbohydrate recognition by binding to mixtures of glycosphingolipids.
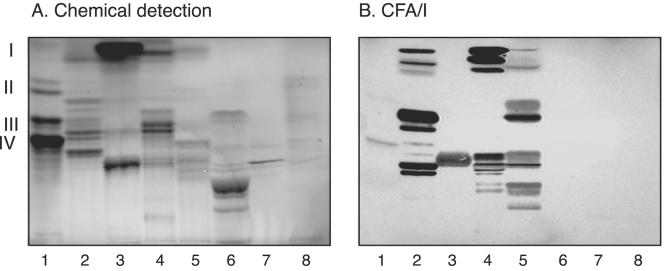
The initial screening for carbohydrate recognition by CFA/I fimbriae was done with mixtures of glycosphingolipids from various sources separated on thin-layer plates, in order to expose the fimbriae to a large number of potentially binding-active carbohydrate structures. Thereby, a selective interaction with several compounds in the nonacid glycosphingolipid fractions of guinea pig intestine, guinea pig erythrocytes, rat intestine, and human meconium was obtained (Fig. 1, lanes 2 to 5). Some features of the binding pattern obtained with CFA/I fimbriae were reminiscent of the binding patterns of bacteria classified as “lactosylceramide-binding” bacteria (19), such as, e.g., the binding to the di- and triglycosylceramide region in the nonacid glycosphingolipid fraction of guinea pig intestine (lane 2). However, the CFA/I fimbriae also bound in the monoglycosylceramide region and to a number of slow-migrating compounds, a pattern that is not seen with lactosylceramide-binding bacteria.
FIG. 1.
Binding of 125I-labeled CFA/I fimbriae to mixtures of glycosphingolipids on thin-layer chromatograms. Chemical detection by anisaldehyde (A) and an autoradiogram obtained by binding of 125I-labeled CFA/I fimbriae (B) are shown. The glycosphingolipids were separated on aluminum-backed silica gel plates with chloroform-methanol-water (60:35:8, by volume) as the solvent system, and the binding assay was performed as described in Materials and Methods. Lanes: 1, nonacid glycosphingolipids of human blood group A erythrocytes (40 μg); 2, nonacid glycosphingolipids of guinea pig intestine (40 μg); 3, nonacid glycosphingolipids of mouse feces (40 μg); 4, nonacid glycosphingolipids of rat intestine (40 μg); 5, nonacid glycosphingolipids of human meconium (40 μg); 6, calf brain gangliosides (40 μg); 7, acid glycosphingolipids of human erythrocytes (40 μg); 8, acid glycosphingolipids of human hypernephroma (40 μg). Autoradiography was performed for 12 h. The roman numerals to the left indicate the approximate numbers of carbohydrate residues in the bands.
The nonbinding of CFA/I fimbriae to the acid glycosphingolipid fractions (Fig. 1, lanes 6 to 8) suggested that sialic acid-containing glycosphingolipids were not recognized. Furthermore, absence of binding to the Galα4Gal motif was indicated by the nonbinding to globotriaosylceramide and globotetraosylceramide, the major compounds of the nonacid glycosphingolipid fractions of human erythrocytes (Fig. 1, lane 1).
Binding of CFA/I fimbriae to pure reference glycosphingolipids.
The concentrations of the various glycosphingolipids in mixtures from natural sources, used in the initial screening for carbohydrate binding, are highly variable. To further define the binding characteristics of the CFA/I fimbriae, a number of pure glycosphingolipids at defined concentrations were next tested in a chromatogram binding assay. The results are summarized in Table 1. The majority of the glycosphingolipids tested, including sulfated compounds (compound 3 in Table 1; Fig. 1, lane 8), gangliosides (compounds 27 to 38; Fig. 1, lanes 6 to 8), and glycosphingolipids with Galα4Gal core (compounds 21, 23, and 25; Fig. 1, lanes 1 and 2) were not recognized by the fimbriae. Furthermore, lactosylceramide with sphingosine and nonhydroxy fatty acids (compound 4; Fig. 2, lane 2, and presented in Fig. 1, lane 1) was not bound by the CFA/I fimbriae.
TABLE 1.
Binding of 125I-labeled native CFA/I fimbriae, 35S-labeled recombinant CFA/I-expressing E. coli Top10-CFA/I, and 125I-labeled CFA/I fimbriae with deletion of the CfaE subunit (CFA/I/E−) to pure glycosphingolipids on thin-layer chromatograms
| Compound no., trivial name | Structure | CFA/I fimbriae | E. coli Top10-CFA/I | CFA/I/E− fimbriae |
|---|---|---|---|---|
| Simple compounds | ||||
| 1, Galactosylceramide | Galβ1Cer | −a | − | − |
| 2, Glucosylceramide | Glcβ1Cer | + | + | + |
| 3, Sulfatide (d18:1-16:0 and 24:0)b | SO3-Galβ1Cer | − | − | NDc |
| 4, LacCer (d18:1-16:0-24:0) | Galβ4Glcβ1Cer | − | − | − |
| 5, LacCer (t18:0-h16:0-h24:0) | Galβ4Glcβ1Cer | + | + | + |
| Ganglioseries | ||||
| 6, GgO3 (d18:1-16:0 and 24:0) | GalNAcβ4Galβ4Glcβ1Cer | + | + | + |
| 7, GgO4 (t18:0-h16:0 and h24:0) | Galβ3GalNAcβ4Galβ4Glcβ1Cer | + | + | + |
| Neolactoseries | ||||
| 8, Neolactotetra (d18:1-16:0 and 24:1) | Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 9, H5-2 (d18:1-16:0/24:0) | Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 10, Lex-5 (t18:0-h16:0-h24:0) | Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 11, B5 (d18:1-16:0/24:0) | Galα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 12, Ley-6 (t18:0-h16:0-h24:0) | Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 13, B6-2 (d18:1-16:0/24:0) | Galα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − |
| 14, A6-2 (d18:1-16:0/24:0) | GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| 15, A7-2 | GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − |
| 16, Neolactohexa (d18:1-16:0-24:0) | Galβ4GlcNAcβ6(Galβ4GlcNAcβ3)Galβ4Glcβ1Cer | − | − | ND |
| Lactoseries | ||||
| 17, Lea-5 (t18:0-h16:0-h24:0) | Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | + | + | + |
| 18, Leb-6 (t18:0-h16:0-h24:0) | Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − |
| 19, A7-1 (t18:0-h16:0-h24:0) | GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | − |
| 20, B7-1 | Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| Globoseries | ||||
| 21, Globotri (d18:1-16:0 and 24:0) | Galα4Galβ4Glcβ1Cer | − | − | − |
| 22, Isoglobotri (t18:0-h22:0 and h24:0) | Galα3Galβ4Glcβ1Cer | + | + | + |
| 23, Globotetra (d18:1-16:0 and 24:0) | GalNAcβ3Galα4Galβ4Glcβ1Cer | − | − | − |
| 24, Isoglobotetra (d18:1-16:0 and 24:0) | GalNAcβ3Galα3Galβ4Glcβ1Cer | − | − | − |
| 25, Forssman (d18:1-16:0 and 24:0) | GalNAcα3GalNAcβ3Galα4Galβ4Glcβ1Cer | − | − | |
| 26, (t18:0-h22:0 and h24:0) | Galα3Galα3Galβ4Glcβ1Cer | + | + | + |
| Gangliosides | ||||
| 27, GM3 (d18:1-18:0/d20:1-18:0) | NeuAcα3Galβ4Glcβ1Cer | − | − | ND |
| 28, GM2 | GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | − | ND |
| 29, GD2 | GalNAcβ4(NeuGcα8NeuGcα3)Galβ4Glcβ1Cer | − | − | ND |
| 30, GD3 | NeuAcα8NeuAcα3Galβ4Glcβ1Cer | − | ND | ND |
| 31, GM1 (d18:1-18:0 and d18:1-20:0) | Galβ3GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | − | ND |
| 32, GD1b (d18:1-18:0/d20:1-18:0) | Galβ3GalNAcβ4(NeuAcα8NeuAcα3)Galβ4Glcβ1Cer | − | − | ND |
| 33, NeuAcα3SPG (d18:1-16:0/24:0) | NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| 34, NeuGcα3SPG | NeuGcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | ND | ND |
| 35, NeuAcα6SPG | NeuAcα6Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| 36, NeuAcα-Lea | NeuAcα3Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| 37, NeuAcα-Lex | NeuAcα3Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | − | ND |
| 38 | Galβ4GlcNAcβ6(NeuAcα6Galβ4GlcNAcβ3)Galβ4Glcβ1Cer | − | − | ND |
Binding is defined as follows: +, binding when 4 μg of the glycosphingolipid was applied to the thin-layer chromatogram; −, no binding even at 4 μg.
In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length and the number after the colon gives the total number of double bonds in the molecule. Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation, e.g., h16:0. For long-chain bases, d denotes dihydroxy and t denotes trihydroxy. Thus, d18:1 is sphingosine (1,3-dihydroxy-2-aminooctadecene) and t18:0 is phytosphingosine (1,3,4-trihydroxy-2-aminooctadecene).
ND, not determined.
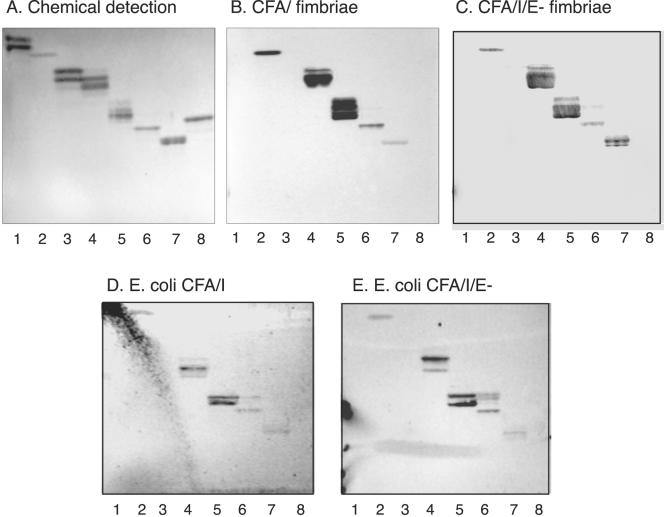
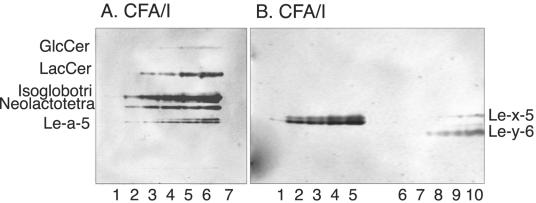
FIG. 2.
Binding of CFA/I fimbriae, CFA/I/E− fimbriae, and recombinant bacterial cells expressing CFA/I fimbriae and CFA/I/E− fimbriae to pure glycosphingolipids on thin-layer chromatograms. Shown are chemical detection by anisaldehyde (A) and autoradiograms obtained by binding of CFA/I fimbriae (B), CFA/I/E− fimbriae (C), CFA/I-expressing E. coli (strain Top10-CFA/I) (D), and E. coli with CFA/I/E− fimbriae (strain Top10-CFA/I/E−) (E). The glycosphingolipids were separated on aluminum-backed silica gel plates with chloroform-methanol-water (60:35:8, by volume) as the solvent system, and the binding assays were performed as described in Materials and Methods. Autoradiography was performed for 12 h. Lanes: 1, galactosylceramide (Galβ1Cer) (2 μg); 2, glucosylceramide (Glcβ1Cer) (2 μg); 3, lactosylceramide (Galβ4Glcβ1Cer) with d18:1-16:0-24:0 (2 μg); 4, lactosylceramide (Galβ4Glcβ1Cer) with t18:0-h16:0-h24:0 (2 μg); 5, isoglobotriaosylceramide (Galα3Galβ4Glcβ1Cer) (2 μg); 6, neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); 7, Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 8, globotetraosylceramide (GalNAcβ3Galα4Galβ4Glcβ1Cer) (2 μg).
However, like lactosylceramide-binding bacteria, the CFA/I fimbriae bound to lactosylceramide with hydroxy long chain base and/or fatty acids (compound 5 in Table 1; Fig. 2, lane 4), isoglobotriaosylceramide (compound 22; Fig. 2, lane 5), gangliotriaosylceramide (compound 6), gangliotetraosylceramide (compound 7; see Fig. 7A, lanes 1 to 4), and neolactotetraosylceramide (compound 8; Fig. 2, lane 6). But the CFA/I fimbriae also interacted with several compounds not recognized by lactosylceramide-binding bacteria, as the Lea pentaglycosylceramide (compound 17; Fig. 2, lane 7; Fig. 3, lane 1), the Lex pentaglycosylceramide (compound 10; Fig. 3, lane 3), the Ley hexaglycosylceramide (compound 12; Fig. 3, lane 7), and the H5 type 2 pentaglycosylceramide (compound 9; Fig. 3, lane 9). Interestingly, although the bacteria bound to glycosphingolipids with terminal Lea, Lex, and Ley determinants, binding to glycosphingolipids carrying the Leb determinant (compounds 18 to 20; Fig. 3, lanes 5 and 6) never occurred; i.e., the binding pattern was in sharp contrast to that of BabA-expressing H. pylori, which recognizes the Leb-6, A7 type 1, and B7 type 1 glycosphingolipids (3).
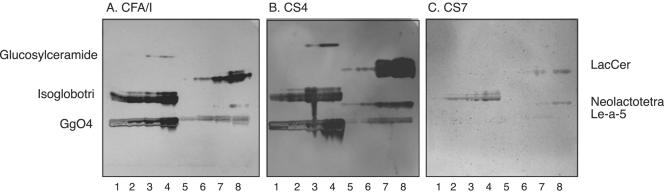
FIG. 7.
Comparison of glycosphingolipid recognition of CFA/I and heterologous CF fimbriae of ETEC. Autoradiograms were obtained by binding of 125I-labeled CFA/I fimbriae (A), CS4 fimbriae (B), and CS7 fimbriae (C) to serial dilutions (0.4 to 2.0 μg) of glycosphingolipids in a chromatogram binding assay. The binding assay was done as described in Materials and Methods. The solvent system used was chloroform-methanol-water (60:35:8, by volume). Autoradiography was performed for 12 h. Lanes: 1 to 4, glucosylceramide (Glcβ1Cer), isoglobotriaosylceramide (Galα3Galβ4Glcβ1Cer), and gangliotetraosylceramide (Galβ3GalNAcβ4Galβ4Glcβ1Cer) (0.4 to 2.0 μg of each compound); 5 to 8, lactosylceramide (Galβ4Glcβ1Cer) with t18:0-h16:0-h24:0, neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer), and Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (0.4 to 2.0 μg of each compound).
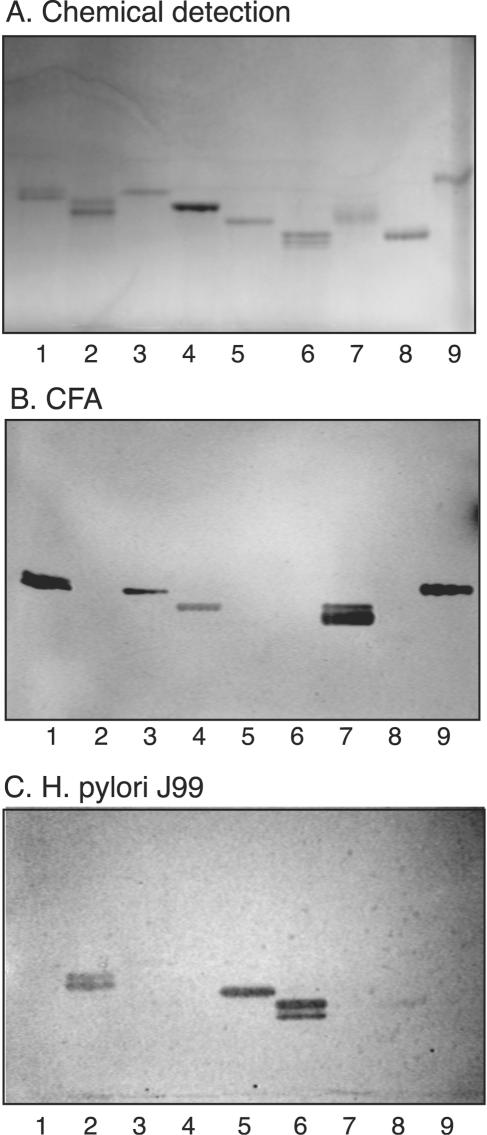
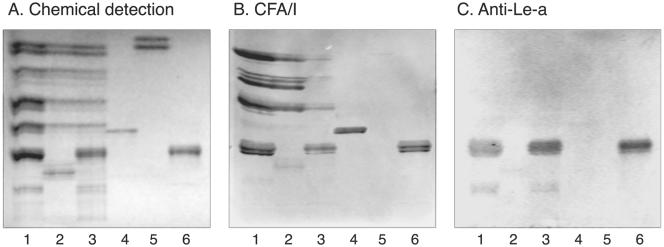
FIG. 3.
Comparison of glycosphingolipid recognition by CFA/I fimbriae of ETEC and BabA-expressing H. pylori. The glycosphingolipids were chromatographed on aluminum-backed silica gel plates and visualized with anisaldehyde (A). Duplicate chromatograms were incubated with 125I-labeled CFA/I fimbriae (B) and 35S-labeled H. pylori strain J99 (C), followed by autoradiography for 12 h, as described in Materials and Methods. The solvent system used was chloroform-methanol-water (60:35:8, by volume). Lanes: 1, Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 2, B type 1 hexaglycosylceramide [Galα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 3, Lex pentaglycosylceramide [Galβ4(Fucα3)GlcNAcβ3Galβ4 Glcβ1Cer] (2 μg); 4, B hepta-glycosylceramide (Galα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); 5, Leb hexaglycosylceramide [Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 6, A type 1 heptaglycosylceramide [GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 7, Ley hexaglycosylceramide [Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 8, A type 2 heptaglycosylceramide [GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 9, H type 2 pentaglycosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (2 μg). Autoradiography was performed for 12 h.
Substitution of the terminal Galβ of neolactotetraosylceramide or the Lea or Lex structure with α3-linked NeuAc was not tolerated (compounds 33, 36, and 37; Fig. 4, lanes 2, 4, and 6). Substitution of the Ley element with GalNAcα3 was also rejected by the fimbriae (compound 15; Fig. 3, lane 7), and substitution of the H-5 type 2 glycosphingolipid with GalNAcα3 or Galα3 (compounds 13 and 14) also abrogated binding.
FIG. 4.
Binding of CFA-1 fimbriae of ETEC to pure glycosphingolipids on thin-layer chromatograms. Chemical detection by anisaldehyde (A) and an autoradiogram obtained by binding of 125I-labeled CFA-1 fimbriae (B) are shown. The glycosphingolipids were separated on aluminum-backed silica gel plates with chloroform-methanol-water (60:35:8, by volume) as the solvent system, and the binding assays were performed as described in Materials and Methods. Autoradiography was performed for 12 h. Lanes: 1, neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); 2, sialyl-neolactotetraosylceramide (NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (2 μg); 3, Lex pentaglycosylceramide [Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 4, sialyl-Lex hexaglycosylceramide [NeuAcα3Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 5, Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (2 μg); 6, sialyl-Lea hexaglycosylceramide [NeuAcα3Galβ3(Fucα4) GlcNAcβ3Galβ4Glcβ1Cer] (2 μg).
Another difference from lactosylceramide-binding bacteria was the binding in the monoglycosylceramide region obtained with CFA/I fimbriae. Here the fimbriae had a preference for glucosylceramide (compound 2; Fig. 2, lane 2), while galactosylceramide (compound 1; Fig. 2, lane 1) was not recognized.
To estimate the relative affinity of the CFA/I fimbriae for various binding-active glycosphingolipids, the binding of radiolabeled fimbriae to serial dilutions of glycosphingolipids on thin-layer chromatograms was determined (Fig. 5). In this assay, lactosylceramide with phytosphingosine and hydroxy fatty acids (compound 5 in Table 1), isoglobotriaosylceramide (compound 22), neolactotetraosylceramide (compound 8), and the Lea-5 glycosphingolipid (compound 17) were the preferred ligands (detection limit, 0.1 to 0.4 μg), followed by the Lex-5 (compound 10) and Ley-6 (compound 12) glycosphingolipids (detection limit, 0.6 to 0.8 μg), while glucosylceramide (compound 2) gave no signal.
FIG. 5.
Binding of CFA/I fimbriae of ETEC to serial dilutions of glycosphingolipids. Autoradiograms were obtained by binding of 125I-labeled CFA/I fimbriae to serial dilutions (0.1 to 1.0 μg) of glycosphingolipids in a chromatogram binding assay. The binding assay was done as described in Materials and Methods. Autoradiography was performed for 12 h. Lanes in panel A: 1 to 6, glucosylceramide (Glcβ1Cer), lactosylceramide (Galβ4Glcβ1Cer) with t18:0-h16:0-h24:0, isoglobotriaosylceramide (Galα3Galβ4Glcβ1Cer), neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer), and Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (0.1 to 1.0 μg of each compound); 7, negative control globotetraosylceramide (GalNAcβ3Galα4Galβ4Glcβ1Cer) (4 μg). Lanes in panel B: 1 to 5, Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (0.1 to 0.8 μg); 6 to 10, Lex pentaglycosylceramide [Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] and Ley hexaglycosylceramide [Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer] (0.1 to 0.8 μg of each compound).
Glycosphingolipid recognition of recombinant CFA/I-fimbriated E. coli.
Next, the glycosphingolipid binding of E. coli expressing intact CFA/I fimbriae (strain Top10-CFA/I) was tested in chromatogram binding assays. The results are presented in Table 1. Thus, all of the glycosphingolipids recognized by 125I-labeled CFA/I fimbriae were also bound by CFA/I-expressing E. coli (exemplified in Fig. 2D).
Glycosphingolipid recognition of CFA/I fimbriae without the tip protein (CFA/I/E−).
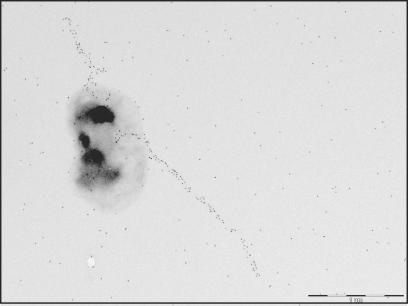
To evaluate the relative roles of the major CfaB subunit and the tip subunit CfaE in the glycosphingolipid interaction, a CfaE deletion mutant was constructed. Although the minor tip protein has been proposed to be involved in fimbrial assembly (30), fimbrial structures were produced by the recombinant bacteria as shown in Fig. 6, demonstrating that CfaE is not indispensable for the assembly of CFA/I fimbriae.
FIG. 6.
Electron micrograph of immunolabeled and negatively stained recombinant E. coli expressing CFA/I fimbriae without tip protein CfaE (strain Top10-CFA/I/E−). The bacteria were labeled with monoclonal anti-CFA/I antibody 1:6. The bar represents 1 μm.
In glycosphingolipid-binding assays with purified, 125I-labeled CFA/I/E− fimbriae, a binding pattern identical to that of native CFA/I fimbriae was obtained (exemplified in Fig. 2C and summarized in Table 1). In addition, the bacteria with CFA/I/E− fimbriae bound in the same manner (Fig. 2E). Thus, minor tip protein CfaE is not involved in CFA/I glycosphingolipid recognition.
Comparison of glycosphingolipid recognition of CFA/I and heterologous CF fimbriae.
To estimate the role of the fimbrial structure in the glycosphingolipid recognition process, the binding of 125I-labeled CS1 and CS4 fimbriae, belonging to the CFA/I group, and CS7 fimbriae, of the CS5 group, was also tested in the chromatogram binding assay. The results are exemplified in Fig. 7, and summarized in Table 2. Thus, the glycosphingolipids recognized by the CFA/I fimbriae were also recognized by fimbriae with high sequence similarity to CFA/I, i.e., CS1 (major subunit, 55% homology to CFA/I) and CS4 (major subunit, 60% homology). In contrast, CS7 (major subunit, 20% homology) displayed weak binding to isoglobotriaosylceramide and lactosylceramide with phytosphingosine and hydroxy fatty acids but not to the other glycosphingolipids recognized by CFA/I. The detection limit of CS7 for isoglobotriaosylceramide and lactosylceramide was approximately 1 μg, while the detection limits of both CFA/I and CS4 for all binding-active compounds, except glucosylceramide, was below 0.4 μg, in line with the results presented above.
TABLE 2.
Comparison of glycosphingolipid binding by 125I-labeled CFs
| Compound no., trivial name | Structure | CFA/I | CS1 | CS4 | CS7 |
|---|---|---|---|---|---|
| 1, Glucosylceramide | Glcβ1Cer | +a | + | + | − |
| 2, LacCer | Galβ4Glcβ1Cer | +++ | +++ | +++ | + |
| 3, Isoglobotri | Galα3Galβ4Glcβ1Cer | +++ | +++ | +++ | + |
| 4, GgO4 | Galβ3GalNAcβ4Galβ4Glcβ1Cer | +++ | +++ | +++ | − |
| 5, Neolactotetra | Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | +++ | +++ | + |
| 6, Lea-5 | Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | +++ | +++ | +++ | − |
Binding is defined as follows: +++, binding when 0.4 μg of the glycosphingolipid was applied on the thin-layer chromatogram; +, binding at 2 μg; −, no binding at 2 μg.
Binding of CFA/I fimbriae to nonacid glycosphingolipids of human small intestine.
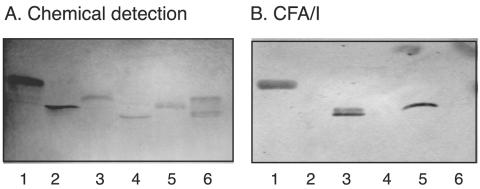
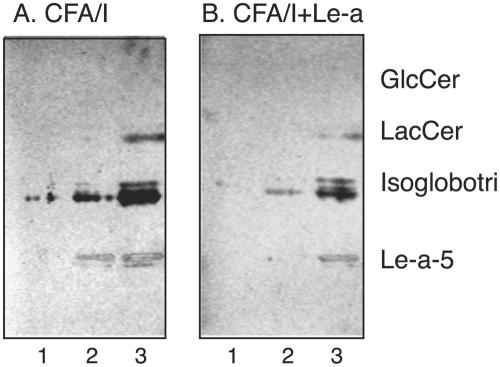
To assess the potential role of glycosphingolipid recognition by CFA/I in target tissue adherence, the binding of CFA/I fimbriae to whole nonacid glycosphingolipid fractions from human small intestine was determined. Thereby, binding in the mono-, di-, and triglycosylceramide regions was observed in the nonacid intestinal fractions from the three individuals tested (Fig. 8, lanes 1 to 3), and in two of these fractions, binding to a compound comigrating with the Lea pentaglycosylceramide was obtained (Fig. 8, lanes 1 and 3). Monoclonal antibodies directed against the Lea determinant also bound to compounds migrating in this region (Fig. 8C).
FIG. 8.
Binding of CFA/I fimbriae of ETEC to nonacid glycosphingolipids of human small intestine. The glycosphingolipids were separated on aluminum-backed silica gel plates and visualized with anisaldehyde (A). Duplicate chromatograms were incubated with 125I-labeled CFA/I fimbriae (B) and monoclonal antibodies directed against the Lea determinant (C), followed by autoradiography for 12 h, as described in Materials and Methods. The solvent system used was chloroform-methanol-water (60:35:8, by volume). Lanes: 1 to 3, nonacid glycosphingolipids of human small intestine of three different individuals (40 μg/lane); 4, reference neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer) (4 μg); 5, reference galactosylceramide (Galβ1Cer) (4 μg); 6, reference Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (4 μg).
Competition experiments.
The ability of soluble oligosaccharides to interfere with the binding of CFA/I to glycosphingolipids was examined by incubating the fimbriae with free saccharides before binding to glycosphingolipids on chromatograms. The interaction of CFA/I fimbriae with glycosphingolipids was reduced by incubation with both of the saccharides used, i.e., lactose and the Lea pentasaccharide. Thus, both lactose and the Lea pentasaccharide interfered with fimbrial binding to lactosylceramide, isoglobotriaosylceramide, Lea pentaglycosylceramide (shown in Fig. 9), and neolactotetraosylceramide (not shown).
FIG. 9.
Effect of preincubation of CFA/I fimbriae with oligosaccharides. Radiolabeled CFA/I fimbriae were incubated with Lea pentasaccharide (1 mg/ml) in PBS for 2 h at room temperature. The suspensions were then utilized in a chromatogram binding assay. Panels: A, binding of CFA/I fimbriae alone; B, binding of CFA/I fimbriae incubated with Lea pentasaccharide. The lanes contained dilutions of glucosylceramide (Glcβ1Cer), lactosylceramide with hydroxy ceramide (Galβ4Glcβ1Cer), isoglobotriaosylceramide (Galα3Galβ4Glcβ1Cer), and Lea pentaglycosylceramide [Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer] (0.1 to 0.4 μg of each compound). The glycosphingolipids were separated on aluminum-backed silica gel plates with chloroform-methanol-water (60:35:8, by volume) as the solvent system, and the binding assay was performed as described in Materials and Methods. Autoradiography was performed for 12 h.
DISCUSSION
In the present study, carbohydrate recognition by CFA/I fimbriae was investigated by testing the binding of either radiolabeled fimbriated bacteria or purified fimbriae to glycosphingolipids separated on thin-layer chromatograms. The binding patterns obtained both with bacteria and with the purified fimbriae had several features in common with those of previously described lactosylceramide-binding bacteria (19), with binding to lactosylceramide with hydroxy fatty acids and/or phytosphingosine, isoglobotriaosylceramide, neolactotetraosylceramide, gangliotriaosylceramide, and gangliotetraosylceramide. However, binding to compounds that are not recognized by lactosylceramide-binding bacteria was also observed. These included glucosylceramide, the H5 type 2 pentaglycosylceramide, and glycosphingolipids with terminal Lea, Lex, and Ley determinants. Previous studies had suggested that sialylated glycoconjugates, such as the GM2 ganglioside (9) or sialoglycoproteins, may act as CFA/I receptors (4, 26, 35). In the present study, however, no binding of CFA/I to gangliosides was observed.
Requirement of a certain ceramide species for binding to occur, like the requirement of hydroxy fatty acids and/or phytosphingosine for CFA/I binding to lactosylceramide, has been reported for antibodies, bacterial toxins, and K99-fimbriated E. coli, as well as other lactosylceramide-binding bacteria, including H. pylori (reference 2 and references therein). In the case of lactosylceramide recognition, it has been proposed that the selectivity is due to binding of a conformation of lactosylceramide in which the oxygen of the fatty acid hydroxyl group forms a hydrogen bond with the hydroxymethyl group of the glucose (2). Unlike other lactosylceramide-recognizing bacteria, CFA/I-fimbriated E. coli binds to glucosylceramide. However, the glucosylceramide binding is relatively weak, indicating that although the binding epitope includes parts of the internal glucose, addition of terminal β4-linked galactose, yielding lactosylceramide, results in a more optimal binding epitope.
In order to investigate which fimbrial proteins were associated with the carbohydrate-binding properties observed in these experiments, a mutant was generated in which the cfaE gene encoding the minor tip protein was deleted. Despite a dramatic reduction in CFA/I expression in this strain, fimbriae could be detected by inhibition enzyme-linked immunosorbent assay, observed by electron microscopy, and purified sufficiently for use in binding assays. In agreement with previous findings in which a point mutation in the tip protein was able to compromise the hemagglutination capacity of CFA/I fimbriae (1), the recombinant bacteria expressing the tipless fimbriae were no longer able to agglutinate human erythrocytes. However, the bacteria expressing these tipless fimbriae still bound to the glycosphingolipids recognized by native fimbriae, demonstrating that the glycosphingolipid-binding site(s) resides within the major CfaB subunit. It thus seems that CFA/I fimbriae have multiple binding sites. Glycosphingolipid binding is mediated by the major CfaB subunit, whereas interaction with unidentified receptors on human erythrocytes and Caco-2 cells is mediated by CfaE, the minor tip protein (1). Indeed, previous findings do support a binding capacity residing with CfaB, since monoclonal antibodies directed against this protein could inhibit the binding of CFA/I-expressing cells to human jejunal cells and prevent fluid accumulation induced by CFA/I-positive bacteria in rabbit intestinal loops (29).
An interesting parallel to our observations is found in the S fimbriae of meningitis-associated E. coli. The minor tip protein SfaS interacts with NeuAcα3Gal-carrying glycoproteins (23, 24), whereas the major subunit SfaA binds to sulfatide, seminolipid, galactosylceramide, and lactosylceramide (27). Hemagglutination induced by S-fimbriated E. coli is abolished when the sfaS gene is deleted (12), but these bacteria still adhere to human endothelial cells (25). Also, the well-characterized Galα4Gal-binding P fimbriae of uropathogenic E. coli seems to have multiple binding capacities, since it interacts with fibronectin in a manner that is independent of Galα4Gal-binding tip protein PapG (36).
The broader carbohydrate recognition pattern of CFA/I fimbriae compared to lactosylceramide-binding bacteria suggests either that the binding site is more permissive or that there is more than one binding site within the protein. However, the binding of lactosylceramide, isoglobotriaosylceramide, neolactotetraosylceramide, and the Lea pentaglycosylceramide could all be inhibited by incubating the CFA/I fimbriae with lactose or Lea pentasaccharide, suggesting that these compounds are accommodated in the same carbohydrate binding. Final resolution of this issue must, however, await cocrystallization of CfaB with binding-active saccharides.
The relevance of the glycosphingolipid-binding capacities shown in this study to the CFA/I-mediated adhesion of ETEC to host cells during colonization has yet to be determined. However, experiments with glycosphingolipid fractions isolated from human small intestine demonstrated that CFA/I bound in the mono-, di-, and triglycosylceramide regions in the nonacid fractions from the three individuals tested. In two of these individuals, binding to a compound comigrating with the Lea pentaglycosylceramide was observed. In the epithelial cells of human small intestine monohexosylceramides (galactosylceramide and glucosylceramide), blood group ABH (type 1 chain) and Lewis glycolipids with five to seven sugar residues are the major glycolipid constituents and the expression of major blood groups glycosphingolipids is in agreement with the ABO, Lewis, and secretor phenotype of the individuals (5). There are also trace amounts of Lex- and Ley-terminated glycosphingolipids. In addition, Lex, Ley, and H type 2 determinants are found on glycoproteins of human small intestinal epithelial cells (10). Several of the CFA/I-binding compounds such as glucosylceramide-, Lea-, Lex-, Ley-, and H type 2-terminated glycoconjugates may thus feasibly act as targets for CFA/I-mediated adherence. Isoglobotriaosylceramide, on the other hand, has been found in, e.g., dog intestine (14) but not in humans, while gangliotriaosylceramide and gangliotetraosylceramide have not been chemically identified in peripheral human tissues.
In conclusion, we have demonstrated that the major CfaB subunit of CFA/I fimbriae is a carbohydrate-binding protein which specifically interacts with a number of carbohydrate sequences that are present in human small intestinal glycosphingolipids and glycoproteins in substantial quantities. Our findings suggest that the carbohydrate-binding activity contributes to the attachment of CFA/I-fimbriated E. coli to host intestinal epithelium and may be a basis for the rational design of receptor saccharide analogues for inhibition of the adhesion of CFA/I-expressing ETEC and also ETEC carrying CFA/I-related fimbriae.
Acknowledgments
This study was supported by the Swedish Medical Research Council (grants 12628 [S.T.] and 16X-09084 [A.M.S.]), the Swedish Cancer Foundation, the Volvo Assar Gabrielssons Foundation, and the Swedish Medical Society/The Foundations of the National Board of Health and Welfare.
Editor: A. D. O'Brien
REFERENCES
- 1.Anantha, R. P., A. L. McVeigh, L. H. Lee, M. K. Agnew, F. J. Cassels, D. A. Scott, T. S. Whittam, and S. J. Savarino. 2004. Evolutionary and functional relationships of colonization factor antigen I and other class 5 adhesive fimbriae of enterotoxigenic Escherichia coli. Infect. Immun. 72:7190-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ångström, J., S. Teneberg, M. Abul Milh, T. Larsson, I. Leonardsson, B.-M. Olsson, M. Ölwegård Halvarsson, D. Danielsson, I. Näslund, Å. Ljungh, T. Wadström, and K.-A. Karlsson. 1998. The lactosylceramide binding specificity of Helicobacter pylori. Glycobiology 8:297-309. [DOI] [PubMed] [Google Scholar]
- 3.Aspholm-Hurtig, M., G. Dailide, M. Lahmann, A. Kalia, D. Ilver, N. Roche, S. Vikström, R. Sjöström, S. Lindén, A. Bäckström, A. Arnqvist, J. Mahdavi, U. J. Nilsson, B. Velapatiño, R. H. Gilman, M. Gerhard, T. Alarcon, M. López-Brea, T. Nakazawa, J. G. Fox, P. Correa, M. G. Dominguez-Bello, G. I. Perez-Perez, M. J. Blaser, S. Normark, I. Carlstedt, S. Oscarson, S. Teneberg, D. E. Berg, and T. Borén. 2004. Functional adaptation of BabA, the Helicobacter pylori blood-group antigen binding adhesin. Science 305:519-522. [DOI] [PubMed] [Google Scholar]
- 4.Bartus, H., P. Actor, E. Snipes, D. Sedlock, and I. Zajac. 1985. Indications that the erythrocyte receptor involved in enterotoxigenic Escherichia coli attachment is a sialoglycoconjugate. J. Clin. Microbiol. 21:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björk, S., M. E. Breimer, G. C. Hansson, K.-A. Karlsson, and H. Leffler. 1987. Structures of blood group glycosphingolipids in human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J. Biol. Chem. 262:6758-6765. [PubMed] [Google Scholar]
- 6.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 7.Bühler, T., H. Hoschutzky, and K. Jann. 1991. Analysis of colonization factor antigen I, an adhesin of enterotoxigenic Escherichia coli O78:H11: fimbrial morphology and location of the receptor-binding site. Infect. Immun. 59:3876-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans, D. G., R. P. Silver, D. J. Evans, Jr., D. G. Chase, and S. L. Gorbach. 1975. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect. Immun. 12:656-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faris, A., M. Lindahl, and T. Wadström. 1980. GM2-like glycoconjugate as a possible erythrocyte receptor for the CFA/I and K99 hemagglutinins of enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 7:265-269. [Google Scholar]
- 10.Finne, J., M. E. Breimer, G. C. Hansson, K.-A. Karlsson, H. Leffler, J. F. Vliegenthart, and H. van Halbeek. 1989. Novel polyfucosylated N-linked glycopeptides with blood group A, H, X, and Y determinants from human small intestinal epithelial cells. J. Biol. Chem. 264:5720-5735. [PubMed] [Google Scholar]
- 11.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 12.Hacker, J., H. Kestler, H. Hoschutzky, K. Jann, F. Lottspeich, and T. K. Korhonen. 1993. Cloning and characterization of the S fimbrial adhesin II complex of an Escherichia coli O18:K1 meningitis isolate. Infect. Immun. 61:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansson, G. C., K.-A. Karlsson, G. Larson, J. M. McKibbin, M. Blaszczyk, M. Herlyn, Z. Steplewski, and H. Koprowski. 1983. Mouse monoclonal antibodies against human cancer cell lines with specificities for blood group and related antigens. Characterization by antibody binding to glycosphingolipids in a chromatogram binding assay. J. Biol. Chem. 258:4091-4097. [PubMed] [Google Scholar]
- 14.Hansson, G. C., K.-A. Karlsson, G. Larson, J. M. McKibbin, N. Strömberg, and J. Thurin. 1983. Isoglobotriaosylceramide and the Forssman glycolipid of dog small intestine occupy separate tissue compartments and differ in ceramide composition. Biochim. Biophys. Acta 750:214-216. [DOI] [PubMed] [Google Scholar]
- 15.Hansson, G. C., K.-A. Karlsson, G. Larson, N. Strömberg, and J. Thurin. 1985. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal. Biochem. 146:158-163. [DOI] [PubMed] [Google Scholar]
- 15a.IUPAC-IUB Commission on Biochemical Nomenclature. 1998. Nomenclature of glycolipids: recommendations 1997. Eur. J. Biochem. 257:293-298. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, A. A. C., B. H. Simons, and F. K. de Graaf. 1987. The role of lysine-132 and arginine-136 in the receptor-binding domain of the K99 fibrillar subunit. EMBO J. 30:1805-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordi, B. J. A. M., G. A. Willshaw, B. A. M. van der Zeist, and W. Gaastra. 1992. The complete nucleotide sequence of region 1 of the CFA/I fimbrial operon of human enterotoxigenic Escherichia coli. DNA Seq. 2:257-263. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson, K.-A. 1987. Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138:212-220. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson, K.-A. 1989. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu. Rev. Biochem. 58:309-350. [DOI] [PubMed] [Google Scholar]
- 20.Koerner, T. A. W., Jr., J. H. Prestegard, P. C. Demou, and R. K. Yu. 1983. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry 22:2676-2687. [DOI] [PubMed] [Google Scholar]
- 21.Lindberg, F., B. Lund, L. Johansson, and S. Normark. 1987. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature 328:84-87. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Vidal, Y., P. Kleem, and A.-M. Svennerholm. 1988. Monoclonal antibodies against different epitopes on colonization factor antigen I of enterotoxin-producing Escherichia coli. J. Clin. Microbiol. 26:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parkkinen, J., J. Finne, M. Achtman, V. Vaisanen, and T. K. Korhonen. 1983. Escherichia coli strains binding neuraminyl α 2-3 galactosides. Biochem. Biophys. Res. Commun. 111:456-461. [DOI] [PubMed] [Google Scholar]
- 24.Parkkinen, J., G. N. Rogers, T. Korhonen, W. Dahr, and J. Finne. 1986. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect. Immun. 54:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkkinen, J., A. Ristimäki, and B. Westerlund. 1989. Binding of Escherichia coli S fimbriae to cultured human endothelial cells. Infect. Immun. 57:2256-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieroni, P., E. A. Worobec, W. Paranchych, and G. D. Armstrong. 1988. Identification of a human erythrocyte receptor for colonization factor antigen I expressed by H10407 enterotoxigenic Escherichia coli. Infect. Immun. 56:1334-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasadarao, N. V., C. A. Wass, J. Hacker, K. Jann, and K. S. Kim. 1993. Adhesion of S-fimbriated Escherichia coli to brain glycolipids mediated by sfaA gene-encoded protein of S-fimbriae. J. Biol. Chem. 268:10356-10363. [PubMed] [Google Scholar]
- 28.Rudin, A., and A.-M. Svennerholm. 1996. Identification of a cross-reactive continuous B-cell epitope in enterotoxigenic Escherichia coli colonization factor antigen I. Infect. Immun. 64:4508-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudin, A., L. Olbe, and A.-M. Svennerholm. 1996. Monoclonal antibodies against subunits of colonization factor antigen I (CFA/I) inhibit binding to human enterocytes and protect against enterotoxigenic Escherichia coli expressing heterologous CFs. Microb. Pathog. 21:35-45. [DOI] [PubMed] [Google Scholar]
- 30.Sakellaris, H., and J. R. Scott. 1998. New tools in an old trade: CS1 pilus morphogenesis. Mol. Microbiol. 30:681-687. [DOI] [PubMed] [Google Scholar]
- 31.Sakellaris, H., G. P. Munson, and J. R. Scott. 1999. A conserved residue in the tip proteins of CS1 and CFA/I pili of enterotoxigenic Escherichia coli that is essential for adherence. Proc. Natl. Acad. Sci. USA 96:12828-12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuelsson, B. E., W. Pimlott, and K.-A. Karlsson. 1990. Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 193:623-646. [DOI] [PubMed] [Google Scholar]
- 33.Stellner, K., H. Saito, and S.-I. Hakomori. 1973. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch. Biochem. Biophys. 155:464-472. [DOI] [PubMed] [Google Scholar]
- 34.Waldi, D. 1962. Sprühreagentien für die Dünnschicht-Chromatographie, p. 496-515. In E. Stahl (ed.), Dünnschicht-Chromatographie. Springer-Verlag, Berlin, Germany.
- 35.Wennerås, C., J. Holmgren, and A.-M. Svennerholm. 1990. The binding of colonization factor antigens of enterotoxigenic Escherichia coli to intestinal cell membrane proteins. FEMS Microbiol. Lett. 54:107-112. [DOI] [PubMed] [Google Scholar]
- 36.Westerlund, B., P. Kuusela, T. Vartio, I. van Die, and T. K. Korhonen. 1989. A novel lectin-independent interaction of P fimbriae of Escherichia coli with immobilized fibronectin. FEBS Lett. 243:199-204. [DOI] [PubMed] [Google Scholar]
- 37.Wu, S., D. W. Pascual, J. L. VanCott, J. R. McGhee, D. R. Maneval, Jr., M. M. Levine, and D. M. Hone. 1995. Immune responses to novel Escherichia coli and Salmonella typhimurium vectors that express colonization factor antigen I (CFA/I) of enterotoxigenic E. coli in the absence of the CFA/I positive regulator cfaR. Infect. Immun. 63:4933-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang, H., and S.-I. Hakomori. 1971. A sphingolipid having a novel ceramide and lacto-N-fucopentose III. J. Biol. Chem. 246:1192-1200. [PubMed] [Google Scholar]