Abstract
Sequestration of Plasmodium falciparum-infected erythrocytes (Pf-IRBC) in postcapillary brain endothelium is a hallmark of cerebral malaria (CM) pathogenesis. There is a correlation between adherent Pf-IRBC and increased expression of intercellular cell adhesion molecule 1 (ICAM-1), which is also a receptor for Pf-IRBC on human brain microvascular endothelial cells (HBMEC). The underlying mechanism for the increased ICAM-1 expression has not been clearly defined. Therefore, we investigated the mechanisms of ICAM-1 expression on isolated HBMEC after exposure to Pf-IRBC. Ultrastructural characterization of the model confirmed that there was attachment through both Pf-IRBC knobs and HBMEC microvillus protrusions. Pf-IRBC induced a dose- and time-dependent increase in ICAM-1 expression on HBMEC that was specific for human brain endothelium and was not observed with human umbilical vein endothelium. Involvement of both membrane-associated Pf-IRBC proteins and parasite-derived soluble factors with the increase in ICAM-1 expression was demonstrated by surface trypsinization and fractionation. Pf-IRBC exposure induced nuclear translocation of NF-κB in HBMEC, which was linked to ICAM-1 expression, as shown by use of specific inhibitors of the transcription factor NF-κB and immunocytochemistry. In addition, inhibition of reactive oxygen species decreased Pf-IRBC-induced ICAM-1 expression on HBMEC. Parasite-induced ICAM-1 expression explains the localization of this molecule on brain endothelium in postmortem CM brain samples. By increasing ICAM-1 expression, Pf-IRBC may increase their sequestration, thereby perpetuating CM.
Cerebral malaria (CM) accounts for more than 200,000 clinical episodes per year in Africa alone and has a high mortality rate (20%) (32). CM often results in permanent neurological sequelae, including seizures. Histopathologic studies at autopsy have shown that small blood vessels in the brain are tightly packed with adherent Plasmodium falciparum-infected red blood cells (Pf-IRBC) that appear to be closely associated with the blood-brain barrier (BBB) endothelium via Pf-IRBC surface knob-like protrusions (37). The knobs contain 200-kDa or larger, parasite-derived variant surface antigens that comprise a family of erythrocyte membrane proteins (PfEMP1) that act as ligands for Pf-IRBC attachment (5).
The attachment of Pf-IRBC is mediated by specific host endothelial receptor molecules, such as predominately intercellular cell adhesion molecule 1 (ICAM-1) in the brain, chondroitin sulfate A (CSA) in the placenta, and CD36 in other organs. Peripheral parasites isolated from CM patients bind to ICAM-1, and postmortem brain samples from CM patients have indicated that Pf-IRBC sequestration correlates with increased endothelial ICAM-1 expression (33, 38, 40, 49).
In CM, immunocytochemical staining for ICAM-1 in vessels has been associated with sequestration of Pf-IRBC (49). General host inflammatory conditions, including endogenous pyrogens, especially tumor necrosis factor alpha (TNF-α), whose levels are elevated during malaria infection, have been postulated to account for the observed increase in endothelial ICAM-1 expression (12, 27, 29, 31, 48). However, Peyron et al. (35) found that serum levels of TNF-α do not correlate with soluble ICAM-1 levels in plasma of P. falciparum malaria patients. In a review, Gimenez et al. (17) concluded that although increased levels of TNF-α in plasma and the brain could be responsible for upregulation of intercellular adhesion molecule 1 on endothelium, there was no clear correlation between the TNF-α levels and the occurrence and severity of CM. Moreover, patients with Plasmodium vivax malaria infections have higher TNF-α levels than patients with P. falciparum infections have and do not have increased brain endothelial ICAM-1 expression (25). On the other hand, induction of ICAM-1 during malaria infection was positively correlated with disease severity (38). Thus, it appears that ICAM-1 plays a role in infection, but TNF-α may not be solely responsible for the increased levels of ICAM-1, like the levels found in brain vessels containing Pf-IRBC, as shown by Turner et al. (49), and additional factors may contribute to this. This raises the possibility that Pf-IRBC may modulate ICAM-1 expression and possibly their own sequestration, but the underlying mechanisms by which this occurs are unclear.
Several studies of the interactions of Pf-IRBC with nonbrain endothelial cells, such as human umbilical vein endothelial cells (HUVEC), dermal cells derived from foreskin, or brain endothelium from other species, have generated valuable information, including information on, for example, adherence factors. However, there is significant heterogeneity in endothelial cells from different organs, and the phenotypic and biochemical properties of macrovascular endothelial cells differ from the phenotypic and biochemical properties of microvascular endothelial cells (6, 16, 21, 23, 34). HUVEC, however, do not possess CD36 or the chemokine receptor CXCR4 (43, 44), whereas dermal cells are CD36 positive and brain endothelium is CXCR4 positive. Therefore, results obtained with one tissue endothelial system, like human umbilical vein endothelial cells, are not likely to be directly applicable to other endothelium systems, such as brain endothelium in the case of CM. Thus, in order to study CM, it is necessary to use human endothelium derived from the brain.
We used isolated characterized human brain microvascular endothelial cells (HBMEC) that were previously isolated in our laboratory to construct an in vitro model of the human BBB. This in vitro model of the human BBB has been used to examine interactions with various pathogens, including bacteria (Escherichia coli, group B Streptococcus, Streptococcus pneumoniae, Citrobacter), monocytes, viruses (human immunodeficiency virus), fungi (Candida albicans, Cryptococcus), and parasites (trypanosoma), which enter the brain (8, 19, 22, 24, 42, 44, 45). This model provides a unique way to explore how Pf-IRBC interact with the BBB. To further investigate the presence of ICAM-1 on brain endothelial vessels, we used isolated brain endothelial cells and exposed them to Pf-IRBC. In this paper we describe the ability of Pf-IRBC to increase ICAM-1 expression on human brain microvascular endothelium.
MATERIALS AND METHODS
Isolation, characterization, and culture of HBMEC.
HBMEC were isolated from human brain specimens obtained postmortem or by surgical resection during surgery for seizure disorder, and they were extensively characterized as described previously (43, 44). Department of Health and Human Services and internal review board regulations for use of deidentified human brain tissue for isolation of endothelial cells were followed. Briefly, HBMEC were purified (>90%) either by isolation of small clones of endothelial cells with cloning cylinders or by fluorescence-activated cell sorting using fluorescently labeled 1,1′-dioctaadecyl-1-3,3′3′-tetramethyl-indocarbocyanaineperchlorate-labeled acetylated low-density lipoproteins. The HBMEC were positive for factor VIII-Rag staining, took up fluorescent 1,1′-dioctaadecyl-1-3,3′3′-tetramethyl-indocarbocyanaineperchlorate-labeled acetylated low-density lipoprotein, and contained gamma-glutamyl transpeptidase and the drug transporter P-glycoprotein, and functional junction proteins, as revealed by ZO1 staining at cell-cell borders, demonstrated their intact brain endothelial cell characteristics. HBMEC cultures were maintained in RPMI-based medium that included 10% fetal bovine serum (FBS) and 10% NuSerum (BD Biosciences) at 37°C in a humid atmosphere containing 5% CO2.
HUVEC were obtained from Clonetics and were maintained in EGM with bulletkit as directed by the supplier.
P. falciparum culture and treatments.
P. falciparum 3D7 (obtained from MR4-ATCC) was cultured with 10% human serum in RPMI 1640 medium by using the method of Trager and Jenson (46). P. falciparum clones FCR-CSA and ItG-ICAM-1 were kindly donated by Joseph Smith (SBRI, Seattle, WA). Use of human erythrocytes to support the growth of P. falciparum was approved by the internal review board. For all experiments we used a sorbitol-synchronized trophozoite stage that was purified by Percoll density gradient centrifugation to obtain more than 90% parasitemia (26, 28). To obtain preparations containing more than 75% ring-stage parasites, we incubated Percoll-purified mature stages with equal numbers of uninfected red blood cells (RBC) overnight and then treated them with sorbitol to remove the remainder of the mature stages. The preparations containing HBMEC were incubated in 5% fetal bovine serum, and the numbers of Pf-IRBC indicated below settled by gravity to form monolayers. In our experience and as judged by morphology and replication vitality, P. falciparum parasites are competent in this growth medium overnight. We also noted that fetal bovine serum can support the growth of P. falciparum parasites (41).
After incubation of HBMEC with up to 108 Pf-IRBC per well for up to 20 h or as indicated below, Pf-IRBC were removed, HBMEC were monolayers washed and fixed with acetone-methanol (1:1, vol/vol), and ICAM-1 expression was determined by an enzyme-linked immunosorbent assay (ELISA) as described below. The reversibility of ICAM-1 expression was assessed after initial exposure of Pf-IRBC for 6 h, followed by subsequent removal of Pf-IRBC and extensive washing of HBMEC. At 24, 48, and 72 h after withdrawal, HBMEC were fixed and ICAM-1 expression was determined. Unless indicated otherwise, 0.6 × 108 Pf-IRBC/well were added to ELISA plates to determine ICAM-1 expression. Binding studies were performed in 24-well plates, and 108 Pf-IRBC/well were added in these studies.
Pf-IRBC were trypsinized to remove extracellularly exposed proteins as described by Gardner et al. (14), with minor modifications. Briefly, Pf-IRBC were washed twice in calcium-free Hanks balance salt solution, incubated in serum-free RPMI 1640 medium with 1 mg/ml trypsin to obtain a final hematocrit of 10%, and incubated for 3 min at room temperature. To block trypsin activity, medium with 5% FBS (serum contains trypsin inhibitors) was added to Pf-IRBC, and the preparation was washed three times with at least 5 volumes of RPMI containing 5% FBS. To inactivate parasites inside Pf-IRBC, Percoll-purified parasite cultures were incubated with 1 μM artemisinin for 4 h. Drug-treated cultures were washed twice with RPMI medium before they were added to HBMEC.
Percoll-purified Pf-IRBC (P. falciparum clone 3D7 at the trophozoite stage) were lysed by three cycles of quick freezing on dry ice and thawing as previously described by Artavanis-Tsakonas and Riley (3). To prepare subfractions of Pf-IRBC, lysates were centrifuged at 18,000 rpm for 10 min and 4°C. Each supernatant was filtered through 0.2-μm syringe filters to remove contaminating micromembrane particles. To remove remaining soluble fraction components, the pellet fraction was washed twice in RPMI 1640 medium and subsequently resuspended in medium supplemented with serum. The pellet fraction was characterized by microscopy and contained erythrocyte membrane fragments and hemozoin residual bodies with surrounding membranes. Western blot analysis showed that parasite aldolase was in the soluble fraction and erythrocyte band 3 was only in the membrane fraction (data not shown).
Culture supernatant was collected from Percoll-purified Pf-IRBC with P. falciparum in the early trophozoite stage to the schizont stage over a 24-h period. Pf-IRBC culture supernatant was added to confluent HBMEC in ELISA plates, and ICAM-1 expression was determined as indicated below.
Ultrastructural visualization of Pf-IRBC-HBMEC interaction.
To visualize the interaction of HBMEC with Pf-IRBC at the ultrastructural level, HBMEC were seeded onto collagen-coated glass coverslips and grown to confluence. HBMEC were then exposed to Pf-IRBC and normal RBC for up to 11 h. As a control, an HBMEC monolayer was incubated with medium alone. HBMEC were then washed after 1, 6, and 11 h with RPMI medium and fixed in 2% glutaraldehyde-2% paraformaldehyde-3 mM CaCl2 in 100 mM HEPES buffer (pH 7.3). After fixation, coverslips were washed in 0.1 M cacodylate buffer (pH 7.2), postfixed in osmium tetraoxide, and dehydrated in a graded ethanol series. For scanning electron microscopy (SEM), the samples were coated with chromium and viewed with a Leo 1530 field emission in-lens SEM operating at 1 kV. For subsequent transmission electron microscope (TEM) analysis the samples used for SEM were cut vertically and embedded in Eponate 12 (Ted Pella, Redding, CA). These samples were viewed and photographed with a Phillips CM 120 transmission electron microscope operating at 60 to 80 kV.
ELISA for endothelial cell surface adhesion molecules.
Expression of ICAM-1 was assessed by ELISA combined with the ABC alkaline phosphatase method (Vector labs, Burlingame, CA), as previously described (45). Briefly, HBMEC were seeded into collagen-coated 96-well plates and grown to confluence in RPMI-based growth medium. Different amounts of Pf-IRBC or RBC were added to the HBMEC, incubated for 6 or 24 h, and washed. The HBMEC were fixed with 50% methanol—50% acetone for 5 min, air dried, and stored at −70°C until they were used.
For ELISA, HBMEC were rehydrated with phosphate-buffered saline (PBS) containing 0.2% Triton X-100, blocked with 10% normal goat serum for 15 min, and incubated with primary monoclonal anti-ICAM-1 antibody at a dilution of 1:1,000 (Beckman Coulter, Fullerton, CA) for 2 h. After this, the HBMEC were washed, incubated with biotinylated horse anti-mouse antibody (1:200) for 1 h, and washed again, and ABC alkaline phosphatase (Vector Labs, Burlington, CA) was added for 30 min. After a final washing step, color was developed using p-nitrophenyl phosphate substrate in the presence of levamisole. Absorbance at 405 nm was determined with a microplate reader (Beckman). The results were expressed as relative optical densities and as percentages of the values for control nonexposed HBMEC.
Determination of NF-κB nuclear translocation.
For immunolocalization, HBMEC were seeded onto collagen-coated glass coverslips placed in a 24-well plate and grown until they were confluent. The cells were exposed to 108 Pf-IRBC for up to 6 h. TNF-α was used as a positive control, and medium alone was used as a negative control. After treatment HBMEC were washed twice with ice-cold PBS to remove medium components and parasitic materials, and this was followed by fixation with 4% paraformaldehyde. After 15 min of fixation, HBMEC were washed twice with PBS, permeabilized with 0.5% Triton X-100, blocked with 10% normal goat serum, and incubated with rabbit anti-p65 (NF-κB) antibody for 2.5 h. Subsequently, HBMEC were washed three times, and anti-rabbit immunoglobulin G rhodamine (1:200) was applied for 1 h. After washing with PBS, the coverslips were mounted on slides, and HBMEC were observed using an inverted Olympus IX-70 fluorescence microscopy system equipped with a cooled charge-coupled device camera (OlymPix TE3/A/S) with a standard rhodamine filter set. The real-time fluorescent images were displayed on a monitor and stored on a hard drive for subsequent analysis using the UltraView software (Perkin-Elmer Co., Massachusetts). Pictures were selected, transferred to Adobe Photoshop, and cropped without further processing.
Statistical analysis.
All data were expressed as means ± standard deviations of at least triplicate observations. Statistical significance was determined by the nonparametric Mann-Whitney U test. Means ± standard deviations were considered significantly different when the P value was <0.05.
RESULTS
Attachment of Pf-IRBC to HBMEC.
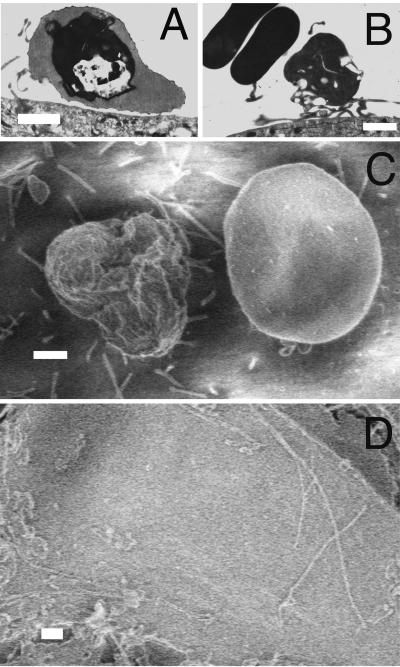
The nonselected parasites settled onto HBMEC through gravity, and the specific interactions between Pf-IRBC and HBMEC were visualized at the ultrastructural level. HBMEC were closely associated with the apical HBMEC surface. Typical knob attachment of the Pf-IRBC surface to the HBMEC was observed by TEM (Fig. 1A). Also observed were microvillus extensions of HBMEC to Pf-IRBC (Fig. 1B). Possible rouleaux of uninfected RBC were occasionally seen close to Pf-IRBC (not shown). SEM confirmed that there was Pf-IRBC adherence 1 h after addition to HBMEC (Fig. 1C). The rough knobby surface of a Pf-IRBC was in contrast to the smooth surface of an uninfected RBC next to it. In addition, microvilli protruding from HBMEC were observed touching Pf-IRBC but not the uninfected RBC. Very few microvilli were seen in control HBMEC monolayers that were not exposed to Pf-IRBC (Fig. 1D).
FIG. 1.
Parasite knob and host endothelial cell microvillus adherence. Confluent HBMEC monolayers were incubated with Pf-IRBC for 1 to 6 h and processed for TEM and SEM. (A) Adherence of Pf-IRBC on HBMEC, showing the typical knob attachment at 1 h. (B) Pf-IRBC adherence by host endothelial microvillus after 6 h of incubation. (C) SEM micrograph showing adherence of Pf-IRBC with the presence of knobs on the Pf-IRBC surface. Endothelial microvilli cluster around Pf-IRBC on the right and not around smooth uninfected RBC on the left. (D) Control endothelial cells with few microvillus extensions. Bars = 1 μm.
Dose and stage dependence of the Pf-IRBC-mediated increase in ICAM-1 expression on HBMEC.
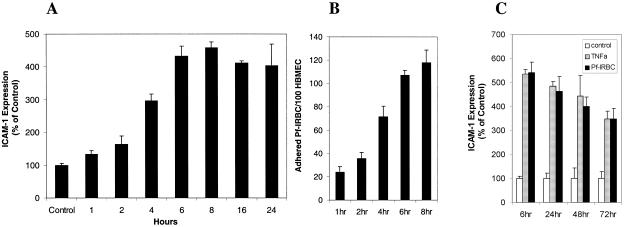
To determine the ability of Pf-IRBC (P. falciparum 3D7) to affect cell adhesion molecule expression on brain endothelium, Pf-IRBC were added to HBMEC and incubated for different times. Subsequently, ICAM-1 expression on the endothelium was quantified by ELISA. Pf-IRBC exposure resulted in a time-dependent increase in ICAM-1 expression on HBMEC, and a maximum-expression plateau was reached between 6 and 8 h and lasted for at least 24 h (Fig. 2A). Concomitantly, binding of Pf-IRBC was observed 1 to 2 h after addition, and the number of adherent Pf-IRBC increased gradually over time (Fig. 2B). After 8 h, the binding ratio for nonselected Pf-IRBC (P. falciparum 3D7) was 118 ± 10.8 Pf-IRBC per 100 HBMEC. Clone 3D7 is known to have low binding ability. For comparison, the binding of clone ITG-ICAM-1 to HBMEC was assessed and was found to be 3.9-fold greater than the binding of clone 3D7(not shown). The ICAM-1 expression on HBMEC that was induced by Pf-IRBC was reversible. Although ICAM-1 expression declined over time, it remained elevated for at least 72 h after withdrawal of Pf-IRBC (Fig. 2 C). For comparison, a similar pattern was observed with TNF-α.
FIG. 2.
Time course of induction of ICAM-1 expression on HBMEC. (A) Surface ICAM-1 expression on confluent HBMEC monolayers after incubation with Pf-IRBC (P. falciparum 3D7; 0.6 × 108 cells/well) for different times. The P value was <0.05 for all times compared to the medium control (n = 4). (B) Time course of binding of Pf-IRBC (P. falciparum 3D7; 0.6 × 108 cells/well) to confluent HBMEC monolayers. The P value was <0.05 for comparisons of different times (n = 4). No binding was observed with control RBC. (C) Reversibility of ICAM-1 expression after incubation of confluent HBMEC monolayers with Pf-IRBC (0.6 × 108 cells/well) for 6 h compared with the results for TNF-α. Subsequently, Pf-IRBC and TNF-α were removed, and regression of the induced surface ICAM-1 expression was assessed for up to 72 h. The P value was <0.05 for all times compared to the medium control (n = 4).
To determine dose dependence, different amounts of Pf-IRBC with the P. falciparum at mature trophozoite stages were added to confluent HBMEC and incubated for up to 20 h. We found that Pf-IRBC exposure led to a time- and concentration-dependent increase in ICAM-1 expression. The increase in ICAM-1 expression was proportional to the number of Pf-IRBC coincubated for 6 h with the HBMEC (Fig. 3A). Addition of 105 Pf-IRBC to 106 HBMEC (multiplicity of infection [MOI], 0.1) increased the ICAM-1 expression to 180% of the expression observed with controls containing medium alone. Addition of 106 or 107 Pf-IRBC (MOI, 1 to 10) resulted in an approximately 350% increase in ICAM-1 expression. With 5 × 107 or 108 Pf-IRBC (MOI, 50 to 100) a plateau corresponding to a 550% increase in ICAM-1 expression was observed; this increase in ICAM-1 expression was not observed when 108 uninfected RBC were added to HBMEC. Other P. falciparum clones (clones ITG and FCR selected for ICAM-1 and CSA binding, respectively) were also able to induce ICAM-1 expression (Fig. 3B). In addition, 108 Pf-IRBC failed to induce expression of ICAM-1 on HUVEC (Fig. 3C), whereas the positive control TNF-α increased ICAM-1 expression on HUVEC more than 300%. This indicates that P. falciparum infection of erythrocytes can lead to increased ICAM-1 expression on brain endothelial cells and that this phenomenon is more specific to brain endothelium than to HUVEC.
FIG. 3.
Dose dependence and specificity of Pf-IRBC-mediated ICAM-1 expression on HBMEC: HUVEC do not increase ICAM-1 expression in response to Pf-IRBC. (A) Surface ICAM-1 expression on confluent HBMEC monolayers after 6 h of incubation with different numbers of Pf-IRBC (solid bars) and control uninfected RBC (open bars). The P value was <0.001 for comparisons with the medium control and the RBC controls (n = 8). (B) Surface ICAM-1 expression on confluent HBMEC monolayers after 6 h of incubation with Pf-IRBC (0.6 × 108 cells/well), ITG-ICAM-1, and FCR-CSA compared to P. falciparum 3D7. The P value was <0.05 for comparisons with the medium control for all strains (n = 4). (C) Confluent HUVEC monolayers were incubated with 108 Pf-IRBC/well or TNF-α (10 ng/ml) for 6 h. The difference between the medium control and Pf-IRBC was insignificant. For TNF-α the P value was <0.001 for a comparison with the medium control (n = 8).
Next, we wondered whether the increase in ICAM-1 expression on HBMEC was limited to the mature stage of the P. falciparum parasite or whether younger stages of the parasite could also increase ICAM-1 expression. Only a marginal nonsignificant increase in ICAM-1 expression was observed when Pf-IRBC containing ring stages (108 cells/well) were added to HBMEC (not shown). This showed that the increase in ICAM-1 expression on HBMEC is dependent on the later and more mature stages of P. falciparum.
Surface and soluble parasite proteins affect ICAM-1 expression on HBMEC.
Pf-IRBC with P. falciparum in the mature trophozoite and schizont stages exhibit maximal expression of PfEMP1 and also have more knobs on their surfaces than Pf-IRBC with P. falciparum in the ring stage (20). The ability of surface components like PfEMP1 on the membranes of intact Pf-IRBC to induce ICAM-1 expression on HBMEC was examined further by enzymatic removal of extracellular Pf-IRBC membrane proteins with trypsin. Alternatively, the intraerythrocytic trophozoite-stage parasites were inactivated with artemisinin, which left the membrane surface structures of Pf-IRBC intact. Trypsin treatment reduced the increase in ICAM-1 expression on HBMEC by 30% at 6 h (Fig. 4A). At this time, artemisinin treatment also reduced induction of ICAM-1 expression by 21%. However, the difference disappeared upon further incubation of artemisinin-treated Pf-IRBC with HBMEC for up to 24 h. Artemisinin and trypsin treatment together resulted in a 52% decrease in ICAM-1 expression on HBMEC compared to the expression on HBMEC incubated with untreated Pf-IRBC.
FIG. 4.
Surface and soluble protein dependence of the Pf-IRBC increase in ICAM-1 expression. (A) Confluent monolayers of HBMEC were incubated with trypsinized Pf-IRBC and/or artemisinin (1 μM)-treated Pf-IRBC for 6 h. The P value was <0.001 for Pf-IRBC and for different treatments compared to the medium control (n = 8). The differences between different treatments and the Pf-IRBC treatment were also significant (P < 0.001; n = 8). (B) Intact Pf-IRBC and supernatant and pellet fractions of Pf-IRBC were added to confluent HBMEC monolayers and incubated for 6 h. The P value was <0.001 for comparisons with the medium control (n = 8).
To determine whether the ICAM-1 induction was due to an interaction of the Pf-IRBC surface proteins with HBMEC or whether additional soluble factors were involved, we fractionated whole Pf-IRBC lysate into separate membrane and soluble fractions and added these fractions to HBMEC (Fig. 4B). The ICAM-1 expression on HBMEC induced by the different fractions was assessed and compared with the ICAM-1 expression with intact Pf-IRBC from the same initial preparation. As expected, addition of intact Pf-IRBC resulted in an approximately 730% increase in ICAM-1 expression. The supernatant fraction increased ICAM-1 expression on HBMEC by 430% compared with the control, and the pellet faction increased ICAM-1 expression on HBMEC by 320% compared with the control. The pellet and supernatant fractions from noninfected RBC did not increase ICAM-1 expression (not shown). As the pellet fraction contained hemozoin, the abilities of both hemozoin and beta-hematin to affect ICAM-1 expression were examined. At low concentrations (1 μM), beta-hematin did not increase ICAM-1 expression. However, at concentrations of 5 to 20 μM, beta-hematin increased ICAM-1 expression. As a control, heme at similar concentrations did not affect ICAM-1 expression (not shown).
To show that Pf-IRBC secrete factors that affect activation of HBMEC, culture supernatants of Pf-IRBC with P. falciparum in the early trophozoite to schizont stages were collected. Addition of these culture supernatants increased ICAM-1 expression on HBMEC (not shown). Pf-IRBC at the trophozoite and schizont stages mature further and eventually lyse, which may also affect ICAM-1 expression. Therefore, supernatant of naturally lysed whole Pf-IRBC in the late schizont stage was collected. This supernatant was shown to increase ICAM-1 expression on the HBMEC surface approximately three- to fourfold compared to the expression in the medium control (not shown). A high concentration (20 mM) of the Pf-IRBC exported metabolite lactate in medium at pH 6.8 did not affect ICAM-1 expression (not shown).
Together, these data show that the increase in ICAM-1 expression on HBMEC is a multifactor process that involves both membrane-associated and soluble components of the Pf-IRBC.
Mechanism of Pf-IRBC-induced increase in ICAM-1 expression.
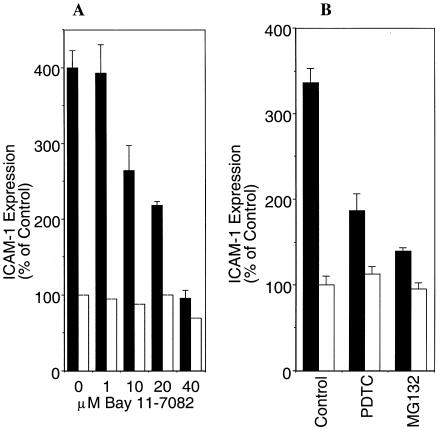
Regulation of adhesion molecule expression has been shown to involve the endothelial NF-κB system (39). Analyses of promoter elements of adhesion molecule genes, including ICAM-1, have revealed the presence of one to three NF-κB binding sites. To investigate the underlying signaling mechanisms of Pf-IRBC-induced ICAM-1 expression, we included two inhibitors of NF-κB, Bay 11-7082 and MG132. In addition, we also included pyrrolidine dithiocarbamate (PDTC), an antioxidant and indirect inhibitor of NF-κB activation, which acts by removing the reactive oxygen species (ROS) intermediates (39). All the inhibitors tested reduced the Pf-IRBC-induced ICAM-1 expression (Fig. 5A and B). At a concentration of 40 mM, Bay 11-7082 completely inhibited the Pf-IRBC-induced increase in ICAM-1 expression. All three inhibitors tested also inhibited a TNF-α-mediated increase in ICAM-1 expression (not shown). At all concentrations tested, the inhibitors alone did not affect the basal level of ICAM-1 expression on HBMEC. This indicates that the Pf-IRBC-mediated increase in ICAM-1 expression on HBMEC is mediated through NF-κB and may involve ROS.
FIG. 5.
NF-κB inhibitors reverse the Pf-IRBC increase in ICAM-1 expression on HBMEC. Confluent monolayers of HBMEC were pretreated with Bay 11-7082 (A), PDTC (100 μM) (B), or MG132 (10 μM) for 1 h, and this was followed by 6 h of incubation with and without Pf-IRBC. MG132 was removed after the 1-h pretreatment, and Bay 11-7082 and PDTC were present during Pf-IRBC incubation. The inhibition of ICAM-1 expression was significant compared to the data obtained with Pf-IRBC alone (P < 0.05; n = 4).
Pf-IRBC-induced nuclear translocation of NF-κB in HBMEC.
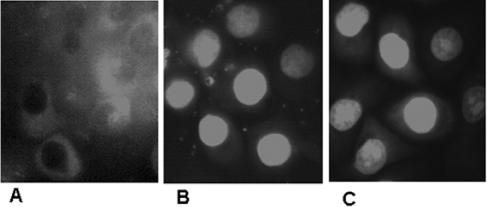
The inhibitor studies indicated that NF-κB is involved in the activation of HBMEC by Pf-IRBC. To verify that Pf-IRBC activation indeed results in translocation of NF-κB subunit P65 from the cytoplasm to the nuclei, we used immunocytochemistry with an antibody raised against the P65 subunit of NF-κB. This showed that the NF-κB P65 subunit was localized in the cytoplasm of nonstimulated HBMEC (Fig. 6A). HBMEC exposed to Pf-IRBC showed bright fluorescent staining of the NF-κB P65 subunit in the nucleus (Fig. 6B). TNF-α treatment was used as a positive control and also resulted in bright staining of the NF-κB P65 subunit inside the nucleus (Fig. 6C). Immunoblotting of cytosolic and nuclear fractions of Pf-IRBC-activated HBMEC produced similar results (not shown). This analysis showed that activation of HBMEC by Pf-IRBC involves NF-κB and translocation of the P65 subunit from the cytoplasm to the nucleus.
FIG. 6.
Pf-IRBC-induced nuclear translocation of NF-κB in HBMEC. (A) In control HBMEC, the majority of the NF-κB P65 subunit signal was in the cytoplasm. (B) Pf-IRBC exposure (6 h), showing staining for the NF-κB P65 subunit in the nuclei of HBMEC. (C) TNF-α treatment, as a positive control, also induced staining of the NF-κB P65 subunit inside the nucleus. A representative photograph is shown (n = 2).
DISCUSSION
The clinical effects of CM are thought to be mediated by a number of factors, including cytoadherence and sequestration of Pf-IRBC in brain endothelial vessels. Adhesion of Pf-IRBC is a prerequisite for the sequestration in brain endothelial high venules. Several host molecules have been reported to mediate cytoadherence and sequestration of Pf-IRBC; these molecules include ICAM-1, CSA, CD36, thrombospondin, and CD31. ICAM-1 is predominantly involved in adherence to brain endothelium in CM (38, 49). However, the events at the BBB that occur during and after Pf-IRBC binding and sequestration are unknown. Therefore, in this investigation we initially visualized the interaction of Pf-IRBC with HBMEC in vitro and subsequently examined whether Pf-IRBC can activate the BBB endothelium.
Adherence of Pf-IRBC on brain endothelium is considered a primary event in the transition from febrile illness to complicated and fatal CM. We visualized the interaction between Pf-IRBC and HBMEC at the ultrastructural level using scanning and transmission electron microscopy. Our results show that Pf-IRBC are close to the membrane surface of the HBMEC and that knob structures on the Pf-IRBC surface are involved. In addition, we observed involvement of the microvilli protruding from HBMEC to Pf-IRBC, and this involvement has remarkable similarities to the involvement observed in brain biopsies of human CM patients (30). The presence of microvilli appears to be a result of activation of HBMEC and compromises the smoothness of the endothelium. After lysis of Pf-IRBC and reopening of the venules, the microvilli on the activated BBB endothelial surface still may affect the blood flow and clearance of Pf-IRBC lysed fragments and may interfere with recovery.
We hypothesized that adhesion of Pf-IRBC on host endothelium could lead to brain endothelial activation, such as increased cell adhesion molecule expression. ICAM-1 is a marker for activation of endothelial cells and has been shown to be a ligand for PfEMP1, and in addition, ICAM-1 expression has been shown to correlate with vessels containing sequestrated Pf-IRBC in CM (38, 49). To explore the possibility that Pf-IRBC could be responsible for the observed expression of ICAM-1 in the vessels containing the sequestered Pf-IRBC, we first tested the ability of Pf-IRBC to modulate ICAM-1 expression on HBMEC. In response to Pf-IRBC, HBMEC surface ICAM-1 expression quickly increased. After 1 h, a significant increase was observed, and then there was a further time-dependent increase and maximal expression occurred 6 h after addition of Pf-IRBC. The ICAM-1 level then remained high for up to 24 h. The upregulation of ICAM-1 levels in response to Pf-IRBC was dose dependent, and there was saturation when higher numbers of Pf-IRBC were added to HBMEC. After removal of Pf-IRBC, which can occur in vivo after maturation and lysis of Pf-IRBC, the ICAM-1 expression slowly decreased, but it remained significantly above the baseline level for at least 72 h. This implies that after an initial cycle of Pf-IRBC adherence, in which other possibly non-PfEMP1 adhesion factors may play a role, the remaining increase in ICAM-1 expression may provide ligands for subsequent rounds of Pf-IRBC adhesion.
Immature ring-stage parasites that did not have surface knobs with the PfEMP1 ligand did not increase ICAM-1 expression, which showed that there was stage dependence. The specificity of the response was also illustrated by the fact that an increase in ICAM-1 expression was not observed when commercially obtained HUVEC were exposed to Pf-IRBC. Our observations are in contrast to those of Esslinger et al. (11) and Viebig et al. (50), who described expression of ICAM-1 by HUVEC in response to direct activation with Pf-IRBC. The difference between our data and the data of these workers could be due to a difference in parasite clones, alternate var gene expression, or different genetic backgrounds of the endothelial cells. Alternatively, serum containing activators could predispose certain endothelial cells to Pf-IRBC activation, which is in agreement with the findings of Chakravorty and Craig (7). Involvement of extracellular membrane-associated factors was demonstrated by trypsinization of extracellular protein components on the Pf-IRBC surface. Trypsinization reduced total ICAM-1 expression by 30%. Under the trypsinization conditions used, the Pf-IRBC remained intact (as determined by microscopy), but soluble factors may still have leaked out. These soluble factors may have accounted for the remaining ability of trypsinized Pf-IRBC to activate HBMEC, resulting in increased ICAM-1 expression. Fractionation of Pf-IRBC and addition of Pf-IRBC culture supernatants showed that in addition to the membrane-associated factors, soluble components indeed contributed to the ability of the parasite to induce ICAM-1 expression on HBMEC. It has been shown that the membranes of Pf-IRBC are very fragile and permeable (2); thus, it is possible that parasite factors may diffuse out of Pf-IRBC. That factors “leak” out of the Pf-IRBC was also shown by the addition of Pf-IRBC culture supernatants to HBMEC, which led to increased ICAM-1 expression. Alternatively, this residual effect may have been mediated by Pf-IRBC membrane components that are not trypsin sensitive.
Artemisinin treatment of Pf-IRBC, which is lethal to the intracellular parasite but leaves Pf-IRBC surface molecules intact, decreased Pf-IRBC-induced ICAM-1 expression by 21%. This reduction was observed only with shorter incubations times and did not occur after prolonged incubation. Although the artemisinin treatment used was lethal for the parasite, soluble parasite factors may still have been inside the treated Pf-IRBC. During subsequent prolonged incubation, these soluble factors may have diffused out and thus may have been partially responsible for the observed induction of ICAM-1 expression on HBMEC. When both Pf-IRBC surface proteins were removed and intraerythrocytic parasites were inactivated by concomitant trypsin and artemisinin treatments, 48% of the original effect remained. Again, the remaining activity could have been the result of residual parasite soluble factors inside Pf-IRBC.
During sequestration, Pf-IRBC mature and subsequently lyse, which releases the contents of the infected erythrocytes. Significant induction of ICAM-1 was also observed in response to Pf-IRBC lysates but not with control RBC lysates, again suggesting that parasite-specific factors are involved. In addition, supernatants of naturally ruptured Pf-IRBC with P. falciparum at the schizont stage were able increased ICAM-1 expression. The nature of the soluble factors involved is unclear. Hemoglobin is released from both infected and noninfected RBC. Since noninfected RBC do not increase ICAM-1 expression, hemoglobin appears not to be a contributing factor. Low concentrations (up to 1 μM) of hemozoin or beta-hematin, present in Pf-IRBC, did not affect ICAM-1 expression, whereas higher concentrations (5 to 10 μM) were able to increase ICAM-1 expression and thus could have contributed to the effect observed.
So far, the data on activation of HBMEC by whole Pf-IRBC, culture supernatant, soluble and membrane Pf-IRBC fractions, and trypsin-artemisinin treatment show that the Pf-IRBC-induced increase in ICAM-1 expression on HBMEC is multifactorial, involving both membrane-associated and soluble factors.
During CM, an increase in ICAM-1 expression on brain endothelial cells has been observed, and we were able to mimic this increase in vitro using our HBMEC. However, the intracellular signal transduction mechanisms are still not clear. Involvement of the transcription factor NF-κB in ICAM-1 expression pathways has been shown previously (10). NF-κB can be activated in response to various extracellular stimuli, such as TNF-α, which leads to translocation of NF-κB from the cytoplasm into the nucleus (10). With the aid of several direct and indirect NF-κB inhibitors, we demonstrated that Pf-IRBC-induced ICAM-1 expression on the HBMEC surface is mediated through NF-κB. The compound Bay11-7082 inhibits phosphorylation of I-κB (36), and MG132 inhibits the 26S proteasome subunit (1, 47), which is required to cleave I-κB alpha in order to activate NF-κB. Both Bay11-708 and MG132 inhibit Pf-IRBC-induced ICAM-1 expression, confirming that NF-κB is involved. For comparison, TNF-α signaling has been shown to activate proteasomal degradation of the I-κB subunit of the NF-κB complex, leading to translocation of the transcription factor into the nucleus (10). Immunocytochemistry confirmed that there is Pf-IRBC-induced translocation of NF-κB subunit p65 from the cytoplasm into the nuclei of HBMEC. NF-κB can be activated in the presence of ROS (9, 15). PDTC is an antioxidant which clears out reactive oxygen species and through this pathway can inhibit NF-κB. PDTC was also able to partially inhibit the Pf-IRBC-mediated increase in ICAM-1 expression These observations suggest that in addition to the proteasome pathway, ROS may be involved in Pf-IRBC-mediated signaling events that lead to NF-κB activation. The origin of the ROS is not clear. Extracellular Pf-IRBC-derived ROS may be involved, but it is also possible that ROS are generated inside the HBMEC by certain parasite-derived factors. Growth and proliferation of the malaria parasite in hemoglobin-laden Pf-IRBC can generate a wide variety of ROS (4, 18). The periodic chill and fever events during chronic malaria infection are due to the synchronous rupture of mature schizonts (13). When schizonts rupture, the contents of the erythrocytes and specific parasite factors, including ROS, are released. Synchronized rupture of adherent Pf-IRBC on brain endothelium could indicate that there is a high local concentration of parasitic factors close to the BBB endothelium. A sudden increase in the amount of these factors could initiate a cascade of molecular events in host cells that leads to localized activation of the endothelium. Upon rupture, the venules are open again to blood flow, which allows erythrocyte debris and contents and parasite factors to be washed away for clearance by immune cells. This may allow restoration from the activation state of the BBB. This is in accordance with the reversible nature of CM pathogenesis. However, Pf-IRBC-induced ICAM-1 on HBMEC may lead to a subsequent round of adhesion and sequestration, possibly perpetuating disease.
In conclusion, we show that Pf-IRBC are able to activate the endothelium of the BBB. Here we present direct evidence that Pf-IRBC are able to increase ICAM-1 expression on the HBMEC surface. Our data support the notion that Pf-IRBC membrane-mediated interactions with brain endothelial cells are involved in CM pathogenesis. In addition, we show that parasite-specific soluble factors play an important role in HBMEC monolayer activation. Our findings explain the observations of Porta et al. (38) and Turner et al. (49), who found that in postmortem brain tissues sequestration of high endothelial venules correlates with ICAM-1 expression and not with plasma TNF-α levels in CM patients. Pf-IRBC elicits intracellular HBMEC signaling pathways involving NF-κB. To our knowledge, this is the first report showing that NF-κB is involved in ICAM-1 upregulation by malaria parasites. Detailed characterization of the pathway for NF-κB activation by Pf-IRBC could be important for developing a therapeutic intervention for CM pathogenesis. Importantly, identification and characterization of the membrane and soluble factors from Pf-IRBC that are responsible for mediating activation of the BBB could lead to novel targets for development of drugs for CM.
Acknowledgments
This work was supported by a pilot grant from the Malaria Research Institute of the Johns Hopkins Bloomberg School of Public Health to M.F.S. and by grants NIH RO1 A145774 (to D.J.S.) and NCCR GPDGCRC RR0052 for isolation and culture of human erythrocytes. A.K.T. was the recipient of a d'Arbeloff Fellowship from the Millipore Foundation for 2004-2005.
We thank Carlum Shiu for his help and Donna Pierce for her excellent technical assistance.
Editor: V. J. DiRita
REFERENCES
- 1.Adam, D., K. Wiegmann, S. dam-Klages, A. Ruff, and M. Kronke. 1996. A novel cytoplasmic domain of the p55 tumor necrosis factor receptor initiates the neutral sphingomyelinase pathway. J. Biol. Chem. 271:14617-14622. [DOI] [PubMed] [Google Scholar]
- 2.Alkhalil, A., J. V. Cohn, M. A. Wagner, J. S. Cabrera, T. Rajapandi, and S. A. Desai. 2004. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood 104:4279-4286. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas, K., and E. M. Riley. 2002. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169:2956-2963. [DOI] [PubMed] [Google Scholar]
- 4.Atamna, H., and H. Ginsburg. 1993. Origin of reactive oxygen species in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 61:231-241. [DOI] [PubMed] [Google Scholar]
- 5.Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Howard. 1995. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. [DOI] [PubMed] [Google Scholar]
- 6.Buzby, J. S., E. M. Knoppel, and M. S. Cairo. 1994. Coordinate regulation of Steel factor, its receptor (Kit), and cytoadhesion molecule (ICAM-1 and ELAM-1) mRNA expression in human vascular endothelial cells of differing origins. Exp. Hematol. 22:122-129. [PubMed] [Google Scholar]
- 7.Chakravorty, S. J., and A. Craig. 2005. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur. J. Cell Biol. 84:15-27. [DOI] [PubMed] [Google Scholar]
- 8.Chang, Y. C., M. F. Stins, M. J. McCaffery, G. F. Miller, D. R. Pare, T. Dam, M. Paul-Satyaseela, K. S. Kim, and K. J. Kwon-Chung. 2004. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi, J. H., W. J. Chung, S. J. Han, H. B. Lee, I. W. Choi, H. K. Lee, K. Y. Jang, D. G. Lee, S. S. Han, K. H. Park, and S. Y. IM. 2000. Selective involvement of reactive oxygen intermediates in platelet-activating factor-mediated activation of NF-kappaB. Inflammation 24:385-398. [DOI] [PubMed] [Google Scholar]
- 10.Collins, T., M. A. Read, A. S. Neish, M. Z. Whitley, D. Thanos, and T. Maniatis. 1995. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 9:899-909. [PubMed] [Google Scholar]
- 11.Esslinger, C. W., S. Picot, and P. Ambroise-Thomas. 1994. Intra-erythrocytic Plasmodium falciparum induces up-regulation of inter-cellular adhesion molecule-1 on human endothelial cells in vitro. Scand. J. Immunol. 39:229-232. [DOI] [PubMed] [Google Scholar]
- 12.Friedland, J. S., M. Ho, D. G. Remick, D. Bunnag, N. J. White, and G. E. Griffin. 1993. Interleukin-8 and Plasmodium falciparum malaria in Thailand. Trans. R. Soc. Trop. Med. Hyg. 87:54-55. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, C. R., R. P. Markus, and L. Madeira. 2001. Tertian and quartan fevers: temporal regulation in malarial infection. J. Biol. Rhythms 16:436-443. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, J. P., R. A. Pinches, D. J. Roberts, and C. I. Newbold. 1996. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 93:3503-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg, A. K., and B. B. Aggarwal. 2002. Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 39:509-517. [DOI] [PubMed] [Google Scholar]
- 16.Garlanda, C., and E. Dejana. 1997. Heterogeneity of endothelial cells. Specific markers. Arterioscler. Thromb. Vasc. Biol. 17:1193-1202. [DOI] [PubMed] [Google Scholar]
- 17.Gimenez, F., L. S. de Barraud, C. Fernandez, P. Pino, and D. Mazier. 2003. Tumor necrosis factor alpha in the pathogenesis of cerebral malaria. Cell. Mol. Life Sci. 60:1623-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginsburg, H., and H. Atamna. 1994. The redox status of malaria-infected erythrocytes: an overview with an emphasis on unresolved problems. Parasite 1:5-13. [DOI] [PubMed] [Google Scholar]
- 19.Grab, D. J., O. Nikolskaia, Y. V. Kim, J. D. Lonsdale-Eccles, S. Ito, T. Hara, T. Fukuma, E. Nyarko, K. J. Kim, M. F. Stins, M. J. Delannoy, J. Rodgers, and K. S. Kim. 2004. African trypanosome interactions with an in vitro model of the human blood-brain barrier. J. Parasitol. 90:970-979. [DOI] [PubMed] [Google Scholar]
- 20.Gruenberg, J., D. R. Allred, and I. W. Sherman. 1983. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 97:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta, S. K., P. G. Lysko, K. Pillarisetti, E. Ohlstein, and J. M. Stadel. 1998. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J. Biol. Chem. 273:4282-4287. [DOI] [PubMed] [Google Scholar]
- 22.Huang, S. H., M. F. Stins, and K. S. Kim. 2000. Bacterial penetration across the blood-brain barrier during the development of neonatal meningitis. Microbes Infect. 2:1237-1244. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, D. E., R. O. Loo, M. T. Holyst, and P. J. Newman. 1997. Identification and characterization of functional cation coordination sites in platelet endothelial cell adhesion molecule-1. Biochemistry 36:9395-9404. [DOI] [PubMed] [Google Scholar]
- 24.Jong, A. Y., M. F. Stins, S. H. Huang, S. H. Chen, and K. S. Kim. 2001. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 69:4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karunaweera, N. D., G. E. Grau, P. Gamage, R. Carter, and K. N. Mendis. 1992. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. USA 89:3200-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer, K. J., S. C. Kan, and W. A. Siddiqui. 1982. Concentration of Plasmodium falciparum-infected erythrocytes by density gradient centrifugation in Percoll. J. Parasitol. 68:336-337. [PubMed] [Google Scholar]
- 27.Kwiatkowski, D., A. V. Hill, I. Sambou, P. Twumasi, J. Castracane, K. R. Manogue, A. Cerami, D. R. Brewster, and B. M. Greenwood. 1990. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336:1201-1204. [DOI] [PubMed] [Google Scholar]
- 28.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 29.Lyke, K. E., R. Burges, Y. Cissoko, L. Sangare, M. Dao, I. Diarra, A. Kone, R. Harley, C. V. Plowe, O. K. Doumbo, and M. B. Sztein. 2004. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 72:5630-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacPherson, G. G., M. J. Warrell, N. J. White, S. Looareesuwan, and D. A. Warrell. 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am. J. Pathol. 119:385-401. [PMC free article] [PubMed] [Google Scholar]
- 31.Mshana, R. N., J. Boulandi, N. M. Mshana, J. Mayombo, and G. Mendome. 1991. Cytokines in the pathogenesis of malaria: levels of IL-I beta, IL-4, IL-6, TNF-alpha and IFN-gamma in plasma of healthy individuals and malaria patients in a holoendemic area. J. Clin. Lab. Immunol. 34:131-139. [PubMed] [Google Scholar]
- 32.Mung'Ala-Odera, V., R. W. Snow, and C. R. Newton. 2004. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 71:64-70. [PubMed] [Google Scholar]
- 33.Newbold, C., P. Warn, G. Black, A. Berendt, A. Craig, B. Snow, M. Msobo, N. Peshu, and K. Marsh. 1997. Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am. J. Trop. Med. Hyg. 57:389-398. [DOI] [PubMed] [Google Scholar]
- 34.Petzelbauer, P., J. R. Bender, J. Wilson, and J. S. Pober. 1993. Heterogeneity of dermal microvascular endothelial cell antigen expression and cytokine responsiveness in situ and in cell culture. J. Immunol. 151:5062-5072. [PubMed] [Google Scholar]
- 35.Peyron, F., C. Caux-Menetrier, P. Roux-Lombard, T. Niyongabo, P. Aubry, and P. Deloron. 1994. Soluble intercellular adhesion molecule-1 and E-selectin levels in plasma of falciparum malaria patients and their lack of correlation with levels of tumor necrosis factor alpha, interleukin 1 alpha (IL-1 alpha), and IL-10. Clin. Diagn. Lab Immunol. 1:741-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 37.Pongponratn, E., M. Riganti, T. Harinasuta, and D. Bunnag. 1985. Electron microscopy of the human brain in cerebral malaria. Southeast Asian J. Trop. Med. Public Health 16:219-227. [PubMed] [Google Scholar]
- 38.Porta, J., A. Carota, G. P. Pizzolato, E. Wildi, M. C. Widmer, C. Margairaz, and G. E. Grau. 1993. Immunopathological changes in human cerebral malaria. Clin. Neuropathol. 12:142-146. [PubMed] [Google Scholar]
- 39.Schreck, R., B. Meier, D. N. Mannel, W. Droge, and P. A. Baeuerle. 1992. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 175:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silamut, K., N. H. Phu, C. Whitty, G. D. Turner, K. Louwrier, N. T. Mai, J. A. Simpson, T. T. Hien, and N. J. White. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava, K., and S. K. Puri. 2004. Plasmodium falciparum: modified medium composition supports continuous cultivation with foetal bovine serum. Exp. Parasitol. 108:74-75. [DOI] [PubMed] [Google Scholar]
- 42.Stins, M. F., J. Badger, and K. K. Sik. 2001. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30:19-28. [DOI] [PubMed] [Google Scholar]
- 43.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 44.Stins, M. F., P. V. Nemani, C. Wass, and K. S. Kim. 1999. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect. Immun. 67:5522-5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stins, M. F., D. Pearce, C. F. Di, A. Erdreich-Epstein, C. A. Pardo, and K. K. Sik. 2003. Induction of intercellular adhesion molecule-1 on human brain endothelial cells by HIV-1 gp120: role of CD4 and chemokine coreceptors. Lab. Investig. 83:1787-1798. [DOI] [PubMed] [Google Scholar]
- 46.Trager, W., and J. B. Jenson. 1978. Cultivation of malarial parasites. Nature 273:621-622. [DOI] [PubMed] [Google Scholar]
- 47.Tsubuki, S., Y. Saito, M. Tomioka, H. Ito, and S. Kawashima. 1996. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. J. Biochem. 119:572-576. [DOI] [PubMed] [Google Scholar]
- 48.Turner, G. D., V. C. Ly, T. H. Nguyen, T. H. Tran, H. P. Nguyen, D. Bethell, S. Wyllie, K. Louwrier, S. B. Fox, K. C. Gatter, N. P. Day, T. H. Tran, N. J. White, and A. R. Berendt. 1998. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am. J. Pathol. 152:1477-1487. [PMC free article] [PubMed] [Google Scholar]
- 49.Turner, G. D., H. Morrison, M. Jones, T. M. Davis, S. Looareesuwan, I. D. Buley, K. C. Gatter, C. I. Newbold, S. Pukritayakamee, B. Nagachinta, et al. 1994. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 145:1057-1069. [PMC free article] [PubMed] [Google Scholar]
- 50.Viebig, N. K., U. Wulbrand, R. Forster, K. T. Andrews, M. Lanzer, and P. A. Knolle. 2005. Direct activation of human endothelial cells by Plasmodium falciparum-infected erythrocytes. Infect. Immun. 73:3271-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]