Abstract
The opportunistic pathogen Pseudomonas aeruginosa utilizes a type III secretion system (T3SS) to intoxicate eukaryotic host cells. Transcription of the T3SS is induced under calcium-limited growth conditions or following intimate contact of P. aeruginosa with host cells. In the present study, we demonstrate that expression of the T3SS is controlled by two distinct regulatory mechanisms and that these mechanisms are differentially activated in a host cell-dependent manner. The first mechanism is dependent upon ExsC, a regulatory protein that couples transcription of the T3SS to the activity of the type III secretion machinery. ExsC is essential for induction of the T3SS under low-calcium-growth conditions and for T3SS-dependent cytotoxicity towards social amoebae, insect cells, and erythrocytes. The second regulatory mechanism functions independently of ExsC and is sufficient to elicit T3SS-dependent cytotoxicity towards certain types of mammalian cells. Although this second pathway (ExsC independent) is sufficient, an exsC mutant demonstrates a lag in the induction of cytotoxicity towards Chinese hamster ovary cells and is attenuated for virulence in a mouse pneumonia model. We propose that the ExsC-dependent pathway is required for full cytotoxicity towards all host cell types tested whereas the ExsC-independent pathway may represent an adaptation that allows P. aeruginosa to increase expression of the T3SS in response to specific types of mammalian cells.
Pseudomonas aeruginosa is a versatile opportunistic pathogen capable of causing disease in humans and a variety of plants, invertebrates, and vertebrates. The broad host range of P. aeruginosa has been exploited to identify virulence determinants required for pathogenesis in disparate hosts (16, 18, 21). One such determinant, a type III secretion system (T3SS), is important for pathogenesis towards social amoebae, insect larvae, and mammals (1, 18, 20, 25). T3SSs are found in many gram-negative pathogens and function by translocating bacterial effector proteins directly into the cytoplasm of eukaryotic host cells. P. aeruginosa utilizes the T3SS to translocate four effector proteins (ExoS, ExoT, ExoU, and ExoY) with antihost properties. ExoS and ExoT are related bifunctional toxins with Rho GTPase-activating protein and ADP-ribosyltransferase domains (3). The Rho GTPase-activating protein activities interfere with the rearrangement of the actin cytoskeleton to inhibit phagocytosis (10). The ADP-ribosyltransferase activity of ExoT further inhibits phagocytosis by disrupting CrkI/II-mediated signal transduction (27). In contrast, ExoS ADP-ribosylates Ras-signaling molecules and is cytotoxic towards host cells (9). ExoU has a patatin-like phospholipase domain with broad activity towards phospholipids and neutral lipids and functions as an acute cytotoxin, resulting in rapid cell death following translocation into the host cell cytoplasm (23, 24). The final effector protein, ExoY, is an adenylate cyclase (36).
The P. aeruginosa T3SS consists of nearly 40 coordinately regulated genes encoding structural components of the secretion and translocation machinery, effector proteins, and regulatory factors (6). Transcription of the T3SS is induced under calcium-limited growth conditions (hereafter referred to as low Ca2+) or following intimate contact of P. aeruginosa with eukaryotic host cells (6, 30). Although the signaling mechanism involved in host cell contact is poorly understood, two distinct physiological responses to low Ca2+ contribute to regulation of the T3SS. The first involves a membrane-bound adenylate cyclase (CyaB) and a cyclic AMP (cAMP)-dependent transcriptional regulator (Vfr). Vfr is a global regulator of P. aeruginosa virulence determinants, including the T3SS, type IV pili, flagella, and a type II secretion system (26, 33). In response to low Ca2+, CyaB-dependent cAMP production leads to Vfr activation. Consequently, mutants lacking either cyaB or vfr are defective for expression of the T3SS (33). The mechanism by which Vfr regulates expression of the T3SS has not been determined.
The second physiological response to low Ca2+ occurs directly at the level of secretion. Through a poorly understood mechanism, low Ca2+ converts the type III secretion machinery from a secretion-incompetent to a secretion-competent state (17). Recent studies have demonstrated that transcription of the T3SS is intimately linked to secretion competence (5, 17, 29). Whereas transcription of the T3SS is repressed when the secretion machinery is inactive, transcription is derepressed when the secretion machinery is activated under low-Ca2+ conditions. The mechanism of coupling transcription to secretion competence involves a cascade of four interacting regulatory proteins (ExsA, ExsD, ExsC, and ExsE). ExsA is a positive regulator of transcription and binds to a defined nucleotide sequence in each of the T3SS promoters (7, 14). ExsD functions as an antiactivator by directly binding to and inhibiting ExsA activity, while ExsC functions as an anti-antiactivator by binding to and inhibiting the negative regulatory activity of ExsD (5, 17). ExsC also functions as a chaperone for ExsE (5, 29). Finally, ExsE directly binds to and inhibits ExsC activity (22, 29). The mechanism of coupling secretion to transcription lies in the fact that ExsE is itself secreted by the T3SS under low-Ca2+ conditions. When the secretion machinery is inactive (high Ca2+), elevated intracellular levels of ExsE sequester ExsC, and ExsD inhibits ExsA-dependent transcription. When the type III secretion machinery is active, however, ExsE is secreted from cells, thereby reducing the intracellular concentration of ExsE. This allows ExsC to bind to and sequester ExsD, and liberated ExsA is available to activate transcription of the T3SS. As predicted by this model, mutants lacking either exsA or exsC are defective for transcription of the T3SS under low-Ca2+ conditions, whereas exsD or exsE mutants are constitutive for transcription irrespective of growth conditions (5, 17, 29).
In this study, we examine the role of ExsC in regulating expression of the T3SS in tissue culture infection models. Of note, we report a conditional requirement for ExsC dependent upon the type of host cell used in the assay. Whereas exsC is essential for T3SS-dependent cytotoxicity towards Sf9 insect cells, social amoebae, and erythrocytes, this requirement was eliminated when a variety of mammalian cell lines were examined. Our data suggest that two independent regulatory mechanisms are involved in regulation of the T3SS and that host factors contribute to transcriptional induction of the T3SS.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and β-galactosidase assays.
The bacterial strains and plasmids used in this study are described in Table 1. The P. aeruginosa strains were maintained on Vogel-Bonner minimal medium (31) with antibiotics as required (300 μg/ml carbenicillin, 100 μg/ml tetracycline, 100 μg/ml gentamicin). For expression of the T3SS in bacteriological medium, strains were grown at 37°C with vigorous aeration in Trypticase soy broth supplemented with 1% glycerol, 100 mM monosodium glutamate, and 2 mM EGTA as previously described (17). Strains harboring the PexsD-lacZ reporters were grown under the indicated conditions until the absorbance at 540 nm reached 1.0. β-Galactosidase activity was measured using the substrate p-nitrophenyl-β-d-galactopyranoside as previously described (5).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain, genotype, or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strain | ||
| PA103 | Wild-type parental strain | 17 |
| Genotypes | ||
| exsA::Ω | Insertional mutant lacking ExsA transcriptional activator; no T3SS expression | 8 |
| ΔexsC | Deletion mutant lacking ExsC anti-anti-activator; conditionally repressed for T3SS expression | 5 |
| ΔexsD | Deletion mutant lacking ExsD anti-activator; constitutive T3SS expression | 17 |
| Δvfr | Deletion mutant lacking Vfr; minimal T3SS expression | This study |
| Δvfr ΔexsC | Double mutant lacking both Vfr and ExsC; no T3SS expression | This study |
| Δvfr ΔexsD | Double mutant lacking both Vfr and ExsD; constitutive T3SS expression | This study |
| Plasmids | ||
| pUCP18 | E. coli-P. aeruginosa multicopy shuttle vector | 32 |
| pexsC | ExsC overexpression clone under transcriptional control of native promoter in pUCP18 | This study |
| pvfr | vfr overexpression clone under transcriptional control of native promoter in pUCP18 | This study |
| Mini-CTX-PexsD-lacZ | Vector for single copy integration of the PexsD-lacZ reporter onto the chromosomal attB site | 17 |
Mutant and plasmid construction.
Oligonucleotide primers were used to amplify the upstream (5′-EcoRI-CCCAAAGAATTCTCGGTTGCTTGCGGCTCTGCC and 5′-BamHI-CCCAAAGGATCCGCGCAGCTTGTCTAGGTGTTTG) and downstream (5′-BamHI-CCCAAAGGATCCACCCATGAAAAAGGCCG and 5′-HindIII-CCCAAAAAGCTTGGCATTCAACTGGCCCACGATG) flanking regions of vfr by PCR. PCR products were sequentially ligated into pEX18Tc (12), resulting in pEX18TcΔvfr with an in-frame deletion of Vfr codons 18 to 215. A Gmr-GFP cassette flanked by FLP recombination sites was excised from pPS858 (12) and ligated into the BamHI site of pEX18TcΔvfr, yielding pEX18TcΔvfr::Gmr-GFP. This construct was mobilized into wild-type, exsC, and exsD strains by electroporation, and gentamicin-resistant, tetracycline-sensitive transformants were isolated. Plasmid pFLP2 (12), expressing the FLP recombinase, was introduced into the mutants to excise the Gmr-GFP cassette. The vfr mutations were confirmed by PCR and Southern blot hybridization. The PexsD-lacZ transcriptional reporter was introduced into each of the strains as previously described (17). The pvfr expression plasmid was constructed by cloning a 1.3-kb XhoI PCR fragment encoding Vfr under the transcriptional control of its native promoter into pUCP18 (32).
Coculture cytotoxicity assays.
All tissue culture cells were obtained from the American Type Culture Collection. Chinese hamster ovary (CHO) cells (CCL-61) were maintained in 75-mm culture dishes in Ham's F-12 nutrient medium supplemented with 10% fetal calf serum, 50 units of penicillin and streptomycin/ml, 2 mM l-glutamine, 0.12% sodium bicarbonate, and 2.5 mM HEPES (Invitrogen Corp., Carlsbad, California) at 37°C in 5% CO2. A549 (CCL-165), Calu-3 (HTB-55), HeLa (CCL-2), and MDCK (NBL-2) cells were maintained in 75-mm dishes in Dulbecco's modified Eagle's medium with Earl's salts (Invitrogen), supplemented with 10% fetal calf serum and 50 units of penicillin and streptomycin/ml at 37°C in 5% CO2. RAW264.7 (TIB-71) cells were maintained in Dulbecco's modified Eagle's medium supplemented with l-glutamine (4 mM), sodium bicarbonate (1.5 g/liter), and glucose (4.5g/liter). Sf9 insect cells (CRL-1711) were cultivated in Sf900 II complete medium (Invitrogen) supplemented with 2 mM l-glutamine in T-25 culture flasks at 28°C under serum-free conditions. For coculture studies, mammalian cells were seeded in 24-well tissue culture plates in their respective media without antibiotics and incubated for 16 to 18 h at 37°C in 5% CO2. Under these conditions, cells typically reached 80 to 85% confluence with approximately 2 × 105 cells/well. The medium was removed, and cells were washed once with phosphate-buffered saline before the addition of the bacterial inoculum. The bacterial inoculum was prepared by growing P. aeruginosa strains on Vogel-Bonner minimal medium plates for 16 to 18 h at 37°C. Cells were suspended (2 × 106 CFU/ml) in prewarmed tissue culture medium, and 1 ml of the suspension was transferred to tissue culture wells (multiplicity of infection [MOI] of 10:1). Plates were centrifuged (500 × g, 5 min, 25°C) and then incubated at 37°C in 5% CO2 for the indicated times. The Sf9 coculture assays were performed as described above, with the following modifications. Sf9 cells were harvested from a T-25 flask by gentle pipetting and then seeded in 24-well plates at 1 × 106 cells per well. After 1 hour of incubation at 28°C, the medium was removed and replaced with 1 ml of the bacterial inoculum (107 CFU) suspended in Sf900 II medium (MOI of 10:1). The plate was centrifuged and incubated at 28°C for 4 h.
Following the coculture incubation, the plates were centrifuged (500 × g, 5 min, 25°C) and 50 μl of the supernatant was transferred to a 96-well plate and assayed for lactate dehydrogenase (LDH) release using the Cytotox 96 system according to the manufacturer's instructions (Promega, Madison, WI). Control wells lacking bacteria were used to calculate the background level of LDH release (normalized to 0%). To calculate the total amount of LDH present (100%), cells were treated with 0.1 ml of the lysis solution provided in the kit prior to performing the assay.
Hemolysis assays.
Sheep erythrocytes were obtained from Elmira Biologicals (Iowa City, Iowa). Just prior to coculture, the erythrocytes were washed with phosphate-buffered saline (1 ml) three times and suspended (108 cells/ml) in RPMI 1640 (Invitrogen) medium. The P. aeruginosa strains were grown to mid-log phase in LB medium at 37°C, washed three times with prewarmed RPMI medium, and suspended at 109 CFU/ml. For the hemolysis assay, 0.1 ml of the erythrocyte suspension was combined with 0.1 ml of the bacterial suspension, mixed gently, and centrifuged (500 × g, 5 min, 25°C). The cocultures were incubated at 37°C for 1 h. To assay for hemoglobin release, the erythrocyte/bacterial pellet was gently suspended and then centrifuged (500 × g, 5 min, 25°C) to sediment-intact erythrocytes. Supernatant (100 μl) from each tube was transferred to a clear, flat-bottom, 96-well plate and read (absorbance at 550 nm) in a microtiter plate reader. An uninfected control was used to calculate the background level of hemolysis. This value was subtracted from those for all of the remaining samples. The total amount of hemoglobin in the cells (100%) was determined by lysing erythrocytes with 0.1% sodium dodecyl sulfate.
Bacterial adherence assay.
For adherence assays, CHO cells were seeded in 24-well plates on collagen-coated coverslips. To minimize ExoU-dependent cytotoxicity, the wells were treated with 27 μM methyl-arachidonyl-fluorophosphonate (MAFP; Sigma Chemical Co.) as previously described (19). After the addition of the bacterial inoculum (2 × 106 bacteria), the plates were centrifuged (5 min, 500 × g, 25°C) and incubated at 37°C for 1 h. The wells were washed three times with Ham's F-12 (1 ml) medium, fixed with methanol for 10 min at room temperature, stained with Giemsa stain for 2 h (4), and then washed with water. The coverslips were removed from the wells, dried, and mounted on microscope slides. The reported values represent the average number of adherent bacteria per randomly selected CHO cell (n > 20) as determined by microscopic counting. The adherence assays were performed in duplicate and repeated twice.
Dictyostelium discoideum and mouse pneumonia infection models.
The D. discoideum infection model was performed by adding serial dilutions of D. discoideum to each of the P. aeruginosa strains and plating them on SM/5 medium as previously described (20). For the mouse pneumonia model, groups of C57BL/6 mice (n = 4, female, age 6 to 8 weeks) (Harlan Laboratories, Indianapolis, IN) were anesthetized with ketamine-xylazine and infected intratracheally with a 50-μl (5 × 105 CFU) inoculum of the indicated P. aeruginosa strains (2). At 6 or 16 h postinfection, the animals were euthanized according to Animal Care Guidelines, and blood samples were obtained from the right ventricle. Organs were harvested, perfused free of intravascular cells, and homogenized. The bacteria were enumerated by plate counting, and the reported values were normalized to volume (blood) or weight (lung and liver). Statistical analyses were performed using analysis of variance (ANOVA) followed by Tukey's test for multiple comparisons (Prism GraphPad software, San Diego, CA). The animal studies were approved by the University of Iowa Institutional Animal Care and Use Committee.
RESULTS
ExsC is not required for T3SS-dependent cytotoxicity towards CHO cells.
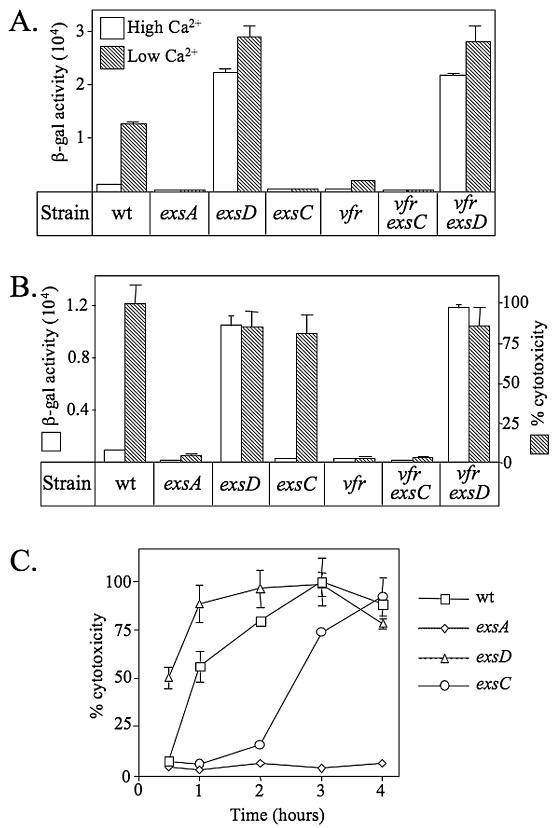
We previously reported that a transcriptional reporter (PexsD-lacZ) consisting of an ExsA-dependent promoter (PexsD) fused to lacZ is induced when P. aeruginosa is grown under low-Ca2+ conditions (Fig. 1A) (17). Furthermore, transcription is derepressed in a mutant lacking the ExsD antiactivator and is repressed in a mutant lacking the ExsC anti-antiactivator (5, 17). To determine whether ExsC is required for expression of the T3SS in response to host cell contact, the T3SS-dependent cytotoxicity of an exsC mutant towards CHO cells was monitored in a coculture assay. In this assay, the translocation of the ExoU cytotoxin into host cells by the T3SS leads to rapid CHO cell lysis and the release of LDH. When P. aeruginosa strains are cultured in tissue culture medium in the absence of CHO cells, the transcription of the PexsD-lacZ reporter resembles the pattern seen in bacteriological medium lacking EGTA (Fig. 1A versus 1B, white bars). Under both conditions, there is minimal expression of the lacZ reporter in the wild-type, exsA, and exsC strains and derepressed expression in the exsD mutant. When cocultured with CHO cells, both wild-type P. aeruginosa and an exsD mutant induce a strong cytotoxic response (100% and 86% LDH release, respectively), whereas an exsA mutant is minimally cytotoxic (<4% LDH release) (Fig. 1B, hatched bars). These data are consistent with previous reports demonstrating contact-dependent expression of the T3SS (13, 30).
FIG. 1.
Genes required for T3SS expression in response to low-Ca2+ and CHO cell signals. (A) The indicated strains carrying the PexsD-lacZ reporter were grown under noninducing (lacking EGTA; white bars) or inducing (with EGTA; hatched bars) conditions for the expression of the T3SS and assayed for β-galactosidase (β-gal) activity (reported in Miller units). (B) P. aeruginosa strains were cultured in Ham's F12 medium alone (white bars) or in the presence (hatched bars) of CHO cells (10:1 MOI) for 4 h and assayed for the expression of the PexsD-lacZ reporter and for T3SS-dependent cytotoxicity, respectively. Percent cytotoxicity (based on the release of LDH) was calculated relative to an uninfected control (0% cytotoxicity) and the amount of LDH released by coculture with the wild-type (wt) strain (100% cytotoxicity). Under these conditions, the wild-type strain released 72% of the LDH compared to cells completely lysed with Triton X-100. (C) Time course analysis of T3SS-dependent cytotoxicity towards CHO cells. P. aeruginosa strains were cocultured with CHO cells (10:1 MOI) for the indicated times and then assayed for LDH release. The reported values represent averages from at least three independent experiments, and error bars indicate the standard errors of the means.
Surprisingly, the exsC mutant, though defective for transcription of the T3SS under low-Ca2+ and tissue culture growth conditions, retained strong cytotoxic activity (81% LDH release) when cocultured with CHO cells (Fig. 1B, hatched bars). Time course experiments revealed that the exsC mutant demonstrates an initial lag in the induction of cytotoxicity compared to the parental strain (Fig. 1C). By 4 h of coculture, however, there is essentially no difference in the amount of LDH released by the wild type, exsD, or exsC strain. Although a reduced growth rate under the coculture conditions could account for the delayed cytotoxicity of the exsC mutant, all of the mutants used in this study were found to have growth rates similar to that of the wild-type strain (data not shown). To confirm that the cytotoxicity of the exsC mutant is dependent upon expression of the T3SS, the coculture experiment was repeated in the presence of an inhibitor (MAFP) of ExoU phospholipase activity (19). Compared to the untreated control, MAFP treatment resulted in a 97% inhibition of the LDH release seen with the exsC mutant (data not shown). These data demonstrate that the cytotoxicity of the exsC mutant results from ExoU translocation into host cells and that contact with CHO cells bypasses the ExsC requirement seen under low-Ca2+ conditions for expression of the T3SS.
The cytotoxicity of an exsC mutant is Vfr dependent.
The cAMP-dependent transcription factor Vfr is required for expression of the T3SS in response to low Ca2+ and host cell contact (33). To determine whether Vfr is required for ExsC-independent cytotoxicity towards CHO cells, a markerless in-frame deletion of vfr was introduced into the wild-type, exsC, and exsD backgrounds. In transcriptional assays, the vfr and vfr exsC mutants were significantly impaired in the expression of the PexsD-lacZ reporter under both low-Ca2+ and tissue culture growth conditions (Fig. 1A and B). In contrast, the vfr exsD double mutant constitutively expressed the PexsD-lacZ reporter irrespective of growth conditions. These data demonstrate that the derepressed phenotype of the exsD mutant is dominant over that of the vfr mutant and suggest that Vfr might function by relieving ExsD-dependent repression of the T3SS.
In coculture assays, both the vfr and the vfr exsC mutants were defective in the cytotoxic response towards CHO cells (Fig. 1B). In these assays, cytotoxicity requires the type IV pilus-mediated adherence of P. aeruginosa to host cells and expression of the T3SS (28, 33). Since Vfr regulates expression of type IV pili, the twitching motilities and adherence properties of the mutants were examined. Strains carrying the Δvfr allele demonstrated reductions in twitching motilities compared to that of the parental strain (Table 2). Despite the defect in twitching, however, there was little difference in the properties of the vfr and vfr exsD mutants regarding their adherence to CHO cells (Table 2). These data indicate that the lack of cytotoxicity in the vfr and vfr exsC mutants cannot be attributed to a decrease in adherence. This conclusion is further supported by the fact that the vfr exsD double mutant retains full cytotoxic activity towards CHO cells and suggests that the reduced cytotoxicities of the vfr and vfr exsC mutants do not result from pleiotropic effects of the vfr mutation but rather from a defect in the expression of the T3SS.
TABLE 2.
Twitching motilities and adherence properties of strains used in this study
| Genotype | Twitching zone diam (mm) | CHO cell adherencea |
|---|---|---|
| Wild type | 16.0 ± 3 | 2.0 ± 0.1 |
| exsA::Ω | 15.3 ± 1 | 3.3 ± 1.4 |
| ΔexsC | 19.0 ± 2 | 2.9 ± 1.6 |
| ΔexsD | 16.0 ± 1 | 1.6 ± 0.5 |
| Δvfr | 6.6 ± 2 | 5.3 ± 0.9 |
| ΔexsC Δvfr | 5.6 ± 1.5 | 4.2 ± 1 |
| ΔexsD Δvfr | 6.0 ± 2 | 3.5 ± 3 |
Number of adherent P. aeruginosa bacteria per CHO cell.
Differential requirements of exsC for induction of T3SS-dependent cytotoxicity.
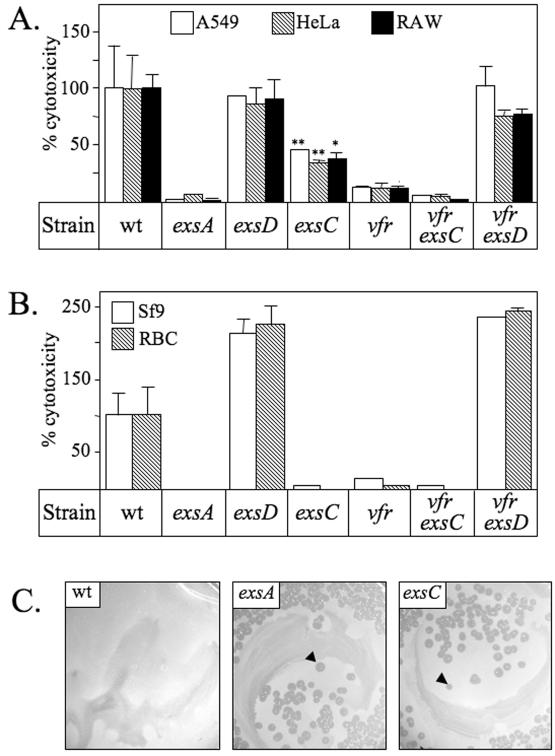
To examine whether the cytotoxicity of an exsC mutant is unique to CHO cells, the LDH release assays were repeated using a variety of cell lines. Initially, a panel of mammalian cell lines, including A549 (alveolar carcinoma), HeLa (cervical adenocarcinoma), MDCK (canine kidney), Calu-3 (lung adenocarcinoma), and RAW264.7 (transformed macrophage), was examined. Coculture of P. aeruginosa strains with these cell lines recapitulated the same general pattern as that seen with CHO cells, whereby the exsC mutant was capable of eliciting T3SS-dependent cytotoxicity (Fig. 2A and data not shown). Unlike with the CHO cells, however, where the cytotoxicities of the exsC mutant and parental strain were nearly identical by 4 h of coculture (Fig. 1B), the cytotoxicity of the exsC mutant was only 40 to 50% of that of the parental strain for A549, HeLa, and Raw264.7 cells following a 4 h coculture. This may reflect differences in the susceptibilities of individual cell lines to T3SS-mediated cytotoxicity, cell line-specific differences in P. aeruginosa adherence, or altered kinetics of T3SS induction dependent upon host cell determinants. Nevertheless, these data indicate that ExsC is not absolutely required for the cytotoxic response of P. aeruginosa and suggest that an ExsC-independent mechanism is involved in induction of the T3SS in response to mammalian epithelial cells and macrophages.
FIG. 2.
Cytotoxicity of the exsC mutant towards mammalian and nonmammalian cells. (A-B) P. aeruginosa strains were cocultured (10:1 MOI) with the indicated eukaryotic cells for 4 h and assayed for LDH release (A549, HeLa, RAW, and Sf9 cells) or hemolysis (erythrocytes). Percent cytotoxicity was calculated relative to an uninfected control (0% cytotoxicity) and to the amount of LDH or hemoglobin released following coculture with wild-type (wt) P. aeruginosa (100% cytotoxicity). The reported values represent averages from at least three independent experiments, and error bars indicate the standard errors of the means. The statistical significance (one-way ANOVA test, 95% confidence interval) between the cytotoxicities elicited by the exsA and exsC mutants for each cell type is indicated (*, P < 0.01; **, P < 0.001). (C) Dictyostelium discoideum plaquing assay. The indicated P. aeruginosa strains were mixed with D. discoideum and plated on nutrient agar. Whereas D. discoideum does not form plaques on a wild-type lawn of P. aeruginosa, plaques (indicated by arrowheads) are readily formed on the lawn formed by strains lacking the expression of the T3SS.
In the next set of experiments, the cytotoxicity of the exsC mutant towards Sf9 insect cells and sheep erythrocytes was examined. The Sf9 insect cell and erythrocyte cytotoxicity assays are based on LDH and hemoglobin release, respectively. Similar to our findings with mammalian cell lines, the wild-type, exsD, and vfr exsD strains elicit a strong cytotoxic response towards Sf9 cells and erythrocytes, whereas the exsA, vfr, and vfr exsC mutants are largely noncytotoxic (Fig. 2B). Surprisingly, the exsC mutant was incapable of eliciting T3SS-dependent cytotoxicity towards Sf9 cells and erythrocytes. Two control experiments suggest that the diminished cytotoxicity of the exsC mutant is specific to the type of host cell used in the coculture rather than the coculture conditions. First, an exsC mutant retains T3SS-dependent cytotoxicity towards CHO cells when cocultured in Sf9 growth medium (data not shown). Second, although the erythrocyte cocultures were conducted in either Ham's F12 medium or Dulbecco's modified Eagle's medium, both of which support ExsC-independent cytotoxicity towards CHO cells, the exsC mutant lacked cytotoxicity towards erythrocytes (data not shown). Combined, these data strongly support the conclusion that host cell determinants, rather than culture conditions, dictate whether the ExsC-independent killing mechanism is functional.
To further examine the host cell requirements for ExsC-independent killing, an infection model using the social amoeba Dictyostelium discoideum was employed. A previous study reported T3SS-dependent killing of D. discoideum by P. aeruginosa (20). To assay for killing, D. discoideum and P. aeruginosa strains were mixed, plated on nutrient agar, and monitored for plaque formation (20). When plated with wild-type P. aeruginosa (Fig. 2C) or the exsD mutant (data not shown), the D. discoideum amoebae are killed and no plaques are formed. When plated with strains defective for expression of the T33S (exsA [Fig. 2C], vfr, and vfr exsC [data not shown]), however, D. discoideum forms plaques on the P. aeruginosa lawns. Similar to our findings for Sf9 cells and erythrocytes, the exsC mutant is defective for expression of the T3SS in the presence of D. discoideum. Since the Sf9 cocultures and D. discoideum plaquing assays were performed at 28°C and 25°C, respectively, the possibility that the ExsC-independent killing mechanism observed for mammalian epithelial cells was nonfunctional at 28°C existed. When cocultured with CHO cells at 28°C, however, the exsC mutant showed a cytotoxic response similar to that observed at 37°C (data not shown). These data suggest that the ExsC-independent killing mechanism observed for epithelial cells is nonfunctional in response to Sf9 cells, erythrocytes, and social amoebae.
Complementation analyses suggest the presence of two distinct regulatory pathways.
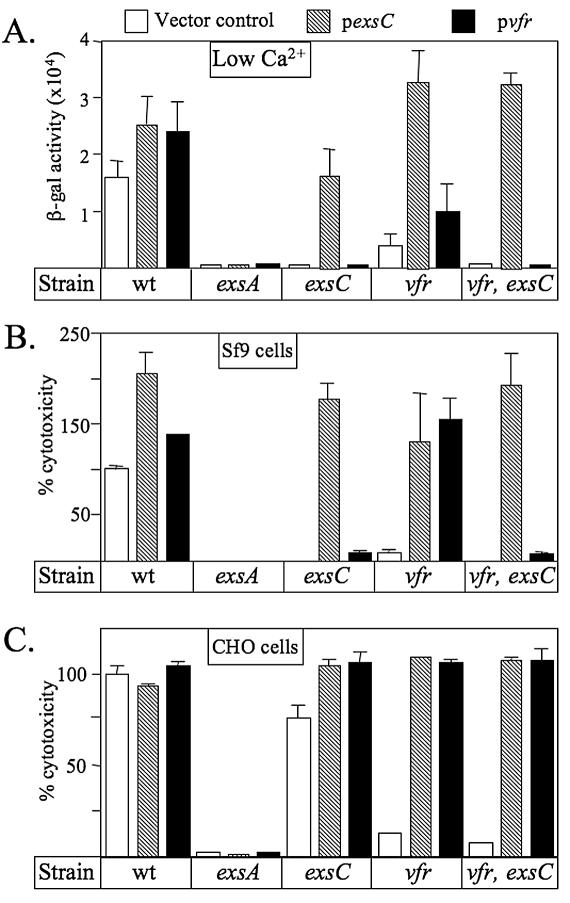
A complementation analysis was performed to further examine the role of ExsC and Vfr in regulation of the T3SS. P. aeruginosa strains were transformed with either a vector control, an ExsC expression plasmid (pexsC), or a Vfr expression plasmid (pvfr) and assayed for transcription of the PexsD-lacZ reporter under low-Ca2+ growth conditions (Fig. 3A) and for T3SS-dependent cytotoxicity towards Sf9 and CHO cells (Fig. 3B and C). In the wild-type background, plasmid-encoded ExsC or Vfr increased expression of the PexsD-lacZ reporter and T3SS dependent cytotoxicity towards Sf9 cells to various degrees (Fig. 3A to C). Conversely, neither plasmid was able to restore expression of the T3SS to an exsA mutant. These findings are consistent with previous studies describing the roles of ExsC, Vfr, and ExsA in regulation of the T3SS (5, 8, 33).
FIG. 3.
Complementation analysis of regulatory mutants. P. aeruginosa strains were transformed with pUCP18 (vector control), an ExsC expression plasmid (pexsC), or a Vfr expression plasmid (pvfr) and assayed for the expression of the PexsD-lacZ reporter (A) or T3SS-dependent cytotoxicity towards Sf9 (B) and CHO (C) cells. The reported values represent averages from at least three independent experiments, and error bars indicate the standard errors of the means. wt, wild type; β-gal, β-galactosidase.
The complementation patterns for the vfr, exsC, and vfr exsC mutants were nearly identical for the low-Ca2+ signal and Sf9 cells. In each case, plasmid-encoded ExsC complemented all three mutants for expression of the PexsD-lacZ reporter and for Sf9 cell cytotoxicity (Fig. 3A and B). In contrast, plasmid-encoded Vfr, though able to complement the Δvfr mutant, did not complement the exsC or vfr exsC mutant for PexsD-lacZ expression or Sf9 cell cytotoxicity. These data demonstrate that ExsC is essential for expression of the T3SS in response to the low-Ca2+ signal and Sf9 cells and suggest that ExsC overexpression can circumvent the requirement for Vfr.
A striking difference in the complementation patterns was seen in the CHO cell coculture assays. Whereas the cytotoxicities of the exsC and vfr exsC mutants towards Sf9 cells were restored only by overexpression of ExsC, the cytotoxicity of the same mutants towards CHO cells was restored by overexpression of either ExsC or Vfr (Fig. 3C). These data suggest that two distinct pathways are involved in the response of P. aeruginosa to mammalian cells. The first regulatory pathway requires both ExsC and Vfr and is essential for T3SS-dependent cytotoxicity towards Sf9 cells. In contrast, the second regulatory pathway is Vfr dependent but ExsC independent, may require host-specific signals (lacking Sf9 cells), and is sufficient to elicit cytotoxicity towards CHO cells.
An exsC mutant is attenuated for virulence in a mouse pneumonia model.
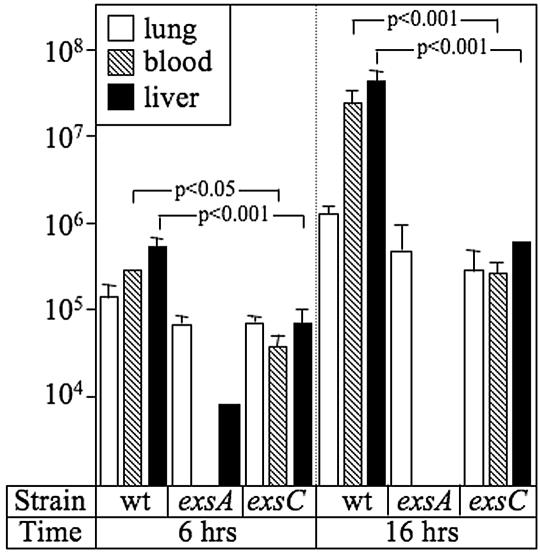
Although the ExsC-independent mechanism is sufficient to elicit cytotoxicity towards mammalian tissue culture cells, we hypothesized that an exsC mutant would be attenuated for virulence in an animal infection model for two reasons. First, a delay in the activation of the T3SS may increase the susceptibility of an exsC mutant to phagocytic clearance in the early stages of an infection. Second, although an exsC mutant retains cytotoxicity, the amount of LDH released in most susceptible cell lines is only 40 to 50% of that seen with wild-type P. aeruginosa (Fig. 2A). To examine the virulence properties of the exsC mutant, a murine pneumonia model was employed. C57B/6 mice were inoculated with wild-type P. aeruginosa and the exsA and exsC mutants (5 × 105 CFU) via tracheal instillation. The mice were euthanized at 6 or 16 h postinfection, and lung, blood, and liver samples were harvested and enumerated for P. aeruginosa by plate counting. At 6 and 16 h postinfection, the bacterial load in the lung was similar for each of the P. aeruginosa strains tested (Fig. 4, white bars). In the blood (hatched bars) and liver (black bars) samples, however, there was a significant difference in the bacterial loads between the wild-type strain and the regulatory mutants. In mice infected with the exsC mutant, the bacterial loads in the blood and liver samples were reduced 10- and 100-fold at 6 and 16 h, respectively, compared to mice infected with wild-type P. aeruginosa. The bacterial loads in the blood and liver samples were reduced even further in mice infected with the exsA mutant, which is consistent with the fact that exsA is essential for expression of the T3SS (8). The finding that the phenotype of the exsC mutant is intermediate to those of the wild-type and exsA strains demonstrates that both the ExsC-dependent and the ExsC-independent regulatory mechanisms contribute to the virulence of P. aeruginosa.
FIG. 4.
ExsC is required for full virulence in a murine pneumonia model. Groups (n = 4) of C57B/6 mice were infected with the indicated P. aeruginosa strains (5 × 105 CFU). At 6 or 16 h postinfection, the animals were euthanized and the bacterial loads in the lung (white bars), blood (hatched bars), and liver (black bars) samples were determined by plate counting. The reported values are numbers of CFU per ml of blood or per 1 g of lung or liver tissue. The statistical significance (one-way ANOVA test) between the bacterial loads in mice infected with the wild-type (wt) strain and the exsC mutant are indicated.
DISCUSSION
Expression of the P. aeruginosa T3SS is highly regulated by environmental signals, such as Ca2+ levels and the presence of eukaryotic cells (6, 30). Although the low-Ca2+ signal has been studied extensively, the physiological significance of this signal is unclear. In the present study, we show for the first time that P. aeruginosa perceives low-Ca2+ and host contact signals differentially and utilizes two genetically distinct regulatory mechanisms to control expression of the T3SS. The first mechanism is ExsC dependent and strictly required for transcription of the T3SS in response to low Ca2+ and for T3SS-dependent cytotoxicity towards sheep erythrocytes, Sf9 insect cells, and D. discoideum amoebae (Fig. 1A and 2B and C). In contrast, the second regulatory mechanism functions independently of ExsC and appears to be specifically involved in the response of P. aeruginosa to certain types of mammalian cells (Fig. 1B). Although ExsC is not essential for T3SS-dependent cytotoxicity towards mammalian cells, an exsC mutant has delayed kinetics for induction of cytotoxicity towards CHO cells and decreased cytotoxicity towards epithelial and macrophage cell lines (Fig. 1C and 2A). These data suggest that the ExsC-dependent mechanism contributes to the cytotoxic response of P. aeruginosa towards all host cell types, albeit to various degrees.
ExsC functions as a component of the ExsECDA regulatory cascade which couples the transcription of the T3SS to the activity of the type III secretion machinery (5, 22, 29). Activation of the signaling cascade requires that ExsE be exported from P. aeruginosa by secretion into the culture medium (29) or by translocation into host cells (M. Urbanowski and T. L. Yahr, unpublished data). The only requirement for the activation of the ExsC-dependent pathway, therefore, would be a host cell surface susceptible to T3SS-mediated translocation and permissive for P. aeruginosa adherence. Based on these observations, we hypothesize that the ExsC-dependent pathway represents a mechanism for generic activation of the T3SS in response to a broad range of eukaryotic cell surfaces. This generic response may be important for the survival of P. aeruginosa in soil and water environments where the organism is constantly challenged by eukaryotic predators.
The model for the induction of the T3SS by the ExsECDA regulatory cascade predicts that the ExsC anti-antiactivator and the ExsD antiactivator form binary complexes with each other and with ExsE and ExsA, respectively (5, 29). Under noninducing conditions, the binding equilibrium favors the formation of the ExsC-ExsE and ExsD-ExsA complexes, and transcription of the T3SS is repressed. When ExsE is secreted under inducing conditions, the binding equilibrium is shifted towards the formation of the ExsC-ExsD complex and the release of the ExsA activator. As predicted from this model, an exsC mutant is repressed for transcription of the T3SS in bacteriological medium due to the unmitigated binding of ExsD to ExsA (5) (Fig. 1A). The same situation likely accounts for the lack of cytotoxicity in the exsC mutant towards social amoebae, erythrocytes, and Sf9 cells. The fact that an exsC exsD double mutant is fully cytotoxic towards Sf9 cells is consistent with the idea that ExsD prevents expression of the T3SS under these conditions (data not shown) and suggests that the lack of cytotoxicity in an exsC mutant reflects a defect in the expression of the T3SS rather than a pleiotropic effect.
Though exsC is required for T3SS expression in response to low Ca2+, amoebae, erythrocytes, and Sf9 cells, this requirement is eliminated in response to most mammalian cell lines (with erythrocytes being the exception). This raises an interesting question. How is ExsD-dependent repression overcome in the absence of exsC? There would appear to be two requirements for this to occur. The first is a mechanism to suppress ExsD-mediated transcriptional repression independently of ExsC. Potential mechanisms include alternative transcription factors, a second anti-antiactivator analogous in function to ExsC, or posttranslational modifications that inhibit or increase the activities of ExsD or ExsA, respectively. The second requirement may be a host factor that activates the ExsC-independent mechanism. This host factor may involve a diffusible signal or a receptor specific to certain types of mammalian cells. Alternatively, cells resistant to ExsC-independent killing (Sf9 cells, amoebae, and erythrocytes) may possess a factor that prevents activation of the pathway.
Both the ExsC-dependent and the ExcC-independent regulatory mechanisms require Vfr; however, the link between Vfr and transcription of the T3SS is unclear. Vfr is required for maximal transcription of the T3SS in response to low Ca2+ and for cytotoxicity towards all eukaryotic cells examined (Fig. 1A and 2A to C). Our complementation analyses of the exsC and vfr mutants and the exsC vfr double mutant shed further light on the requirement for Vfr. As shown in Fig. 3A and B, exsC is necessary for expression of the T3SS in response to both low Ca2+ and Sf9 cells. Although Vfr also contributes to this response, the Vfr-dependent mechanism is not sufficient since vfr cannot complement the exsC vfr double mutant. These data demonstrate that the ExsC-dependent pathway is essential for the response to low Ca2+ and Sf9 cells. In contrast, overexpression of either ExsC or Vfr in the exsC vfr double mutant restores full cytotoxicity towards CHO cells. These data demonstrate that both the ExsC-dependent and the ExsC-independent (possibly mediated through Vfr) pathways contribute to type III-dependent cytotoxicity towards CHO cells. This conclusion is supported by the time course experiment in which the exsC mutant demonstrated a significant lag in cytotoxicity and the animal studies whereby the exsC mutant was attenuated for virulence.
Why might there be two separate regulatory mechanisms? One possibility is that multiple regulatory inputs provide a system for fine-tuning the expression of the T3SS. This may be important in limiting the expression of the T3SS until all of the proper host-specific signals are sensed. A second possibility is that two regulatory mechanisms allow for coordinate regulation of multiple virulence factors. This possibility is consistent with recent studies demonstrating coordinate regulation of the T3SS with polysaccharide and alginate biosynthesis, biofilm formation, and pyocin expression (11, 15, 33-35). At least four two-component regulatory systems have been implicated in the regulation of the T3SS and at least one of these may be involved in the activation of the Vfr and/or ExsC-independent regulatory mechanism. Future studies will be directed towards understanding the relationship between previously defined regulators of the T3SS and the ExsC-independent mechanism and identification and characterization of genes required for ExsC-independent regulation.
Acknowledgments
We thank Bob Wehrle for assistance with the D. discoideum experiments and Mark Urbanowski and Guinevere Lykken for technical assistance.
Support for these studies was provided by the Howard Hughes Medical Institute Biomedical Research Support Faculty Start-up Program (T.L.Y.), the University of Iowa W. M. Keck Microbial Communities and Cell Signaling Program (T.L.Y.), a VA Merit Review Grant (to G.W.H.), and the National Institutes of Health (RO1-AI055042 to T.L.Y., HL-073967-02 to G.W.H., and RR-017700 to A.A.).
Editor: J. T. Barbieri
REFERENCES
- 1.Apodaca, G., M. Bomsel, R. Lindstedt, J. Engel, D. Frank, K. E. Mostov, and J. Wiener-Kronish. 1995. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation-defective host cells are resistant to bacterial killing. Infect. Immun. 63:1541-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashare, A., M. M. Monick, L. S. Powers, T. Yarovinsky, and G. W. Hunninghake. 2006. Severe bacteremia results in loss of hepatic bacterial clearance. American J. Respir. Crit. Care Med. 173:644-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbieri, J. T., and J. Sun. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 152:79-92. [DOI] [PubMed] [Google Scholar]
- 4.Cervin, M. A., D. A. Simpson, A. L. Smith, and S. Lory. 1994. Differences in eucaryotic cell binding of Pseudomonas. Microb. Pathog. 17:291-299. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta, N., G. L. Lykken, M. C. Wolfgang, and T. L. Yahr. 2004. A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol. Microbiol. 53:297-308. [DOI] [PubMed] [Google Scholar]
- 6.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 7.Frank, D. W., and B. H. Iglewski. 1991. Cloning and sequence analysis of a trans-regulatory locus required for exoenzyme S synthesis in Pseudomonas aeruginosa. J. Bacteriol. 173:6460-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank, D. W., G. Nair, and H. P. Schweizer. 1994. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect. Immun. 62:554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganesan, A. K., T. S. Vincent, J. C. Olson, and J. T. Barbieri. 1999. Pseudomonas aeruginosa exoenzyme S disrupts Ras-mediated signal transduction by inhibiting guanine nucleotide exchange factor-catalyzed nucleotide exchange. J. Biol. Chem. 274:21823-21829. [DOI] [PubMed] [Google Scholar]
- 10.Goehring, U. M., G. Schmidt, K. J. Pederson, K. Aktories, and J. T. Barbieri. 1999. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J. Biol. Chem. 274:36369-36372. [DOI] [PubMed] [Google Scholar]
- 11.Goodman, A. L., B. Kulasekara, A. Rietsch, D. Boyd, R. S. Smith, and S. Lory. 2004. A signaling network reciprocally regulates genes associated with acute infection and chronic persistence in Pseudomonas aeruginosa. Dev. Cell 7:745-754. [DOI] [PubMed] [Google Scholar]
- 12.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 13.Hornef, M. W., A. Roggenkamp, A. M. Geiger, M. Hogardt, C. A. Jacobi, and J. Heesemann. 2000. Triggering the ExoS regulon of Pseudomonas aeruginosa: A GFP-reporter analysis of exoenzyme (Exo) S, ExoT and ExoU synthesis. Microb. Pathog. 29:329-343. [DOI] [PubMed] [Google Scholar]
- 14.Hovey, A. K., and D. W. Frank. 1995. Analyses of the DNA-binding and transcriptional activation properties of ExsA, the transcriptional activator of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 177:4427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuchma, S. L., J. P. Connolly, and G. A. O'Toole. 2005. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J. Bacteriol. 187:1441-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 17.McCaw, M. L., G. L. Lykken, P. K. Singh, and T. L. Yahr. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol. Microbiol. 46:1123-1133. [DOI] [PubMed] [Google Scholar]
- 18.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips, R. M., D. A. Six, E. A. Dennis, and P. Ghosh. 2003. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 278:41326-41332. [DOI] [PubMed] [Google Scholar]
- 20.Pukatzki, S., R. H. Kessin, and J. J. Mekalanos. 2002. The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 99:3159-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahme, L. G., F. M. Ausubel, H. Cao, E. Drenkard, B. C. Goumnerov, G. W. Lau, S. Mahajan-Miklos, J. Plotnikova, M. W. Tan, J. Tsongalis, C. L. Walendziewicz, and R. G. Tompkins. 2000. Plants and animals share functionally common bacterial virulence factors. Proc. Natl. Acad. Sci. USA 97:8815-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rietsch, A., I. Vallet-Gely, S. L. Dove, and J. J. Mekalanos. 2005. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 102:8006-8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato, H., and D. W. Frank. 2004. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 53:1279-1290. [DOI] [PubMed] [Google Scholar]
- 24.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 26.Suh, S. J., L. J. Runyen-Janecky, T. C. Maleniak, P. Hager, C. H. MacGregor, N. A. Zielinski-Mozny, P. V. Phibbs, Jr., and S. E. West. 2002. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa. Microbiology 148:1561-1569. [DOI] [PubMed] [Google Scholar]
- 27.Sun, J., and J. T. Barbieri. 2003. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. J. Biol. Chem. 278:32794-32800. [DOI] [PubMed] [Google Scholar]
- 28.Sundin, C., M. C. Wolfgang, S. Lory, A. Forsberg, and E. Frithz-Lindsten. 2002. Type IV pili are not specifically required for contact dependent translocation of exoenzymes by Pseudomonas aeruginosa. Microb. Pathog. 33:265-277. [DOI] [PubMed] [Google Scholar]
- 29.Urbanowski, M. L., G. L. Lykken, and T. L. Yahr. 2005. A secreted regulatory protein couples transcription to the secretory activity of the Pseudomonas aeruginosa type III secretion system. Proc. Natl. Acad. Sci. USA [DOI] [PMC free article] [PubMed]
- 30.Vallis, A. J., T. L. Yahr, J. T. Barbieri, and D. W. Frank. 1999. Regulation of ExoS production and secretion by Pseudomonas aeruginosa in response to tissue culture conditions. Infect. Immun. 67:914-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 32.West, S. E., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyen-Janecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 33.Wolfgang, M. C., V. T. Lee, M. E. Gilmore, and S. Lory. 2003. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 4:253-263. [DOI] [PubMed] [Google Scholar]
- 34.Wu, W., H. Badrane, S. Arora, H. V. Baker, and S. Jin. 2004. MucA-mediated coordination of type III secretion and alginate synthesis in Pseudomonas aeruginosa. J. Bacteriol. 186:7575-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu, W., and S. Jin. 2005. PtrB of Pseudomonas aeruginosa suppresses the type III secretion system under the stress of DNA damage. J. Bacteriol. 187:6058-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]